Back to Journals » International Medical Case Reports Journal » Volume 15
Secondary Macular Holes Post Pars Plana Vitrectomy
Authors Okonkwo ON , Akanbi T, Agweye CT
Received 30 January 2022
Accepted for publication 11 March 2022
Published 5 April 2022 Volume 2022:15 Pages 141—155
DOI https://doi.org/10.2147/IMCRJ.S357655
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ogugua N Okonkwo,1,2 Toyin Akanbi,2 Chineze T Agweye3
1Eye Foundation Retina Institute, Lagos, Nigeria; 2Department of Ophthalmology, Eye Foundation Hospital, Abuja, Nigeria; 3Department of Ophthalmology, University of Calabar Teaching Hospital, Calabar, Nigeria
Correspondence: Ogugua N Okonkwo, Eye Foundation Retina Institute, Lagos, Nigeria, Tel +234 803 502 7308, Email [email protected]
Purpose: To report incidence, clinical presentation, and treatment outcome of full-thickness macular hole (FTMHs) diagnosed post pars plana vitrectomy.
Methods: We retrospectively reviewed the demographics, best-corrected visual acuity (BCVA), indication for the primary vitrectomy, time to diagnose the secondary FTMH, optical coherence tomographic (OCT) appearance, and treatment outcome of FTMHs, occurring after vitrectomy performed between January 2019 and December 2020.
Results: Six of 523 vitrectomized eyes developed FTMHs, an incidence of 1.1%. There were five females and one male, mean age of 56.5 years (range 37– 85). The indication for primary vitrectomy was rhegmatogenous retinal detachment (RRD) in three eyes, one eye each for sub internal limiting membrane hemorrhage from a ruptured macroaneurysm, vitreous hemorrhage from polypoidal choroidal vasculopathy (PCV), and pre-insertion of Ahmed glaucoma drainage device (GDD). FTMHs occurred within one week to three months after vitrectomy (time from primary vitrectomy to the identification of the secondary MH was a mean of 1.03 months). Mean BCVA in all six MH eyes was log MAR 0.9 (Snellen: 6/54). Anatomical closure was achieved after one surgery in three eyes, two surgeries in 1 eye, after photodynamic therapy (PDT) in the PCV eye, and one patient declined surgery. The mean BCVA in the four surgically closed MH eyes improved marginally from log MAR 0.82 (Snellen: 6/38) to log MAR 0.72 (Snellen: 6/30), mean follow-up 7.6 months.
Conclusion: Post-vitrectomy FTMH is rare, and RRD was the commonest indication for initial vitrectomy. We observed that all secondary MHs were closed successfully using the inverted internal limiting membrane (ILM) flap technique with limited improvement in vision. The visual outcome of these secondary MHs trails behind that of idiopathic MHs.
Keywords: full thickness macular hole, vitrectomy, complications of vitrectomy, epiretinal membrane, optical coherence tomogram, inverted internal limiting membrane flap
Introduction
Significant advances have been made over the past three decades in understanding the pathogenesis of idiopathic macular holes (MH); Gass led this effort.1,2 Optical coherence tomography (OCT) has confirmed an earlier hypothesis regarding the significance of tangential and anteroposterior traction in the pathogenesis of idiopathic MHs.3 Formation of secondary MH after vitrectomy (with hypothetical removal of anteroposterior traction component) has been reported by several researchers.4,5 The evolution of post vitrectomy MHs is uncertain, partly because of its rarity and the diversity of indications for the primary vitrectomy before the occurrence of the secondary MH. The causative mechanisms that have been associated with post vitrectomy MH formation include tangential traction from the residual perifoveal vitreous cortex,6 cystoid degeneration,7 and intraoperative (iatrogenic) trauma.8 Epiretinal membranes (ERM) are present in several cases, suggesting a causal association.9 Indication for the primary vitrectomy reported in the literature include rhegmatogenous retinal detachment (RRD), ERM surgery, macular edema, diabetic vitrectomy, vitreous hemorrhage removal, and pre-insertion of Baerveldt glaucoma implant amongst others. This research contributes vitrectomy indications and techniques to close the MH and reviews the current knowledge on this topic.
