Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Secondhand Smoke Exposure and the Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis
Authors Chen P, Li Y, Wu D, Liu F, Cao C
Received 12 January 2023
Accepted for publication 13 May 2023
Published 7 June 2023 Volume 2023:18 Pages 1067—1076
DOI https://doi.org/10.2147/COPD.S403158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Peixv Chen,1,2,* Yiting Li,1,2,* Di Wu,1,2 Fang Liu,1 Chao Cao1
1Department of Respiratory and Critical Care Medicine, Key Laboratory of Respiratory Disease of Ningbo, First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, 315000, People’s Republic of China; 2Medical College of Ningbo University, Ningbo, Zhejiang, 315000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Cao, Department of Respiratory and Critical Care Medicine, Key Laboratory of Respiratory Disease of Ningbo, First Affiliated Hospital of Ningbo University, 59 Liuting Road, Ningbo, Zhejiang, 315000, People’s Republic of China, Email [email protected]
Background: Many studies have shown that active smoking leads to an increasing incidence of chronic obstructive pulmonary disease (COPD). However, studies interested in the effects of secondhand smoke exposure (SHS exposure) on COPD were less or underappreciated.
Methods: A systematic review and meta-analysis was conducted to investigate the association between SHS exposure and the risk of COPD. Three databases (PubMed, Embase and Web of Science) were searched to obtain data. After assessing the study quality, stratified analyses were performed according to the region, gender, and duration of exposure. Cochran’s Q and I2 were utilized for heterogeneity assessment. To assess publication bias, we used a funnel plot and Egger’s test.
Results: A total of 15 studies (6 cross-sectional studies, 6 case-control studies, and 3 cohort studies) with 25,592 participants were involved in this meta-analysis. This study showed that SHS exposure was associated with an increased risk of COPD (odds ratio (OR): 2.25, 95% CI: 1.40– 3.62, I2 = 98%, p < 0.01 for heterogeneity based on a random-effects analysis model), especially in those with a longer time exposure of more than 5 years was 4.38 (95% CI: 1.28– 15.00, I2 = 89%, p < 0.01 for heterogeneity based on a random-effects analysis model). In addition, SHS exposure also increases the risk of COPD in women (odds ratio (OR): 2.02, 95% CI: 1.52– 2.67, I2 = 0%, p = 0.89 for heterogeneity based on a random-effects analysis model).
Conclusion: The findings suggest that SHS exposure is associated with the risk of COPD, especially for individuals with a long time exposed.
Trial Registry: Prospero CRD42022329421.
Keywords: COPD, secondhand smoke exposure, systemic review, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by irreversible obstructive ventilation dysfunction, has been a serious public health problem and impose a huge financial burden on health care worldwide. It was also reported as the third leading cause of death and the fifth of life years lost around the world due to respiratory failure and serious complications.1,2 A large population-based survey in China showed an overall COPD prevalence of 8.2% in people over 40,2 and a study in the United States indicated that people over 65 exhibit a higher prevalence of COPD.3 Unfortunately, as the population age, more than 21% of the population will be over 60 years of age by 2050.4 It is therefore the issue of COPD will receive more attention and become more serious in the future.
Secondhand smoke exposure (SHS exposure) is one of the most common indoor air pollutants in many regions.5 SHS contains a mixture of side-stream smoke from burning cigarettes and mainstream smoke from smokers’ breath, of which the former is in the vast majority. It is a mixture of more than 4000 compounds full of carcinogens and respiratory toxins.6 In addition, SHS exposure has been proven to have an irritating effect on the eyes, nose, respiratory tract, and other organs.7–9 It is also further established that it presents a correlation with many chronic and serious diseases (Ischemic Heart Disease, Stroke, etc.), especially in the respiratory system.10
Many studies have shown that active smoking is the most critical risk factor for developing COPD and the most important modifiable factor that can be intervened to improve the prognosis of COPD.11–13 However, studies focusing on the effects of SHS exposure on COPD may be less or underappreciated. It reflects the fact that public health policy has not yet fully recognized the health effects of SHS exposure.14 In an epidemiological survey from China, the prevalence of COPD among non-smokers has reached 5.2%.15 Although peer-reviewed studies in recent years have concluded that there is some evidence for a causal relationship between SHS exposure and adult respiratory disease,16 their relationship has not been elaborated in clear detail. A study even suggested that SHS is not a risk factor for COPD prevalence.17 Therefore, our study aimed at investigating the association between the specific effects of SHS exposure and the risk of COPD.
