Back to Journals » Clinical Interventions in Aging » Volume 17
Self-Reported Sleep Characteristics Associated with Cardiovascular Disease Among Older Adults Living in Rural Eastern China: A Population-Based Study
Authors Qin Y, Liu R , Wang Y, Tang J, Cong L, Ren J, Tang S, Du Y
Received 10 February 2022
Accepted for publication 6 May 2022
Published 18 May 2022 Volume 2022:17 Pages 811—824
DOI https://doi.org/10.2147/CIA.S361876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Yu Qin,1,2,* Rui Liu,1,2,* Yongxiang Wang,1– 3 Jiyou Tang,4 Lin Cong,1– 3 Juan Ren,1,2 Shi Tang,1– 3 Yifeng Du1– 3
1Department of Neurology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 3Shandong Provincial Clinical Research Center for Neurological Diseases, Jinan, Shandong, People’s Republic of China; 4Department of Neurology, The First Affiliated Hospital of Shandong First Medical University, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yifeng Du; Shi Tang, Department of Neurology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, No. 324 Jingwuweiqi Road, Jinan, Shandong, 250021, People’s Republic of China, Tel +86 531 68776354 ; +86-18678780912, Fax +86 531 68776354, Email [email protected]; [email protected]
Purpose: To investigate the cross-sectional associations of self-reported sleep characteristics with cardiovascular diseases (CVDs) and cardiovascular multimorbidity in older adults living in rural Eastern China.
Patients and Methods: This population-based study included 4618 participants (age ≥ 65 years; 56.5% women) living in rural Eastern China. In March–September 2018, data were collected through interviews, clinical examinations, neuropsychological testing, and laboratory tests. Sleep parameters were assessed using the Pittsburgh Sleep Quality Index, Epworth Sleepiness Score, and Berlin questionnaire. Coronary heart disease (CHD), heart failure (HF), and stroke were defined according to in-person interviews, clinical and neurological examinations, and electrocardiogram examination. Data were analyzed using logistic regression and restricted cubic spline regression.
Results: CHD was diagnosed in 991 participants, HF in 135 participants, and stroke in 696 participants. The multivariable-adjusted odds ratio (OR) of CHD was 1.27 (95% CI, 1.09– 1.49) for sleep duration ≤ 6 hours/night (vs > 6– 8 hours/night), 1.40 (1.20– 1.62) for poor sleep quality, and 1.22 (1.04– 1.43) for high risk for obstructive sleep apnea (OSA). The OR of HF was 2.16 (1.38– 3.39) for sleep duration > 8 hours/night, and 1.76 (1.22– 2.54) for high risk for OSA. In addition, the OR of stroke was 1.23 (1.04– 1.46) for poor sleep quality, 1.32 (1.01– 1.72) for excessive daytime sleepiness, and 1.42 (1.19– 1.70) for high risk for OSA. The associations of poor sleep with cardiovascular multimorbidity (≥ 2 CVDs) were stronger than that of sleep problems with a single CVD.
Conclusion: Extreme sleep duration, high risk for OSA, and other sleep problems were associated with CVDs, especially cardiovascular multimorbidity.
Keywords: sleep, coronary heart disease, heart failure, stroke, cardiovascular multimorbidity
Introduction
Since the 1950s, China’s population has experienced a dramatic increase in life expectancy, leading to fast population aging in China. Population aging contributes to the increasing burden of cardiovascular disease (CVD). A study using a Markov computer simulation model found that along with population growth and aging in China, the annual CVD events would increase by more than 50% between 2010 and 2030.1 Since the 1990s, the age-standardized mortality of CVDs has steadily declined among urban residents in China, but the declining trend was not evident among people living in the rural areas.2 On the other hand, as people age, sleep structure and patterns change. Poor sleep quality and other sleep problems are highly prevalent in the rural-dwelling older adults in China.3 As an indispensable part of human life, sleep is an important modulator of cardiovascular health.
