Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Selecting an Optimal Staging System for Intermediate-Stage Hepatocellular Carcinoma: Comparison of 9 Currently Used Prognostic Models
Authors Zhang YF , Shi M, Lu LH, Wang L, Guo RP
Received 10 February 2021
Accepted for publication 1 April 2021
Published 19 April 2021 Volume 2021:8 Pages 253—261
DOI https://doi.org/10.2147/JHC.S305581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Yong-Fa Zhang,1,2,* Ming Shi,3,* Liang-He Lu,3 Lu Wang,1,2 Rong-Ping Guo3
1Department of Hepatic Surgery, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, 200032, People’s Republic of China; 3The Department of Hepatobiliary Oncology of Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lu Wang
Department of Hepatic Surgery, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China
Tel/Fax +8620-64175590
Email [email protected]
Rong-Ping Guo
Department of Hepatobiliary Oncology, Sun Yat-Sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China
Tel/Fax +8620-87342266
Email [email protected]
Purpose: It remains unknown which staging system is best in predicting the survival of patients with intermediate stage hepatocellular carcinoma (HCC). We aimed to investigate the performance of nine currently used HCC staging systems.
Patients and Methods: Between 2005 and 2014, a large cohort of 880 consecutive patients with intermediate stage HCC and sufficient data for utilization in all staging systems were enrolled. The prognostic performance of each staging system was compared. Independent prognostic variables were also identified.
Results: Multivariate analysis revealed that alkaline phosphatase (ALP), aspartate aminotransferase (AST), etiology, alpha-fetoprotein (AFP), Child-Pugh stage, tumor size, and tumor number were independent prognostic factors for survival. In the entire cohort, the Hong Kong Liver Cancer (HKLC) staging system was associated with the highest Harrell’s c-index and lowest Akaike information criterion value in comparison with other systems. In subgroup analysis according to treatment strategy, the HKLC staging system remained the best prognostic model in patients undergoing hepatic resection (n=222) or transarterial chemoembolization (n=658). Additional prognostic factors of AST, ALP, etiology, and AFP improved the discriminatory ability of HKLC.
Conclusion: The HKLC staging system is stable and consistently the best prognostic model in all patients with intermediate-stage HCC and in patients subjected to different treatment strategies. Selecting an optimal staging system is helpful in improving the design of future clinical trials in intermediate stage HCC.
Keywords: hepatocellular carcinoma, intermediate-stage, staging system, prognosis, overall survival, hepatic resection, transarterial chemoembolization
Introduction
The purposes of cancer staging are to accurately predict the patient’s prognosis and to determine the appropriate intervention. Hepatocellular carcinoma (HCC) is somewhat unique in that it usually affects patients with underlying liver disease, and both the tumor burden and liver function must be carefully evaluated at the time of the prognostic prediction and treatment recommendation. Over the past several decades, many organizations have proposed staging systems for HCC (Table 1) based on tumor burdens and liver reserve to guide accurate treatment programs with a good prognostic value. Among these systems, the Barcelona Clinic Liver Cancer (BCLC) staging system1 has been validated by several groups in the United States and Europe and provides the best stage classification system and guidance for HCC treatment assignments.2,3 However, intermediate-stage (BCLC-B) HCC includes a heterogeneous group of patients with different tumor burdens, liver functions and other associated factors.4
 |
Table 1 The Variables Included in Nine Staging Systems for Intermediate Stage HCC |
Transarterial chemoembolization (TACE) is recommended by the BCLC guideline as the first-line treatment for intermediate-stage HCC. TACE results in varying clinical benefit in the BCLC-B group.5–7 Recently, the role of hepatic resection (HR) was reviewed8,9 and the question “What treatment yields the best outcome for patients with BCLC stage B HCC?” has been under intense debate.10–17 Thus, a staging system is needed to help predict survival outcomes and also help to determine the optimal medical care, especially in this era of controversy regarding choices for the treatment of intermediate-stage HCC. Additionally, there is a pressing need to properly interpret data from clinical trials of patients with intermediate-stage HCC. Whether any of the staging systems have more information in patients with intermediate-stage HCC, and whether other variables not included in these systems have prognostic significance is unknown.