Methods
The meta-analysis was performed according to the 2020 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (It is described in detail in Appendix Table 1).
Search Strategy and Selection Criteria
PubMed, Web of Science and Embase have been searched for studies from the inception of the research to 14 December 2022. The search was conducted using MeSH terms “Tobacco Smoke Pollution” and “Pulmonary Disease, Chronic Obstructive” (search strategy is described in detail in Appendix p2). We also considered articles identified in references of included studies. People who met any of the following points: (1) someone at home or around the workplace smokes; (2) were exposed to tobacco smoke for several hours in a week would be defined as SHS exposure. Considering that active smoking is a high impact confounder and the amount of literature included, we decided to use the criterion that the number of active smokers is less than 50% of the included population.
Inclusion and Exclusion Criteria
The inclusion criteria included:
- Original cross-sectional, case-control, and cohort studies conducted;
- Studies assessing the association between SHS exposure and COPD prevalence;
- The determination of COPD followed the criteria in the 2022 Gold Report (The spirometric criterion for airflow limitation remains a post-bronchodilator fixed ratio of FEV1 (Forced Expiratory Volume in the first second) /FVC (Forced Vital Capacity) < 0.70).
- Include only non-smokers and ex-smokers in the population or less than 50% of current smokers.
The exclusion criteria included:
- Other types of research;
- Not related to SHS exposure and COPD prevalence;
- The definition of COPD in the article did not meet the GOLD criteria or consider other phenotypic diseases as COPD (asthma, etc.).
- Current smoking is over 50% of the included population.
Data Extraction
During the literature screening process, two independent investigators (PC, DW) judged whether the literature met the inclusion criteria based on the title and the abstract of the article. When the two investigators disagreed on whether to include the article, the final decision was made through discussions with the third investigator (YL). We considered people who were not exposed to SHS as the control group and the opposite as the test group. The following data were selected from each study: study type, number of SHS exposures, number of people in the control and test groups, gender, region, location of exposure, exposure measurement, and duration of exposure.
Quality Assessment
The Newcastle-Ottawa scale literature quality evaluation scale was utilized to assess cohort and case-control studies. Case-control studies were assessed in three parts: selection (adequacy of case definition; representativeness of cases; selection and definition of controls), comparability (whether cases and controls controlled for COPD prevalence; whether other important confounders were controlled for), and exposure (criteria for determining exposure; whether exposure assessment methods were the same for case-controls; and whether there was an explanation for the nonresponse rate). All questions were answered in three levels, with only the first level receiving a score of 9 points in total. For the assessment of cross-sectional studies, we used the AHRQ (Agency for Healthcare Research and Quality) cross-sectional study quality assessment scale, which has a total of 11 questions. Only questions with a “yes” answer were scored, with a total of 11 points, each question accounting for one point. For cohort studies and case-control studies, we determined that articles with scores of 7 and above were considered to be high-quality articles that met the requirements and were included. For cross-sectional studies, articles with scores of 8–11 were judged to be eligible.18 The specific results were shown in Appendix Figure 3.
Statistical Analysis
All statistical analyses were conducted with STATA 12 (Stata Corp, College Station, Texas) and R language (4.2.2). Given that most studies were cross-sectional and case-control, and that cohort studies were based on low incidence and had RR values similar to OR values, calculating odds ratio (OR) values and 95% confidence intervals (CI) was the best way to investigate the relationship between SHS exposure and COPD risk. And subgroup analyses were planned according to the region, sex, exposure site, smoking status, and length of exposure to SHS.