Several studies have revealed the associations of sleep problems with CVDs. A meta-analysis suggested that extreme sleep duration (<7 hours or ˃8 hours) and poor sleep quality were associated with a higher risk of cardiovascular events and CVD mortality, such as coronary heart disease (CHD) and stroke.4 However, the results of previous studies are not consistent. For example, a population-based study of 10,657 adults aged ≥15 years in China suggested that, compared to normal sleep duration (7 hours), short sleep duration (<6 hours) was associated with a higher likelihood of CHD.5 Another population-based study of 6538 participants in the US National Health and Nutrition Examination Survey (NHANES) showed that compared to normal sleep duration (6–8 hours), long sleep duration (˃8 hours) had an association with CHD.6 Meanwhile, a population-based study of 32,152 participants (mean age, 47 years) in the US NHANES Study showed that neither short nor long sleep durations was associated with CHD.7 Moreover, most of the previous study focused on middle-aged people. However, the prevalence of poor sleep conditions and CVDs are higher in older adults than that in middle-aged people. Furthermore, little attention has been paid to the Chinese rural-dwelling older adults. However, the prevalence of sleep problems among rural-dwelling older adults was higher than that among urban-dwelling older adults.3,8 The age-standardized mortality of CVDs has steadily declined among urban residents but not rural residents in China.2 Studying the associations of sleep characteristics and CVDs among older adults living in rural areas in China is important because over 50% of people live outside cities.9 In addition, most of the previous studies focused on the associations of sleep duration with CVDs, ignoring the multifaceted sleep problems in older adults. Finally, previous studies focused on a single CVD, rather than cardiovascular multimorbidity. This is important because cardiovascular multimorbidity is highly prevalent among adults, affecting around one-third of adults in the primary care settings.10
Therefore, using the baseline data of the Multidomain Interventions to Delay Dementia and Disability in Rural China (MIND-China) Study,11 we sought to assess the associations of a range of self-reported sleep characteristics (sleep duration, sleep latency, sleep efficiency, sleep disturbances, use of sleep medications, sleep quality, excessive daytime sleepiness [EDS], and risk for obstructive sleep apnea [OSA]) with CVDs (CHD, heart failure [HF], and stroke) and cardiovascular multimorbidity.
Materials and Methods
Study Design and Participants
The population-based study used data from the baseline examination of the MIND-China study,12–14 a participating project in the World-Wide FINGERS Network.11 In March–September 2018, 5765 individuals who were aged ≥60 years and living in the 52 villages of Yanlou Town, Yanggu County in western Shandong Province were enrolled in the MIND-China study. Of these, 519 participants who were aged 60–64 years were excluded due to relatively low participating rate. Of the 5246 participants who were aged 65 years and older, 302 were excluded due to dementia, 311 due to missing data on one or more sleep characteristics, and 15 due to missing information on the diagnosis of CVDs, leaving 4618 for the current analysis (Figure 1).
 |
Figure 1 Flowchart of the study participants. Abbreviation: MIND-China, the Multidomain Interventions to Delay Dementia and Disability in Rural China Study. |
The MIND-China project was approved by the Ethics Committee at Shandong Provincial Hospital in Jinan, Shandong Province. Written informed consent was obtained from all participants or informants. Research within the MIND-China project has been conducted in accordance with the ethical principles for medical research involving human participants expressed in the Declaration of Helsinki. MIND-China was registered in the Chinese Clinical Trial Registry (registration no.: ChiCTR1800017758).
Data Collection and Assessments
Data were collected through face-to-face interviews, clinical examinations, cognitive testing, and laboratory tests by trained medical staff following standard procedures. We collected data on sociodemographics (eg, age, sex, and education), health-related behavioral factors (eg, smoking, alcohol consumption, and leisure-time physical activity), a detailed medical history (eg, a physician’s diagnosis of hypertension, diabetes, and dyslipidemia), use of medications (eg, antihypertensive, hypoglycaemic, and lipid-lowering agents), physical and neurological examination (eg, height, weight, blood pressure, and neurological disorders), electrocardiogram (ECG) examination, and laboratory tests (eg, fasting blood glucose and lipids). All medications were classified and coded according to the Anatomical Therapeutic Chemical (ATC) classification system.12
Education was divided into illiteracy, primary school (1–5 years), and middle school and above (≥6 years). Body mass index (BMI) was calculated from height and weight (kg/m2) and was categorized as underweight (<18.5), normal (18.5–23.9), overweight (24–27.9), and obese (≥28).15 We categorized smoking status and alcohol consumption as never, former, and current. Regular physical activities during leisure time were defined as participating in any physical activities at least once a week. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or current use of antihypertensive drugs (ATC codes C02, C03, and C07-C09).16 Diabetes was defined as fasting blood glucose level ≥7.0 mmol/L, or use of antidiabetic agents (ATC code A10), or having a self-reported history of diabetes.17 Dyslipidemia was defined as total cholesterol ≥6.22 mmol/L, or triglycerides ≥2.27 mmol/L, or low-density lipoprotein cholesterol ≥4.14 mmol/L, or high-density lipoprotein cholesterol <1.04 mmol/L, or use of hypolipidemic agents (ATC code C10).18 The 15-item Geriatric Depression Scale (GDS-15, score range: 0–15) was used to evaluate depression in older individuals. The presence of depressive symptoms was defined as a total GDS-15 score ≥5.19,20