Moreover, when evaluating the performance of a staging system, we believe that the type of treatment and its efficacy should be considered. For this reason, we evaluated the prognostic power of each staging system in a specific cohort of patients after TACE and HR treatments. We also explored whether the staging systems identified as the best for the population of intermediate-stage HCC could be improved by the inclusion of additional prognostic factors identified in a multivariate analysis.
Patients and Methods
Eligibility
We retrospectively identified patients with HCC who were evaluated by clinical oncologists in the Department of Hepatobiliary Oncology of Sun Yat-sen University Cancer Center between February 2005 and August 2014. Patients were included if they were initially diagnosed with intermediate-stage HCC and received TACE or HR as the first-line treatment for HCC at our institute after diagnosis. HCC was diagnosed by histology or radiologic criteria of two imaging studies (eg, ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]) according to the European Association for the Study of the Liver diagnostic criteria.18 Intermediate-stage (BCLC-B) HCC was defined on the basis of BCLC classification as follows: 2–3 tumors, of which at least 1 was >3 cm in diameter; more than 3 tumors of any diameter; and absence of extrahepatic metastasis and absence of tumor invasion into the portal or hepatic veins and performance status 0.19
Patients were excluded if data were missing for the classification of patients in any of the nine staging systems or if there was no follow-up data. Patients with other concurrent malignancies were also excluded.
This study was approved by the Institutional Review Board and complied with the standards of Declaration of Helsinki and current ethical guidelines.
Staging
All data needed to fulfill these nine staging systems were collected for classification according to the recently proposed BCLC B sub-classification,4 Tumor Nodes Metastasis (TNM) seventh edition,20 Cancer of the Liver Italian Program (CLIP),21 Okuda,22 Japan Integrated Scoring Score (JIS score),23 the Chinese University Prognostic Index (CUPI),24 Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire Prognostic classification (GRETCH),25 Tokyo score,26 and Hong Kong Liver Cancer staging system (HKLC).27 An overview of the variables used in the nine staging systems for HCC is presented in Table 1.
Treatment
At our center, HR was offered to patients with resectable disease if functional reserve was sufficient.28,29 Resectable disease was defined as the possibility of completely removing all tumors with an expected remnant liver volume of no less than 250 mL/m2.30 HR was performed using the technique that we have previously described.29,31 TACE was offered to patients with unresectable HCC, or resectable HCC with low predicted remnant liver volume, and patients unwilling to undergo surgery. For TACE, the Seldinger’s technique of arterial embolization was administered as the standard TACE procedure to achieve complete flow stagnation in tumor(s), as we have previously reported.32 In addition, all patients with HBV-related HCC who were prepared for treatment for their HCC in our hospital were counseled by a hepatologist for antiviral therapy regardless of the serum HBV DNA result.
Statistical Analysis
Overall survival (OS) of patients was the single end point used to assess the performance of the different scoring systems. OS was calculated from the date of initial diagnosis with intermediate-stage HCC until death or the end of the follow-up period. Survival time was estimated by the Kaplan–Meier method, and the survival difference among prognostic strata was assessed by the Log rank test. The median of survival was calculated with its 95% confidence interval (CI). The Cox regression model was used to identify independent predictors of survival.
The prognostic performance of each scoring system was statistically assessed following Ueno et al,33 evaluating homogeneity within the classification groups, discriminatory ability, and monotonicity of the gradients in the association between stages and survival rates. The Cox regression model was then used to calculate the likelihood ratio (LR) x2 to determine homogeneity. The linear trend x2 was then used to measure the discriminatory ability of each staging system. Both the linear trend x2 and LR x2 were also used to measure the monotonicity of survival gradients, and the degree of freedom was 1, so two prognostic systems with different stages could be compared. In addition, the consequences of the Cox model were expressed with the Akaike information criterion (AIC), which showed how the explanatory variable (staging systems) affected the dependent variable (survival of HCC).34 In addition, Harrell’s c-index, which requires no assumption in the model, was calculated to verify the discriminatory ability of each staging system. To reduce the confounding effect of treatment on survival response variables, we also performed a separate analysis for subgroups of patients who received HR or TACE.