Methods
We searched the hospital’s surgical log and identified all eyes that developed a full thickness macular hole (FTMH) after undergoing vitrectomy between January 2019 and December 2020. We then extracted information retrospectively from the records. All patients signed informed consent before undergoing the primary vitrectomy and before surgery to repair the secondary MH. The same surgeon performed all the primary vitrectomies and performed the MH repairs.
Ethical approval was sought from the Eye Foundation Hospital Research Ethics Committee (EFHREC), which determined that the research was a retrospective review of case records and no significant risk to the patients and provided a waiver for this research (EFHREC/10/2021). We conducted this research in compliance with the protocol stated in the Helsinki declaration. A deliberate effort was made to ensure the identity of the presented cases was concealed.
All the patients with the exclusion of one (case 4) had a dilated slit-lamp binocular fundus examination using a 90D non-contact lens and optical coherence tomography (OCT) (Optovue, Inc.) imaging of the macula before the diagnosis of post vitrectomy FTMH. In case 4, the diagnosis of FTMH was made during surgery (intraoperatively) at the time of silicone oil removal (SOR). None of the patients had a MH before the primary vitrectomy. All patients who had coexisting FTMH with retinal detachment (RD) at the time of primary vitrectomy were excluded. Demographic information extracted from the case record includes age and gender. Clinical examination findings include laterality, timing and the indication of primary vitrectomy, details of the vitrectomy surgery, documented time of FTMH detection, surgical technique for closure of the MH, best corrected visual acuity (BCVA) in the presence of MH and after MH repair, the status of the MH at the last visit, duration of follow up and the presence of any ocular comorbidity.
The MH surgery was performed using the inverted internal limiting membrane (ILM) flap technique in three eyes and an ILM peel technique in one eye. The inverted ILM flap technique involved staining the foveomacular ILM using DuoBlue (combination of 0.01% trypan blue and 0.05% Brilliant Blue G). Then a small piece of ILM was mobilized from an area beneath the superior arcade and advanced to the edge of the macular hole. This piece of ILM tissue was then flipped over (inverted) to cover the MH and kept fixed to the MH with a bubble of PFCL. A PFCL air exchange was performed, and the air-filled eye was closed using 7–0 vicryl sutures. There was no instruction to the patient to maintain a face down posturing, and the patient could adopt any face position desired apart from facing upwards.
In one eye in which an ILM peel was employed for MH closure surgery, a similar technique of ILM staining was used, and a complete peel of the ILM surrounding the MH was done, and 10% perfluoropropane (C3F8) gas was used as tamponade, with no instruction to adopt a face down posture.
We also conducted a Medline search using the words “secondary macular hole” and “post vitrectomy macular hole.” All publications found in English about post vitrectomy MH were selected and read by the authors (there were no exclusion criteria for article selection). Also, from the references in these articles, other relevant publications were selected and included amongst the reviewed materials.
Details of the pre and post MH repair BCVA are seen in Table 1. Table 1 also summarizes the clinical presentation and outcome of the intervention. Table 2 presents the OCT characteristics of the MHs.