Assessments of Sleep Characteristics
Pittsburgh Sleep Quality Index (PSQI) is a validated self-rated questionnaire that assesses sleep quality and sleep disturbance over the past month.21 PSQI includes 19 items that assess seven sleep components: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. The sum of scores for the seven domains (score for each domain ranges from 0 to 3, no difficulty to severe difficulty) yielded a global score ranging from 0 to 21. We defined poor sleep quality as the overall PSQI score >5. The abnormal sleep in a specific domain was defined as the score ≥2 for that domain.22 The PSQI has demonstrated adequate internal consistency and validity in diverse populations.23
We categorized sleep duration into short (≤6 hours/night), normal (>6–8 hours/night), and long (>8 hours/night) sleep duration according to previous studies.24,25 The Epworth Sleepiness Score (ESS) is a questionnaire that assesses daytime sleepiness. The ESS asks the participants to rate on a scale of 0–3 the chances of dozing in eight different situations commonly met in daily life (sum of scores for the eight items varies from 0 to 24). EDS is defined as the ESS score >10.26
We used Berlin questionnaire (BQ) to screen people with high risk for OSA. BQ is a cost-effective screening tool for risk of OSA, with relatively high sensitivity (87%).27 The BQ includes 10 questions and information on BMI and hypertension, which were divided into 3 categories: snoring severity and cessation of breathing (category 1), symptoms of EDS (category 2), and BMI and hypertension (category 3). When having positive scores in more than one category, participants were considered as having a high risk for OSA.28
Definitions of CVDs and Cardiovascular Multimorbidity
CVDs included CHD, HF, atrial fibrillation (AF), and stroke.29 CHD was defined via self-reported history and physical examination as having angina pectoris, myocardial infarction, received coronary angioplasty, coronary artery bypass grafting, or myocardial infarction in ECG. HF was defined as a combination of self-reported history of HF or the judgement of HF by a physician via clinical examination. AF was defined according to self-reported physician diagnosis of AF or ECG examination. Stroke was defined according to self-reported ischemic and hemorrhagic stroke history or via neurological examination. Hypertension was not included in CVDs because of its high prevalence and it is a risk factor for CHD and stroke.30,31 A single CVD was defined as having only one of the above four CVDs. Cardiovascular multimorbidity was defined as concurrently having two or more of the above four CVDs.
Quality Control Procedures
The Quality Management Committee of MIND-China (project principal investigators, coordinator, specialists, and local town hospital administrators) is responsible for data collection, data assessments, database management, and collection and storage of biological samples. The study protocol and structured questionnaires were developed following brainstorm discussions among national and international scientists. Prior to the start of the assessments, all research staff (eg, clinicians and interviewers) were trained and certified according to the operations manual. Then, we conducted the pilot study to test the feasibility of the assessment procedure and the questionnaire. The automated biochemical analyzer and instruments (eg, electronic blood pressure monitor, 12-lead resting ECG, ultrasonic machine, and automated blood cell analyzer) are regularly calibrated and standardized following the manufacturer’s instructions.13
Statistical Analysis
We performed descriptive analysis about sociodemographic characteristics, lifestyle factors, sleep characteristics and clinical conditions of participants by sex. We presented mean (standard deviation [SD]) for continuous variables and frequencies (%) for categorical variables. We tested the statistical differences using Mann–Whitney U-test for skew distributed continuous variables, the t test for normal distributed continuous variables, and Chi-square test for categorical variables.
We employed binary logistic regression models to estimate the odds ratio (OR) and 95% confidence interval (CI) of sleep characteristics associated with CVDs while adjusting for different potential confounding factors. Multinomial logistic regression models were used to examine the associations of sleep characteristics with a single CVD and cardiovascular multimorbidity. Because of the small number of participants with AF (n = 66), we did not specifically examine the association of sleep characteristics with AF. We reported the main results from two models: Model 1 was controlled for age and sex; and Model 2 was additionally controlled for education, BMI, smoking, alcohol consumption, regular physical activities, hypertension, diabetes, dyslipidemia, and presence of depressive symptoms. Statistical interactions of age groups (<75 vs ≥75 years), sex, and education (illiteracy vs non-illiteracy) with sleep characteristics on CVDs were assessed by simultaneously entering the independent variables and their cross-product term into the same model of binary logistic regression. Stratified analysis was performed when statistical interactions were detected (P for interaction <0.05).
We used restricted cubic spline curves to evaluate the nonlinear associations of sleep duration with a single CVD and cardiovascular multimorbidity, in which logistic regression models were employed with 3 knots at the 10th, 50th, and 90th percentiles of sleep duration. The sleep duration of 7 hours/night was used as the reference group.
IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp, Armonk, NY, USA) was used for all the statistical analyses, except the restricted cubic spline regression analysis where the R package for Windows (version 4.0.4, R Foundation for Statistical Computing, Vienna, Austria) was used. We considered two-tailed P<0.05 to be statistically significant.
Results
Characteristics of the Study Participants
Table 1 details the demographic characteristics of the study participants. Of the 4618 participants, the mean age was 71.11 years (SD 4.85), 56.5% were women, and 37.7% were illiterate. Overall, the mean PSQI score of all participants was 5.94 (SD 4.15), the mean ESS score was 4.41 (SD 4.19), the prevalence of high risk for OSA was 29.2% in the total sample. Overall, 1567 (33.9%) participants were ascertained to have CVDs, including 991 (21.5%) with CHD, 135 (2.9%) with HF, 66 (1.4%) with AF, and 696 (15.1%) with stroke.
 |
Table 1 Characteristics of the Study Participants by Sex |
Associations of Sleep Characteristics with CHD, HF, and Stroke
After controlling for sociodemographic factors, prolonged sleep latency, short sleep duration, low sleep efficiency, sleep disturbances, use of sleep medications, poor sleep quality, and high risk for OSA were significantly associated with an increased likelihood of CHD (Table 2, Model 1). These associations remained significant in the multivariable-adjusted model (Table 2, Model 2).
 |
Table 2 Associations of Self-Reported Sleep Characteristics with Coronary Heart Disease and Heart Failure |
After controlling for sociodemographic factors, long sleep duration, sleep disturbances, use of sleep medications, poor sleep quality, and high risk for OSA were significantly associated with an increased likelihood of HF (Table 2, Model 1). Long sleep duration, sleep disturbances, use of sleep medications, and high risk for OSA were still associated with HF in the multivariable-adjusted model (Table 2, Model 2). Poor sleep quality was associated with HF after controlling for age and sex, but the association became non-significant in Model 2 when additionally controlling for multiple potential confounders.
After adjusting for age and sex, individuals with prolonged sleep latency, low sleep efficiency, sleep disturbances, poor sleep quality, EDS, high risk for OSA were associated with a higher likelihood of stroke (Table 3, Model 1). After multivariable-adjustment for additional potential confounders, these associations were slightly attenuated, except that the associations of prolonged sleep latency and low sleep efficiency with stroke became statistically non-significant (Table 3, Model 2). We found no significant associations of sleep duration and use of sleep medications with stroke.
 |
Table 3 Associations Between Self-Reported Sleep Characteristics and Stroke |
We did not detect any statistical interactions of age, sex, and education level with sleep characteristics on CVDs (data not shown).
Associations of Sleep Characteristics with a Single CVD and Cardiovascular Multimorbidity
After adjusting for sociodemographic factors, prolonged sleep latency, short sleep duration, low sleep efficiency, sleep disturbances, poor sleep quality, and high risk for OSA were significantly associated with increased likelihoods of both a single CVD and cardiovascular multimorbidity (Table 4, Model 1). These associations remained significant in the multivariable-adjusted model (Table 4, Model 2). The associations of above sleep characteristics with cardiovascular multimorbidity were stronger than those with a single CVD. EDS was significantly associated with increased likelihoods of a single CVD in Model 1 but not in Model 2. Long sleep duration and use of sleep medications were significantly associated with cardiovascular multimorbidity, but not with a single CVD (Table 4).
 |
Table 4 Associations of Self-Reported Sleep Characteristics with a Single CVD and Cardiovascular Multimorbidity |
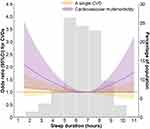
After adjusting for multiple potential confounders, restricted cubic splines showed a U-shaped association of sleep duration with cardiovascular multimorbidity (P for nonlinear association = 0.013). The sleep duration of 6.75 hours/night was associated with the lowest likelihood of cardiovascular multimorbidity. However, restricted cubic splines did not show the nonlinear association of sleep duration with a single CVD (P for nonlinear association >0.05) (Figure 2).
Discussion
In this large-scale population-based study of older adults living in rural Eastern China, we found that sleep problems such as prolonged sleep latency, extreme sleep duration, low sleep efficiency, sleep disturbances, use of sleep medications, poor sleep quality, EDS or high risk for OSA were associated with CVDs and cardiovascular multimorbidity.