Finally, for the best staging system, the additional independent prognostic variables that were previously identified were added to it. A new c-index was calculated to quantify the improvement. The c-index of the resulting model was internally validated using bootstrap to quantify the improvement.35 The statistical analysis was performed using IBM SPSS v.19.0 (SPSS, Armonk, NY, USA) and R 2.13.2 (http://www.r-project.org/). All statistical tests were 2-tailed, and a p value less than 0.05 was considered significant.
Results
Patient Characteristics
Between February 2005 and August 2014, 1027 patients with intermediate-stage HCC were seen by medical oncologists at the Sun Yat-sen University Cancer Center; among these patients, 147 patients either did not fulfill the inclusion or had one or more of the exclusion criteria. Eventually, 880 patients were staged according to each of the nine different HCC staging systems discussed in the Methods section (Table 1). A total of 222 (25%) patients had HR, and 658 (75%) patients underwent TACE. In addition, 129/658 patients undergoing TACE combined with local treatment (radiofrequency ablation (RFA), n=86; microwave ablation (MWA), n=43).
All patients with demographic, clinical, and tumor information are shown in Table 2.
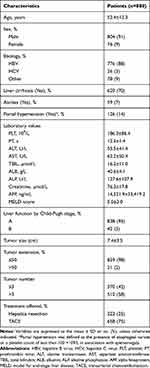 |
Table 2 Baseline Demographic and Clinical Characteristics of Patients with Intermediate Stage HCC |
Survival
When the data were censored, 580 (66%) patients had died, with a median follow-up time of 25.0 months. The 1-, 3-, and 5-year OS rates for all patients were 68.7%, 34.3.0%, and 22.6%, respectively (Figure 1A). The 1-, 3- and 5-year OS rates were 76.3%, 44.0%, and 33.3% for patients who underwent HR, 86.0%, 56.6%, and 26.8% for patients who underwent TACE+RFA/MWA, and 60.9%, 23.4%, and 15.8% for patients who underwent TACE, respectively (Supplementary Figure 1).
Prognostic Factors
Univariate analysis showed that etiology, alanine transaminase (ALT), aspartate aminotransferase (AST), albumin (ALB), alkaline phosphatase (ALP), Child-Pugh stage, serum-fetoprotein (AFP), tumor extension, tumor size, and tumor number were significant baseline predictors of survival in patients with intermediate HCC (Supplementary Table 1). The independent prognostic factors identified by multivariate analysis are reported in Table 3. The most significant of these prognostic factors that are often used as part of the different staging system are tumor size, Child-Pugh stage, and tumor number. Identified other prognostic factors included elevated ALP, AST, AFP levels, and etiology.
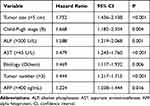 |
Table 3 Independent Prognostic Factors for Overall Survival in Patients with Intermediate Stage HCC According to Multivariate Analysis |
Evaluation of Staging Systems
Because the study focused on patients with intermediate stage HCC, no patients with early cases, such as TNM stage I, CLIP score 0, JIS score 0, and Tokyo score 0, were included. None of the patients were classified as end-stage disease, such as TNM stage IV, CLIP score ≥4, JIS score ≥4, and HKLC stage IV/V (Table 1). The patient distribution among the stage groups is presented in Supplementary Table 2. Interestingly, patients with different HKLC stages were more evenly distributed in compared with other staging systems, with 12.4%, 43.9%, and 43.7% of patients having an HKLC stage of I, II, or III, respectively.