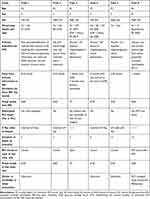 |
Table 1 Presentation and Clinical Characteristics of Six Post-Vitrectomy Macular Hole Patients |
 |
Table 2 OCT Features of Macular Holes (microns) |
A Presentation of the Six Cases and OCT Images
Case 1
A 52-year-old female presented with a significant amount of vitreous in the anterior chamber after left eye phacoemulsification of cataract and intraocular lens implantation. She developed sustained elevated intraocular pressure (>30 mmHg) despite being on maximum medical therapy and advanced stages of glaucomatous damage to the optic nerve. The management plan by the glaucoma specialist was to place an Ahmed glaucoma drainage device (GDD) into the anterior vitreous cavity. A combined surgery was performed using a general anesthetic in the same surgical session. First, a three-port pars plana vitrectomy (PPV), followed by GDD insertion. After surgery, an air-filled vitreous cavity with a well-positioned drainage tube in the anterior vitreous cavity was achieved. A FTMH was noticed following uneventful surgery on fundus examination at the first postoperative clinic visit. Macular OCT showed a large FTMH with intraretinal cystic spaces Figure 1. Significantly, intraoperatively, PPV did not involve any manipulation around the macula. Two attempts using an inverted ILM flap technique were required before the MH was successfully closed. Pre- and postoperative BCVA and other clinical information, including OCT details, are shown in Tables 1 and 2.
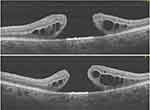 |
Figure 1 A large FTMH which developed one week after primary vitrectomy for the insertion of glaucoma drainage device (GDD) into the anterior vitreous space. |
Cases 2
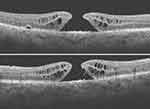
An 85-year-old female complained of reduced vision in her only seeing right eye. On fundus examination, she had a sub ILM hemorrhage covering the right eye macula due to a ruptured macro aneurysm, Figure 2. Vision in her left eye was Hand Motion due to a macular scar. The sub ILM hemorrhage was successfully removed using vitrectomy. FTMH developed three weeks following vitrectomy surgery, as shown in Figure 3. OCT showed perifoveal epiretinal membranes in the inner portion of the MH and intraretinal cystic spaces. The FTMH was closed using the inverted ILM flap technique. More information can be seen in Tables 1 and 2.
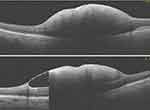 |
Figure 2 Sub-internal limiting membrane (ILM) hemorrhage from a ruptured macro aneurysm. |
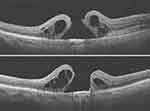 |
Figure 3 FTMH, with accumulation of large intraretinal cystic fluid and ERM occurring three weeks after vitrectomy for the removal of sub ILM hemorrhage. |
Case 3
A 62-year-old female diagnosed with an RRD was referred to the clinic for care. On examination, we noticed she had a left eye superior bullous rhegmatogenous retinal detachment involving the macula, with a superior retinal break present. She underwent a vitrectomy with silicone oil tamponade. Postoperative vision improved to 6/36 (with a visually significant cataract), and she had pre silicone oil removal supplemental retinal laser to peripheral retinal breaks. After six months of initial vitrectomy, she had combined silicone oil removal with phacoemulsification of cataract and lens implantation. BCVA was 6/60 at the first-week post-surgery examination. One-month post-surgery clinic review, her vision decreased to counting fingers (CF). An MH was diagnosed on examination, and an FTMH with ERM and intraretinal cystoid spaces were seen on OCT, Figure 4. She declined further surgery to close the MH. Tables 1 and 2 provides clinical and OCT details.
Case 4
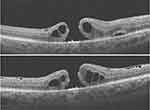
A 37-year-old female myope was diagnosed with a left eye macula involving total retinal detachment with multiple peripheral retinal breaks, reducing BCVA to 6/36. Preoperative OCT showed a detached macula and no MH, Figure 5. She had a combined encircling band, vitrectomy, and silicone oil tamponade. Her vision improved to 6/18. At the time of silicone oil removal, an FTMH was noticed after the silicone oil was removed. Therefore, an ILM flap was created and inverted over the MH. Postoperatively the MH was closed with ILM tissue covering the foveomacular area when examined using the OCT, Figure 6. Vision remained the same, and she received topical ocular hypotensive medications to control intraocular pressure to both eyes.