We found that short sleep duration was associated with CHD and long sleep duration was associated with HF. Similarly, a population-based study of 10,657 adults (age ≥15 years) in China suggested that compared to normal sleep duration (7 hours), short sleep duration (<6 hours) was associated with CHD.5 By contrast, the US NHANES Study (mean age, 62 years) showed that, compared to normal sleep duration (6–8 hours), long sleep duration (˃8 hours) was associated with CHD, short sleep duration (<6 hours) was associated with increased likelihoods of various CVDs (eg, CHD and HF).6 Differences in characteristics of study participants (eg, age, educational attainment, living regions, and race or ethnicities) and control of potential confounders might partly contribute to the inconsistent findings across studies. First, short sleep duration and CVDs are increasingly common as people age.32,33 Our study focus on older adults, which have been underrepresented in the current literature. Second, compared to the whites, Chinese were 2.3 times more likely to have short sleep duration.34 A review showed that poor sleep conditions may differently affect health outcomes of various ethnic groups because genetic differences may increase the susceptibility of particular groups to CVDs.35,36 Third, the US NHANES Study did not adjust for education, alcohol consumption, regular physical activities, diabetes, and depression, which was different with our study.6 Our study focused on older adults living in the rural communities, who have limited education, insufficient medical knowledge, and relatively high prevalence of depression,37 compared to urban older residents. Moreover, alcohol consumption and diabetes are risk factors of CVDs.38 All these factors may potentially bias the associations of sleep with CVDs, and should be taken into consideration in the analysis. Another study from the US NHANES database showed that compared to normal sleep duration (7–9 hours), both short and long sleep duration were associated with a higher likelihood of HF.7 In addition, we found that a high risk for OSA was associated with both HF and CHD. A clinical-based study of 6716 adults (age ≥18 years) showed that OSA measured by polysomnography was associated with CHD and HF,39 which was in line with our study. The population-based Sleep Heart Health Study of 6264 adults (age ≥40 years) showed that OSA had an association with HF but not with CHD.40 Different ethnic characteristics and average age of study participants might partly contribute to the different findings between studies, as described above. The Multi-Ethnic Study of Atherosclerosis (MESA) showed that, compared to the whites, Chinese were more likely to experience OSA.34 The prevalence of OSA and severe OSA was generally higher in older adults than younger individuals.41 Of note, we only assessed the risk of OSA rather than a diagnosis of OSA. Taken together, we found that sleep latency, sleep efficiency, sleep disturbances, use of sleep medications, poor sleep quality were also associated with CHD and that sleep disturbances and use of sleep medications were associated with HF. Yet few population-based studies have focused on the associations of these sleep characteristics with CHD and HF in older adults.
We did not detect association of sleep duration with stroke. Previous studies have shown inconsistent results with regard to the association of sleep duration with stroke. For instance, in line with our study, a population-based study of Chinese older adults (age ≥65 years) showed no association of sleep duration with stroke.42 By contrast, data from the US NHANES survey (mean age, 47 years) showed both short and long sleep duration (vs 7–9 hours) were associated with stroke.7 We found that high risk for OSA was associated with stroke. Similarly, the Sleep Heart Health Study showed that OSA had an association with self-reported history of stroke.40 Taken together, we found that poor sleep quality, sleep disturbances, and EDS were associated with stroke. However, data on the associations of these sleep characteristics with stroke are sparse.
Various CVDs often coexist in older adults. However, previous studies have rarely examined the associations of sleep characteristics with cardiovascular multimorbidity. We found that sleep problems such as prolonged sleep latency, extreme sleep duration, low sleep efficiency, sleep disturbances, use of sleep medications, poor sleep quality, EDS or high risk for OSA were associated with cardiovascular multimorbidity, but not with a single CVD. In addition, the restricted cubic splines showed a U-shaped association of sleep duration with cardiovascular multimorbidity. More research is needed to further characterize sleep problems associated with cardiovascular multimorbidity in older adults.
Several potential mechanisms may explain the cross-sectional associations of self-reported sleep characteristics with CVDs. Some experimental studies showed that shorter sleep duration and poor sleep quality were associated with endothelial dysfunctions, which may lead to CVDs.43,44 In addition, hypoxia caused by OSA may result in oxidative stress and release of inflammatory mediators involved in the progression of atherosclerosis, and arousal-induced reflex sympathetic activation with resultant repetitive blood-pressure rises, all these conditions favouring the cardiovascular damage.45 EDS could involve in the activation of the hypothalamic–pituitary–adrenal axis and sympathetic nervous system, with increased levels of circulating catecholamines, all conditions above favouring the occurrence of cardiovascular events.46,47 Sleep problems may be associated with a variety of CVDs through common mechanisms, such as inflammation,48 metabolic and endocrine effects, activation of the sympathetic nervous system,49 and impaired endothelium-dependent vasodilation.50 On the other hand, physical discomfort related to CVDs may disrupt sleep and cause various sleep problems. Further prospective studies are needed to investigate the direction and reciprocal effect of sleep problems on CVDs and multiple cardiovascular conditions.