In analyses, heterogeneity with the Cochran Q statistic was assessed, with a P value less than 0.10 suggesting evidence of heterogeneity. Then, the magnitude of heterogeneity was measured using the I2 statistic, with approximately 25%, 50%, and 75% representing low, medium, and high heterogeneity, respectively. The funnel plot (Appendix Figure 4) and Galbraith plot (Appendix Figure 5) were used to evaluate the possibility of the risk of bias. The Egger test was conducted to test for the asymmetry of funnel plots. We conducted sensitivity analysis to find the source of heterogeneity as well. (Appendix Figure 6) Statistical results were deemed significant if the 2-sided P value was less than 0.05.
Results
Overall, a total of 15 articles (6 cross-sectional studies, 6 case-control studies, and 3 cohort studies) involving 25,592 participants were included in this meta-analysis. (Specific screening process is shown in Figure 1 and details of the included studies were shown in Table 1).
 |
Table 1 Overview of Selected Literature (1) |
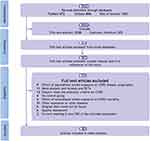 |
Figure 1 Flow chart for study selection. |
It should be pointed out that 4 studies did not provide a specific description of SHS exposure, 7 studies described the duration of exposure, and 4 studies mentioned the number of packs or sticks of secondhand smoke inhaled per year. As the duration of exposure to SHS varies greatly among different studies, 5 years is the most common standard among them,21,24,26 so 5 years is selected as the standard for subgroup analysis. In addition, the sites of SHS exposure were not specifically separated among the populations included in all studies, so the subgroup analysis of exposure sites was eliminated.
Figure 2 showed that SHS exposure was associated with an increased risk of COPD (odds ratio (OR): 2.25, 95% CI: 1.40–3.62, I2 = 98%, p < 0.01 for heterogeneity based on a random-effects analysis model). Subgroup analysis based on geographical distribution was further performed. Observed in Figure 3 that a statistically significant of SHS exposure and risk of COPD was shown in American people (odds ratio (OR): 1.65, 95% CI: 1.30–2.10, I2 = 0.0%, p = 0.56 for heterogeneity based on a random-effects analysis model) without heterogeneity and Asian people (odds ratio (OR): 2.91, 95% CI: 1.23–6.91, I2 = 87%, p < 0.01 for heterogeneity based on a random-effects analysis model). But there was no statistical significance in the European group (odds ratio (OR): 2.08, 95% CI: 0.81–5.33, I2 = 99%, p < 0.01 for heterogeneity based on a random-effects analysis model). The subgroup analysis of gender (Figure 4) showed SHS exposure was significantly associated with high COPD prevalence in group of mostly male (odds ratio (OR): 5.27, 95% CI: 1.01–27.36, I2 = 91%, p < 0.01 for heterogeneity based on a random-effects analysis model) and the same was observed for the only female group (odds ratio (OR): 2.02, 95% CI: 1.52–2.67, I2 = 0%, p = 0.89 for heterogeneity based on a random-effects analysis model). Subgroup analysis of exposure duration (Figure 5) included a total of 7 studies, in which three studies provided both data of exposure time >5 years and <=5 years (Yin et al,21 Zubair et al,24 Sinha et al26). 6 studies provided data with SHS exposure duration >5 years and the OR value for COPD prevalence in those for the SHS exposure group was 4.38 (95% CI: 1.28–15.00, I2 = 89%, p < 0.01 for heterogeneity based on a random-effects analysis model). The results of the 4 studies with a duration of SHS exposure <= 5 years showed SHS exposure was also associated with the risk of COPD (odds ratio (OR): 1.78, 95% CI: 1.17–2.72, I2 = 49%, p = 0.12 for heterogeneity based on a random-effects analysis model) (Table 2).
 |
Table 2 Basic Information About the Studies Included in the Subgroup Analysis of Duration of Exposure and COPD Prevalence |
 |
Figure 2 Meta-analyses of COPD prevalence and SHS exposure. |
 |
Figure 3 Subgroup analysis of the association between region and COPD prevalence. |
 |
Figure 4 Subgroup analysis of the association between gender and COPD prevalence. |
 |
Figure 5 Subgroup analysis of the association between exposure duration and COPD prevalence. |
Publication Bias
To check publication biases, Egger’s test was utilized to confirm the asymmetry of the funnel plot and that the publication bias was not significant (P= 0.09>0.05).