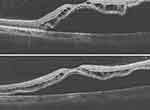 |
Figure 5 Rhegmatogenous retinal detachment involving the macula, and absent macular hole. |
 |
Figure 6 The inverted internal limiting membrane flap is seen over the fovea in the attached retina. The MH is closed and there is a presence and continuity of the outer retinal layers. |
Case 5
A 53-year-old female presented with a superior “macular on” RRD and attached vitreous, Figure 7. Vitrectomy was uneventful with uncomplicated induction of posterior vitreous detachment (PVD), retinal reattachment, and air tamponade. On postoperative examination, the retina was reattached, but an FTMH with ERM and intraretinal cystoid spaces (Figure 8) was noticed at the one-week clinic visit, reducing vision from 6/6 to 3/36. As there was a delay in MH surgery, the MH was observed to begin to self-close, Figure 9. However, re-vitrectomy with ILM peel and gas tamponade resulted in complete closure of the FTMH, Figure 10. Vision improved to 6/36.
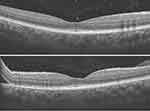 |
Figure 7 Superior macular “on” retinal detachment with attached macula and attached vitreous. |
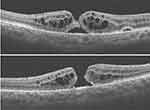 |
Figure 9 Attempted self-closure of the FTMH, following a delay in MH surgery. |
Case 6
A 50-year-old male was referred to the retina clinic and diagnosed with a large subretinal hemorrhage in the right eye secondary to active polypoidal choroidal vasculopathy (PCV). His vision was CF at presentation. He received monthly intravitreal injections of Aflibercept, which resulted in the resolution of hemorrhage and return of vision to 6/12. There was recurrent hemorrhage from the PCV lesion with associated vitreous hemorrhage. A vitrectomy for vitreous hemorrhage removal was performed. One month after this vitrectomy, he developed an FTMH with a large amount of subretinal fluid (SRF) and macular detachment, Figure 11. After undergoing photodynamic therapy (PDT), the SRF resolved, and the macular hole closed (Figure 12). The Snellen visual acuity has remained 6/60, the same as before MH closure.
 |
Figure 11 FTMH and a large amount of subretinal fluid. ERM and minimal intraretinal cystic fluid are also present. |
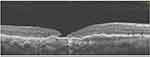 |
Figure 12 Type “2” closure of the FTMH, with exposure of the RPE. There is resolution of the subretinal fluid. Undulating appearance of the RPE due to the PCV. |
Discussion and Review
We report a 1.1% incidence of secondary MH after vitrectomy, the primary indications for vitrectomy, and the treatment outcomes of a series of post vitrectomy FTMHs and review the existing literature. The occurrence of FTMH after vitrectomy is rare.6 The incidence of FTMH after vitreoretinal surgery varies widely. Sokol et al reported the highest incidence of 4.4% after diabetic vitrectomy for tractional retinal detachment.10 The incidence of FTMH after RRD repair has been reported to be between 0.9 to 1.9%.7,11,12 In our study, the incidence after vitrectomy for RRD was 0.57%. After ERM surgery, the incidence of 2.1 to 2.6% has been reported by Rush et al.13 Though the largest series on post vitrectomy FTMH did not report any incidence, in general, for vitrectomy (considering all indications), the incidence of FTMH ranges from 0.24 to 0.60.5,6 Our incidence of 1.1% is higher than the previously reported range. This finding may be because we included eyes that developed MH soon after surgery, which was excluded from other studies.
FTMH after vitrectomy has not been an area of keen research, partly because of low incidence and more interest in the more commonly reported idiopathic macular hole. Our Medline search on this topic showed that the most recent report was Kang et al in 2019.6 Before this, we found less than ten publications (excluding case reports) over the past decade using the Medline search. Therefore, a considerable gap exists in knowledge regarding the causes and pathogenetic mechanisms, treatment, and outcomes.