The strengths of this study include the population-based design of rural-dwelling older people and comprehensive assessments of various sleep characteristics and cardiovascular morbidities. However, our study also has limitations. First, the cross-sectional nature of this study made it impossible to infer a causal relationship of sleep problems with CVDs. Instead, our study aimed to characterize the self-reported sleep characteristics associated with CVDs among older adults living in rural Eastern China. Second, despite our efforts to diagnose various CVDs through in-person interviews, clinical and neurological examinations, and ECG examination, some people with CVDs might still be missed, which might underestimate the association of sleep parameters with CVDs. Third, sleep characteristics was assessed through self-reported information, which was subject to recall bias. However, we excluded people with dementia to minimize this bias. Fourth, although a wide range of confounding factors had been taken into account in the analysis, residual confounding might still exist due to lack of certain factors (eg, income and marital status).51–53 Finally, the study participants were recruited from a single rural area in western Shandong, China, which should be kept in mind when generalizing our findings to other populations.
Conclusion
Our study showed that extreme sleep duration, poor sleep quality, high risk for OSA, and other abnormal sleep characteristics were associated with adverse cardiovascular outcomes, especially cardiovascular multimorbidity. Further longitudinal studies are needed to investigate their temporal causal relationships and the potential mechanisms underlying the associations of sleep characteristics with CVDs.
Abbreviations
AF, atrial fibrillation; BMI, body mass index; BQ, Berlin questionnaire; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; ECG, electrocardiogram; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Score; GDS-15, 15-item Geriatric Depression Scale; HF, heart failure; MESA, Multi-Ethnic Study of Atherosclerosis; MIND-China, Multidomain Interventions to Delay Dementia and Disability in Rural China; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; OSA, obstructive sleep apnea; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
The MIND-China protocol has been approved by the Ethics Committee at Shandong Provincial Hospital, Jinan, Shandong, China. Written informed consent was obtained from all participants or from informants.
Acknowledgments
The authors would like to thank the MIND-China study participants, medical staff at the Yanlou Town Hospital, and the MIND-China research group at the Shandong Provincial Hospital for their invaluable contributions to data collection and management.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agreed to be accountable for all aspects of the work.
Funding
The Multidomain Interventions to Delay Dementia and Disability in Rural China (MIND-China) Study was financially supported in part by the grants from the National Key Research and Development Program of China (grant no.: 2017YFC1310100), the National Natural Science Foundation of China (NSFC, grants no.: 81861138008, and 82011530139), the Academic Promotion Program of Shandong First Medical University (grant no.: 2019QL020), and the Taishan Scholar Program of Shandong Province, China. Tang S received grants from the NSFC (grant no.: 82001397), and the Jinan Science and Technology Bureau (grant no.: 202019187), Shandong, China. Tang J received grants from the Chinese Natural Science Foundation (grant no.: 81471345), the Natural Science Foundational of Shandong Province (grant no.: ZR2012HM068), and the Key Research and Development Project of Shandong Province (grant no.: 2015GGH318011).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16(4):203–212. doi:10.1038/s41569-018-0119-4
2. Sun W, Zhou Y, Zhang Z, Cao L, Chen W. The trends in cardiovascular diseases and respiratory diseases mortality in Urban and Rural China, 1990–2015. Int J Environ Res Public Health. 2017;14(11):1391. doi:10.3390/ijerph14111391
3. Wang P, Song L, Wang K, et al. Prevalence and associated factors of poor sleep quality among Chinese older adults living in a rural area: a population-based study. Aging Clin Exp Res. 2020;32(1):125–131. doi:10.1007/s40520-019-01171-0
4. Kwok CS, Kontopantelis E, Kuligowski G, et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose-response meta-analysis. J Am Heart Assoc. 2018;7(15):e008552. doi:10.1161/JAHA.118.008552
5. Ke J, Zhou X, Qiu H, et al. Sex-specific associations between extreme sleep duration and prevalence of cardio-cerebral vascular disease: a community-based cross-sectional study. Sleep Med. 2018;42:61–67. doi:10.1016/j.sleep.2017.11.1148
6. Aggarwal S, Loomba RS, Arora RR, Molnar J. Associations between sleep duration and prevalence of cardiovascular events. Clin Cardiol. 2013;36(11):671–676. doi:10.1002/clc.22160
7. Krittanawong C, Kumar A, Wang Z, et al. Sleep duration and cardiovascular health in a representative community population (from NHANES, 2005 to 2016). Am J Cardiol. 2020;127:149–155. doi:10.1016/j.amjcard.2020.04.012
8. Lu L, Wang SB, Rao W, et al. The prevalence of sleep disturbances and sleep quality in older Chinese adults: a comprehensive meta-analysis. Behav Sleep Med. 2019;17(6):683–697. doi:10.1080/15402002.2018.1469492
9. Liu Y, Rao K, Wu J, Gakidou E. China’s health system performance. Lancet. 2008;372(9653):1914–1923. doi:10.1016/S0140-6736(08)61362-8
10. Mathur R, Hull SA, Badrick E, Robson J. Cardiovascular multimorbidity: the effect of ethnicity on prevalence and risk factor management. Br J Gen Pract. 2011;61(586):e262–70. doi:10.3399/bjgp11X572454
11. Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078–1094. doi:10.1002/alz.12123
12. Liu R, Tang S, Wang Y, et al. Self-reported sleep characteristics associated with dementia among rural-dwelling Chinese older adults: a population-based study. BMC Neurol. 2022;22(1):5. doi:10.1186/s12883-021-02521-0
13. Wang Y, Han X, Zhang X, et al. Health status and risk profiles for brain aging of rural-dwelling older adults: data from the interdisciplinary baseline assessments in MIND-China. Alzheimers Dement. 2022;8(1):e12254.