Heterogeneity
The sensitivity test demonstrated that the studies by Sinha et al26 and Korsbæk et al28 contributed to high heterogeneity (Appendix Figure 6). It is therefore speculated that high heterogeneity may have come from the selection of the study population. According to the study by Korsbæk et al,28 the average age of participants was around 55, which was older than that in other studies. In addition, all people enrolled were from one community in the study by Sinha et al.26 After excluding two articles, the heterogeneity was better (I2 from 98% to 51%) and the results showed a close relationship between SHS exposure and risk of COPD (odds ratio (OR): 1.52, 95% CI: 1.27–1.81, I2 = 51%, p = 0.02 for heterogeneity based on a random-effects analysis model) (Appendices Figures 7 and 8).
Discussion
Previous studies have related active smoking to the prevalence of chronic obstructive pulmonary disease.34 However, the relationship between SHS exposure and the risk of COPD was still unsatisfactory. Most studies investigated the association between SHS exposure and respiratory symptoms disease in children, but few articles focused on SHS exposure as a risk factor for COPD prevalence in adults.35 In short, this was a comprehensive systemic review and meta-analysis to integrate the relationship between SHS exposure and COPD prevalence.
15 articles involving 25,592 participants were included in this meta-analysis. Overall, the present study demonstrated the relationship between SHS exposure and COPD prevalence. The risk of COPD was 2.25 times higher with SHS exposure compared with controls, and SHS exposure significantly increased the risk of COPD in the US and elsewhere. It should be noted that even with a short time (<= 5 years), SHS exposure also had an increased risk of COPD (odds ratio (OR): 1.78, 95% CI: 1.17–2.72, I2 = 49%, p = 0.12 for heterogeneity based on a random-effects analysis model). Moreover, SHS exposure also increases the risk of developing COPD in women (odds ratio (OR): 2.02, 95% CI: 1.52–2.67, I2 = 0%, p = 0.89 for heterogeneity based on a random-effects analysis model). Those findings may partly explain why many COPD patients are non-smokers, among which SHS exposure is also an important risk factor.
There was a positive correlation between SHS exposure and increased prevalence of COPD. None the less, it should be mentioned that prenatal exposure to SHS was also a risk factor for COPD and can synergize with active smoking later in life.36,37 All these findings underscore the necessary to eliminate smoking at work and public places, which consistent with the claim made by Wheaton et al in a recent paper.38
In 2015, Fischer et al10 performed a similar meta-analysis and suggest that there was a greater association of SHS with COPD among women. However, the results were inconsistent with our findings. It should be noted that only one paper included in the male group in Fischer’s study and the statistical result was not significant, which may lead to a certain degree of bias. In the present study, we included cross-sectional studies to minimize bias. In addition, more databases were searched in our study and all participants included were diagnosed according to GOLD criteria.
The strengths of this paper include an adequate number of participants which increases the capacity for potential associations and reduces biases. Moreover, corresponding quality scales for different types are adopted to ensure the quality of every included article. Both increased the credibility of the study.
Limitations
Several limitations of this meta-analysis should be acknowledged. First, we searched only three databases, which may cause some limitations. Second, the significant heterogeneity was observed in the analysis. In this study, it is identified that the sources of heterogeneity may come from the selection of enrolled participants. And the duration of SHS exposure was varied among included studies, which may lead to heterogeneity and set limits for further analysis. Lastly, only cohort studies, case-control studies, and cross-sectional studies have been conducted in our study and the credibility of the evidence was unclear. As such, the results of this study should be referred to with caution.
Conclusion
In conclusion, this study showed SHS exposure is associated with the risk of COPD, especially in those exposed to SHS for more than 5 years. It should be noted that SHS exposure significantly increases the risk of developing COPD in those with a short time exposure and in females. The findings of this study may partly explain why many COPD patients are non-smokers.