When Kaplan-Meier survival analysis (n = 880) was used to analyze nine prognostic staging systems, the survival probability of each staging system was significantly different across the different stages (log-rank P<0.001 in all cases, Figure 1B–J). Figure 1F, G and I show that the GRETCH (stages low, intermediate and high), Tokyo (scores 2 and 3), and BCLC B sub-classification (stages B3 and B4) systems had poor survival stratification for the intermediate stages HCC, while the HKLC, TNM, CLIP, CUPI, JIS, and Okuda systems had better survival stratification across all stages.
In all patient cohorts, the HKLC system had the highest homogeneity (LRχ2=87.3), indicating the survival of patients in the same stage was less differences (Table 4).
 |
Table 4 Comparison of Prognostic Stratification of Nine HCC Staging Systems |
Compared with other systems, the HKLC classification also had the highest discriminatory score (linear trendχ2=84.0). The HKLC system had the best monotonicity of gradient based on the linear trendχ2 and LRχ2. The AIC of the HKLC system was lowest, indicating that the model containing the HKLC system was the most informative when explaining the survival of patients with intermediate-stage HCC (Table 4). Further evidence showed that the HKLC system provided the best prediction of survival in our cohort with the highest c-index. When patients were stratified according to treatment strategy, the HKLC system remained the most accurate system in patients receiving either HR or TACE treatments (Table 4).
Improvement of HKLC Staging Systems
The addition of ALP, AST, etiology, and AFP improved the discriminatory ability of HKLC with a higher c-index of 0.727 (95% CI: 0.688–0.766) compared with 0.620 (bootstrap validated).
Discussion
Treatment strategies for intermediate-stage HCC are currently a controversial topic in the HCC field.10–12,14,36 To resolve this controversy, identifying patients who are suitable for TACE or HR is essential. In addition, the interpretation of clinical trials in intermediate HCC may also depend on the staging system used. We identified and then compared nine commonly used staging systems in terms of their prognostic abilities using AIC, discriminatory ability (c-index), homogeneity within classification, and monotonicity of the gradient. This study is the first to compare prognostic scores on a specific population of intermediate-stage HCC, and the results indicated that the HKLC is the best prognostic model among the nine currently used staging systems. More importantly, our results were consistent in the subgroup analysis among patients undergoing HR and TACE treatments, indicating that its predictive accuracy is highly stable and independent of the treatment strategy.
The newly developed HKLC classification system introduced substantial amendments that have been verified in a large cohort of almost 4000 consecutively diagnosed and treated patients.27 It incorporates performance status, liver synthetic function, and tumor stage and also provides treatment recommendations. Recently, a study from Singapore showed that HKLC system and BCLC system have the same prognostic value, but HKLC system can better guide treatment.37 Moreover, the HKLC system introduces a multilayered stratification of tumor status using the triad of tumor size (5 cm as the new cut-off diameter), number of tumors, and vascular invasion in various combinations to more precisely characterize the status of the cancer. Therefore, the benefits of the HKLC system are clearly apparent when dealing with patients who have intermediate-stage disease with a performance status score of 0.38 In addition, the HKLC system is a more fluent staging system, with a less demarcated border between curative and palliative treatment arms, enabling aggressive treatment pathways to be pursued in subgroups of patients with an otherwise truly dismal prognosis. Therefore, the HKLC system may be regarded as a more fluent model with better predictive ability of intermediate-advanced stage disease compared with the BCLC system.38–40
In our study, important predictors of prognosis for intermediate-stage HCC were identified. Multivariate analysis showed that independent predictors of survival were mostly related to the tumor burden (ie, tumor size, tumor number, and AFP) and liver function (ie, Child-Pugh stage, elevated AST, and high ALP level). The intermediate-stage HCC patient’s general health status was strictly defined by BCLC classification with a score of zero. Thus, incorporating more information on tumor burden and liver function may result in better predictive abilities in intermediate stage HCC. This inclusion may explain why HKLC was the best predictive model of survival in our cohort of intermediate-stage HCC patients. We also confirmed that the addition of the prognostic variables of ALP, AST, etiology, and AFP improved the discriminatory ability of HKLC.