14. Jia Y, Liu R, Tang S, et al. Associations of the Glycaemic Control of Diabetes with Dementia and Physical Function in Rural-Dwelling Older Chinese Adults: A Population-Based Study. Clin Interv Aging. 2021;16:1503-1513. doi:10.2147/CIA.S319633
15. Sun H, Ren X, Chen Z, et al. Association between body mass index and mortality in a prospective cohort of Chinese adults. Medicine. 2016;95(32):e4327. doi:10.1097/MD.0000000000004327
16. Liu LS, Wu ZS, Wang JG, et al. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. 2019;16(3):182–241. doi:10.11909/j.issn.1671-5411.2019.03.014
17. Liu X, Li Y, Li L, et al. Prevalence, awareness, treatment, control of type 2 diabetes mellitus and risk factors in Chinese rural population: the RuralDiab study. Sci Rep. 2016;6(1):31426. doi:10.1038/srep31426
18. Song P, Zha M, Yang X, et al. Socioeconomic and geographic variations in the prevalence, awareness, treatment and control of dyslipidemia in middle-aged and older Chinese. Atherosclerosis. 2019;282:57–66. doi:10.1016/j.atherosclerosis.2019.01.005
19. Shin C, Park MH, Lee SH, et al. Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J Affect Disord. 2019;259:370–375. doi:10.1016/j.jad.2019.08.053
20. Dias F, Teixeira AL, Guimarães HC, et al. Accuracy of the 15-item Geriatric Depression Scale (GDS-15) in a community-dwelling oldest-old sample: the Pietà Study. Trends Psychiatry Psychother. 2017;39(4):276–279. doi:10.1590/2237-6089-2017-0046
21. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
22. Li J, Yao YS, Dong Q, et al. Characterization and factors associated with sleep quality among rural elderly in China. Arch Gerontol Geriatr. 2013;56(1):237–243. doi:10.1016/j.archger.2012.08.002
23. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi:10.1016/j.smrv.2015.01.009
24. Ji A, Lou H, Lou P, et al. Interactive effect of sleep duration and sleep quality on risk of stroke: an 8-year follow-up study in China. Sci Rep. 2020;10(1):8690. doi:10.1038/s41598-020-65611-y
25. Lao XQ, Liu X, Deng HB, et al. Sleep quality, sleep duration, and the risk of coronary heart disease: a prospective cohort study with 60,586 adults. J Clin Sleep Med. 2018;14(1):109–117. doi:10.5664/jcsm.6894
26. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
27. Pataka A, Daskalopoulou E, Kalamaras G, Fekete Passa K, Argyropoulou P. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15(7):776–781. doi:10.1016/j.sleep.2014.03.012
28. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
29. Liu S, Li Y, Zeng X, et al. Burden of cardiovascular diseases in China, 1990–2016: findings from the 2016 Global Burden of Disease Study. JAMA Cardiol. 2019;4(4):342–352. doi:10.1001/jamacardio.2019.0295
30. Satoh M, Ohkubo T, Asayama K, et al. Lifetime risk of stroke and coronary heart disease deaths according to blood pressure level: EPOCH-Japan (Evidence for Cardiovascular Prevention From Observational Cohorts in Japan). Hypertension. 2019;73(1):52–59. doi:10.1161/HYPERTENSIONAHA.118.11635
31. Harmsen P, Lappas G, Rosengren A, Wilhelmsen L. Long-term risk factors for stroke: twenty-eight years of follow-up of 7457 middle-aged men in Göteborg, Sweden. Stroke. 2006;37(7):1663–1667. doi:10.1161/01.STR.0000226604.10877.fc
32. Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594(8):2061–2073. doi:10.1113/JP270538
33. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19–36. doi:10.1016/j.neuron.2017.02.004
34. Chen X, Wang R, Zee P, et al. Racial/Ethnic differences in sleep disturbances: the multi-ethnic study of atherosclerosis (Mesa). Sleep. 2015;38(6):877–888. doi:10.5665/sleep.4732
35. Chattu VK, Chattu SK, Spence DW, Manzar MD, Burman D, Pandi-Perumal SR. Do disparities in sleep duration among racial and ethnic minorities contribute to differences in disease prevalence? J Racial Ethn Health Disparities. 2019;6(6):1053–1061. doi:10.1007/s40615-019-00607-7
36. Yan J, Zhang J, Jiang Y, Yu L. [Sleep conditions of adult residents in Shandong Province from 2010 to 2012]. Wei Sheng Yan Jiu. 2019;48(6):884–887. Chinese.