Abbreviations
COPD, chronic obstructive pulmonary disease; SHS, Secondhand smoke.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Zhejiang Province and Province jointly established key disciplines of medicine (2022-S01).
Disclosure
All authors declare no competing interests in this work.
References
1. Easter M, Bollenbecker S, Barnes JW, et al. Targeting aging pathways in chronic obstructive pulmonary disease. Int J Mol Sci. 2020;21(18). doi:10.3390/ijms21186924
2. Yang Y, Mao J, Ye Z, et al. Risk factors of chronic obstructive pulmonary disease among adults in Chinese mainland: a systematic review and meta-analysis. Respir Med. 2017;131:158–165. doi:10.1016/j.rmed.2017.08.018
3. Guilleminault L, Rolland Y, Didier A. Characteristics of non-pharmacological interventions in the elderly with COPD. Smoking cessation, pulmonary rehabilitation, nutritional management and patient education. Rev Mal Respir. 2018;35(6):626–641. doi:10.1016/j.rmr.2017.12.004
4. Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44(4):1055–1068. doi:10.1183/09031936.00059814
5. Oberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–146. doi:10.1016/s0140-6736(10)61388-8
6. Health USDO, Human S. Opioid abuse in the United States and department of health and human services actions to address opioid-drug-related overdoses and deaths. J Pain Palliat Care Pharmacother. 2015;29(2):133–139. doi:10.3109/15360288.2015.1037530
7. Pauwels RA, Löfdahl CG, Pride NB, et al. European respiratory society study on chronic obstructive pulmonary disease (EUROSCOP): hypothesis and design. Eur Respir J. 1992;5(10):1254–1261. doi:10.1183/09031936.93.05101254
8. Thurlbeck WM. Pathophysiology of chronic obstructive pulmonary disease. Clin Chest Med. 1990;11(3):389–403. doi:10.1016/S0272-5231(21)00708-5
9. Wright JL, Lawson LM, Paré PD, et al. The detection of small airways disease. Am Rev Respir Dis. 1984;129(6):989–994. doi:10.1164/arrd.1984.129.6.989
10. Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health. 2015;15:1202. doi:10.1186/s12889-015-2489-4
11. Croghan IT, Ebbert JO, Hays JT, et al. Impact of a countywide smoke-free workplace law on emergency department visits for respiratory diseases: a retrospective cohort study. BMC Pulm Med. 2015;15:6. doi:10.1186/1471-2466-15-6
12. Criner GJ, Martinez FJ, Aaron S, et al. Current controversies in chronic obstructive pulmonary disease: a report from the global initiative for chronic obstructive lung disease scientific committee. Ann Am Thorac Soc. 2019;16(1):29–39. doi:10.1513/AnnalsATS.201808-557PS
13. Burghuber OC, Urban M, Hartl S. Lung diseases related to active and passive smoking (except lung cancer and COPD). Atemwegs- Lungenkrankh. 2015;41(8):364–371. doi:10.5414/ATX02091
14. Kawachi I, Colditz GA, Speizer FE, et al. A prospective study of passive smoking and coronary heart disease. Circulation. 1997;95(10):2374–2379. doi:10.1161/01.cir.95.10.2374
15. Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509–518. doi:10.1183/09031936.00084408
16. Jaakkola MS, Jaakkola JJ. Effects of environmental tobacco smoke on the respiratory health of adults. Scand J Work Environ Health. 2002;28:52–70.