Until now, the optimal treatment of intermediate-stage HCC patients still remains controversial. BCLC guidelines recommend patients with intermediate-stage HCC to receive TACE treatment for first-line therapy. However, in fact surgical resection remains the primary curative modality in the management of intermediate-stage HCC. Furthermore, a recent meta-analysis data provided evidence that TACE+RFA as a combination therapy provides outcomes comparable to surgical resection.41 Our results also confirmed the above findings. Therefore, we suggest that in future studies, TACE+RFA should be included in the Hong Kong guiding treatment system and help to determine the optimal medical care for intermediate-stage HCC.
There were a few potential limitations in this study. First, our effort was limited by the single-institution experience and the retrospective nature of the study. Second, with approximately 90% of the patients having evidence of HBV infection, our data require validation from other study groups in which HCV infection or alcohol is the prevailing etiology of chronic liver disease. In addition, this study only included patients with intermediate-stage HCC undergoing resection or TACE. When patients with different disease stages receive different treatments, future multicenter prospective studies are necessary to validate our findings.
Conclusion
For intermediate-stage HCC, our results indicate that the HKLC is the best predictor among the 9 currently used staging systems. The performance of the HKLC is reliable for long-term prognostic prediction and is independent of the treatment strategy. This finding is crucial because selecting an optimal staging system is helpful in improving the design of future clinical trials for intermediate-stage HCC.
Funding
This study was supported by grants from the Shanghai Sailing Program (No. 18YF1404900) and Clinical Research Plan of SHDC (SHDC2020CR4018).
Disclosure
The authors declare no conflicts of interest.
References
1. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi:10.1055/s-2007-1007122
2. Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. doi:10.1093/jnci/djn134
3. Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41(4):707–716. doi:10.1002/hep.20636
4. Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–359. doi:10.1055/s-0032-1329906
5. Hucke F, Pinter M, Graziadei I, et al. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol. 2014;61(6):1287–1296. doi:10.1016/j.jhep.2014.07.002
6. Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565–2570. doi:10.1093/annonc/mdt247
7. Xu L, Peng ZW, Chen MS, et al. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. J Hepatol. 2015;63(1):122–130. doi:10.1016/j.jhep.2015.02.034
8. Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62(2):440–451. doi:10.1002/hep.27745
9. Cucchetti A, Djulbegovic B, Tsalatsanis A, et al. When to perform hepatic resection for intermediate-stage hepatocellular carcinoma. Hepatology. 2015;61(3):905–914. doi:10.1002/hep.27321
10. Roayaie S. TACE vs. surgical resection for BCLC stage B HCC. J Hepatol. 2014;61(1):3–4. doi:10.1016/j.jhep.2014.04.005
11. Zhong JH, Lu SD, Wang YY, Ma L, Li LQ. Intermediate-stage HCC–upfront resection can be feasible. Nat Rev Clin Oncol. 2015;12(5):295. doi:10.1038/nrclinonc.2014.122-c3
12. Forner A, Gilabert M, Bruix J, Raoul JL. Intermediate-stage HCC–upfront resection can be feasible. Nat Rev Clin Oncol. 2015;12(5):295. doi:10.1038/nrclinonc.2014.122-c4
13. Bruix J, Fuster JA. Snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An Observational Study of the HCC East-West Study Group. Ann Surg. 2015;262(1):e30. doi:10.1097/SLA.0000000000000381
14. Forner A, Gilabert M, Bruix J, Raoul JL. Reply: heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol. 2015;12(1):10. doi:10.1038/nrclinonc.2014.122-c2
15. Gao Q, Wang XY, Zhou J, Fan J. Heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol. 2015;12(1):10. doi:10.1038/nrclinonc.2014.122-c1
16. Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11(9):525–535. doi:10.1038/nrclinonc.2014.122
17. Yamakado K, Kudo M. Treatment strategies of intermediate-stage hepatocellular carcinomas in Japan (Barcelona clinic liver cancer stage B). Oncology. 2014;87(Suppl 1):78–81. doi:10.1159/000368149