37. Wang R, Chen Z, Zhou Y, Shen L, Zhang Z, Wu X. Melancholy or mahjong? Diversity, frequency, type, and rural-urban divide of social participation and depression in middle- and old-aged Chinese: a fixed-effects analysis. Soc Sci Med. 2019;238:112518. doi:10.1016/j.socscimed.2019.112518
38. Thomas H, Diamond J, Vieco A, et al. Global atlas of cardiovascular disease 2000–2016: the path to prevention and control. Glob Heart. 2018;13(3):143–163. doi:10.1016/j.gheart.2018.09.511
39. Bouloukaki I, Mermigkis C, Markakis M, et al. Cardiovascular effect and symptom profile of obstructive sleep apnea: does sex matter? J Clin Sleep Med. 2019;15(12):1737–1745. doi:10.5664/jcsm.8074
40. Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi:10.1164/ajrccm.163.1.2001008
41. Hua-Huy T, Rouhani S, Nguyen XY, Luchon L, Meurice JC, Dinh-Xuan AT. Cardiovascular comorbidities in obstructive sleep apnoea according to age: a sleep clinic population study. Aging Clin Exp Res. 2015;27(5):611–619. doi:10.1007/s40520-015-0318-3
42. Li W, Kondracki A, Gautam P, et al. The association between sleep duration, napping, and stroke stratified by self-health status among Chinese people over 65 years old from the China health and retirement longitudinal study. Sleep Breath. 2021;25(3):1239–1246. doi:10.1007/s11325-020-02214-x
43. Weil BR, Mestek ML, Westby CM, et al. Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Can J Physiol Pharmacol. 2010;88(8):777–781. doi:10.1139/Y10-046
44. Cooper DC, Ziegler MG, Milic MS, et al. Endothelial function and sleep: associations of flow-mediated dilation with perceived sleep quality and rapid eye movement (REM) sleep. J Sleep Res. 2014;23(1):84–93. doi:10.1111/jsr.12083
45. Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677–685. doi:10.1038/nrcardio.2010.145
46. Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi:10.1016/j.pcad.2008.10.003
47. Blachier M, Dauvilliers Y, Jaussent I, et al. Excessive daytime sleepiness and vascular events: the Three City Study. Ann Neurol. 2012;71(5):661–667. doi:10.1002/ana.22656
48. Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5(2):93–102. doi:10.2174/157016107780368280
49. Maurovich-Horvat E, Pollmächer TZ, Sonka K. The effects of sleep and sleep deprivation on metabolic, endocrine and immune parameters. Prague Med Rep. 2008;109(4):275–285.
50. Bain AR, Weil BR, Diehl KJ, Greiner JJ, Stauffer BL, DeSouza CA. Insufficient sleep is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation. Atherosclerosis. 2017;265:41–46. doi:10.1016/j.atherosclerosis.2017.08.001
51. Etindele Sosso FA, Matos E. Socioeconomic disparities in obstructive sleep apnea: a systematic review of empirical research. Sleep Breath. 2021;25(4):1729–1739. doi:10.1007/s11325-020-02274-z
52. Etindele Sosso FA, Holmes SD, Weinstein AA. Influence of socioeconomic status on objective sleep measurement: a systematic review and meta-analysis of actigraphy studies. Sleep Health. 2021;7(4):417–428. doi:10.1016/j.sleh.2021.05.005
53. Silva-Perez LJ, Gonzalez-Cardenas N, Surani S, et al. Socioeconomic status in pregnant women and sleep quality during pregnancy. Cureus. 2019;11(11):e6183. doi:10.7759/cureus.6183
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.