17. Kim WJ, Song JS, Park DW, et al. The effects of secondhand smoke on chronic obstructive pulmonary disease in nonsmoking Korean adults. Korean J Intern Med. 2014;29(5):613–619. doi:10.3904/kjim.2014.29.5.613
18. Hu J, Dong Y, Chen X, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. 2015;61:78–89. doi:10.1016/j.comppsych.2015.05.001
19. Ebbert JO, Croghan IT, Schroeder DR, et al. Association between respiratory tract diseases and secondhand smoke exposure among never smoking flight attendants: a cross-sectional survey. Environ Health. 2007;6:28. doi:10.1186/1476-069X-6-28
20. Eisner MD, Balmes J, Katz PP, et al. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4(1):7. doi:10.1186/1476-069X-4-7
21. Yin P, Jiang CQ, Cheng KK, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou biobank cohort study. Lancet. 2007;370(9589):751–757. doi:10.1016/S0140-6736(07)61378-6
22. Wu CF, Feng NH, Chong IW, et al. Second-hand smoke and chronic bronchitis in Taiwanese women: a health-care based study. BMC Public Health. 2010;10:44. doi:10.1186/1471-2458-10-44
23. Chan-Yeung M, Ho AS, Cheung AH, et al. Lam, on behalf of the Hong Kong Thoracic Society COPD study group. determinants of chronic obstructive pulmonary disease in Chinese patients in Hong Kong. Int J Tuberc Lung Dis. 2007;11:502–507.
24. Zubair T, Abbasi A, Khan OA, Amer E. Role of passive smoking in non-smoking related chronic obstructive pulmonary disease. J Pak Med Assoc. 2018;68(9):1310–1315.
25. Johannessen A, Bakke PS, Hardie JA, et al. Association of exposure to environmental tobacco smoke in childhood with chronic obstructive pulmonary disease and respiratory symptoms in adults. Respirology. 2012;17(3):499–505. doi:10.1111/j.1440-1843.2012.02129.x
26. Sinha B, Chowdhury R, Chowdhury R, Chowdhury R. An epidemiological profile of chronic obstructive pulmonary disease: a community-based study in Delhi. J Postgrad Med. 2017;63(1):29–35. doi:10.4103/0022-3859.194200
27. Hagstad S, Bjerg A, Ekerljung L, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298–1304. doi:10.1378/chest.13-1349
28. Korsbaek N, Landt EM, Dahl M. Second-hand smoke exposure associated with risk of respiratory symptoms, Asthma, and COPD in 20,421 adults from the general population. J Asthma Allergy. 2021;14:1277–1284. doi:10.2147/JAA.S328748
29. Sezer H, Akkurt İ, Guler N, et al. A case-control study on the effect of exposure to different substances on the development of COPD. Ann Epidemiol. 2006;16(1):59–62. doi:10.1016/j.annepidem.2004.12.014
30. Waked M, Salame J, Khayat G, et al. Correlates of COPD and chronic bronchitis in nonsmokers: data from a cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2012;7:577–585. doi:10.2147/COPD.S35044
31. Kalandidi A, Trichopoulos D, Hatzakis A, et al. Passive smoking and chronic obstructive lung disease. Lancet. 1987;330(8571):1325–1326. doi:10.1016/s0140-6736(87)91210-4
32. Kalandidi A, Trichopoulos D, Hatzakis A, et al. The effect of involuntary smoking on the occurrence of chronic obstructive pulmonary disease. Soz Praventivmed. 1990;35(1):12–16. doi:10.1007/BF01369539
33. Flexeder C, Zock JP, Jarvis D, et al. Second-hand smoke exposure in adulthood and lower respiratory health during. Respir Res. 2019;20(1). doi:10.1186/s12931-019-0996-z
34. Cunalata-Paredes AV, Gea-Izquierdo E. COPD in the major nonsmoking adult: a systematic review and meta-analysis. Arch Environ Occup Health. 2021;76(6):319–329. doi:10.1080/19338244.2020.1828243
35. Jindal SK, Gupta D. The relationship between tobacco smoke & bronchial asthma. Indian J Med Res. 2004;120(5):443–453.
36. Guerra S, Stern DA, Zhou M, et al. Combined effects of parental and active smoking on early lung function deficits: a prospective study from birth to age 26 years. Thorax. 2013;68(11):1021–1028. doi:10.1136/thoraxjnl-2013-203538
37. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375(9):871–878. doi:10.1056/NEJMra1603287
38. Wheaton AG, Liu Y, Croft JB, et al. Chronic obstructive pulmonary disease and smoking status - United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(24):533–538. doi:10.15585/mmwr.mm6824a1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