18. European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943.
19. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi:10.1055/s-0030-1247133
20. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
21. Llovet JM, Sala M, Castells L, et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31(1):54–58. doi:10.1002/hep.510310111
22. Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56(4):918–928. doi:10.1002/1097-0142(19850815)56:4<918::AID-CNCR2820560437>3.0.CO;2-E
23. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38(3):207–215. doi:10.1007/s005350300038
24. Leung TW, Tang AM, Zee B, et al. Construction of the Chinese university prognostic index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the cancer of the liver Italian program staging system: a study based on 926 patients. Cancer. 2002;94(6):1760–1769. doi:10.1002/cncr.10384
25. Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31(1):133–141. doi:10.1016/S0168-8278(99)80173-1
26. Tateishi R, Yoshida H, Shiina S, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54(3):419–425. doi:10.1136/gut.2003.035055
27. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–1700e1693. doi:10.1053/j.gastro.2014.02.032
28. Zhang YF, Guo RP, Zou RH, et al. Efficacy and safety of preoperative chemoembolization for resectable hepatocellular carcinoma with portal vein invasion: a prospective comparative study. Eur Radiol. 2016;26(7):2078–2088. doi:10.1007/s00330-015-4021-8
29. Luo J, Peng ZW, Guo RP, et al. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259(1):286–295. doi:10.1148/radiol.10101072
30. Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188(3):304–309. doi:10.1016/S1072-7515(98)00301-9
31. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. doi:10.1097/01.sla.0000231758.07868.71
32. Shi M, Lu LG, Fang WQ, et al. Roles played by chemolipiodolization and embolization in chemoembolization for hepatocellular carcinoma: single-blind, randomized trial. J Natl Cancer Inst. 2013;105(1):59–68. doi:10.1093/jnci/djs464
33. Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the liver Italian program. Hepatology. 2001;34(3):529–534. doi:10.1053/jhep.2001.27219
34. Forster MR. Key concepts in model selection: performance and generalizability. J Math Psychol. 2000;44(1):205–231. doi:10.1006/jmps.1999.1284
35. Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28(17):2889–2895. doi:10.1200/JCO.2009.25.9895
36. Spacek LA, Solga SF. When to perform hepatic resection for intermediate-stage hepatocellular carcinoma. Hepatology. 2015;63(3). doi:10.1002/hep.27896
37. Selby LK, Tay RX, Woon WW, et al. Validity of the Barcelona clinic liver cancer and Hong Kong liver cancer staging systems for hepatocellular carcinoma in Singapore. J Hepatobiliary Pancreat Sci. 2017;24(3):143–152. doi:10.1002/jhbp.423
38. Chapiro J, Geschwind JF. Hepatocellular carcinoma: have we finally found the ultimate staging system for HCC? Nat Rev Gastroenterol Hepatol. 2014;11(6):334–336. doi:10.1038/nrgastro.2014.67
39. Yan X, Fu X, Cai C, Zi X, Yao H, Qiu Y. Validation of models in patients with hepatocellular carcinoma: comparison of Hong Kong liver cancer with Barcelona clinic liver cancer staging system in a Chinese cohort. Eur J Gastroenterol Hepatol. 2015;27(10):1180–1186. doi:10.1097/MEG.0000000000000418
40. Wu L, Bartlett A, Plank L, McCall J. Validation of the Hong Kong liver cancer staging system in hepatocellular carcinoma patients treated with curative intent. J Hepatol. 2015;64(4):978–979. doi:10.1016/j.jhep.2015.12.008
41. Gui CH, Baey S, D’Cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - a meta-analysis. Eur J Surg Oncol. 2020;46(5):763–771. doi:10.1016/j.ejso.2020.01.004
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