A. Age
There have been several reports on the age of patients who develop FTMH after vitrectomy. The youngest age was reported by Schlenker et al, who reported this complication in a 9 year old female who had retinal detachment with associate optic pit.7 Lee et al and Garcia-Arumi et al also reported this complication in a 27 year old and a 29 year old, respectively.5,14 The youngest patient in our current series is a 37 year old female myope who had a macula involving RRD. The oldest age in our series is 85 years and is similar to the 86 years reported by Medina et al. The mean age from previous reports falls within the range of 45–69 years. Our mean age of 56.5 years falls within this reported age bracket and agrees with the finding by Schlenker that the age of patients was relatively younger than the age reported for idiopathic macular holes.
B. Sex
Traditionally females have been demonstrated to be at increased risk of developing idiopathic MHs and probably have larger horizontal diameter holes. In the current study, 83% of the patients were females. Rates of females in other reported series are Kang et al 51%, Lee et al 70%, Kumagai et al 53%, and Ido Didi et al. 67%. It, therefore, appears that the female predisposition to idiopathic MHs also extends to this form of secondary MH. However, Schlenker reported a male dominance of 61% in his series of 18 patients, and higher male ratios have also been reported in other smaller case series on MHs after retinal detachment repair. These studies all examined MH formation after retinal reattachment surgery. RRD tends to occur more in males; this may significantly influence and skew the finding in favor of males. Kang et al, however, observed that there was no sex predilection in one of the largest series considering 38 eyes.
C. Pathogenetic Mechanism of Macular Hole Formation
Different mechanisms have been suggested to be responsible for FTMH in post vitrectomy eyes. In post vitrectomized eyes, the absence of vitreous eliminates the concept of anterior-posterior traction, which is known to influence idiopathic MH formation significantly. However, the evidence seems to support the existence of tangential traction as a mechanism for creating this secondary MH. Some studies have used OCT to confirm the presence of ERM in eyes with FTMH post vitrectomy.9,15,16 In some cases, the ERM has occurred before and even after the occurrence of the hole. OCT enables the determination of ERM presence and can demonstrate the tractional effect of the ERM. Khurana et al, in their report of 25 FTMHs occurring after retinal reattachment surgery, concluded that ERM was integral to hole formation.9 Another significant series reported by Kang et al noticed the occurrence of ERM (using OCT for detection) in 71% of eyes.6 In our study, OCT demonstrated the presence of ERM at the time of MH formation in five eyes. In two eyes (cases 3 and 5), there was an inward indentation of the outer retina, which was unusual and could be caused by tangential tractional forces induced by the ERM (Figures 4 and 8).
The presence of cystoid macular is another mechanism thought to be responsible for this complication, as argued by Lee and Schlenker.5,7 Significantly, a minority of studies have reported on the presence of CME before the occurrence of MHs. In the five MHs, we imaged using OCT, intraretinal cystoid spaces were present. The occurrence of this CME, we believe, is secondary to MH formed and not the cause of the MH. Another mechanism of MH formation is iatrogenic trauma during vitrectomy surgery.8 Possible causes of iatrogenic trauma include direct instrument contact, traction induced MH during ILM or ERM peeling, and the direct impact of a jet of high-velocity air or infusion fluid on the macula. A replay of the surgical video, if available, will reveal any unrecognized physical impact on the macula. Eccentric and foveal MHs have been reported to occur after vitrectomy and ILM peeling. The incidence of this complication ranges between 0.6 and 2.2%.17 Grasping of the retinal tissue with the forceps at the time of ILM or ERM peeling, and the weakening of the retina’s glial (Muller cell) structure has been proposed to be responsible for the occurrence of this unique complication.17 None of these forms of iatrogenic trauma occurred in the current study, and there was no attempt at peeling ILM or ERM in any of the six primary vitrectomies. Finally, other suggested mechanisms of secondary MH formation include the presence of a taut internal limiting membrane and residual vitreous contraction.10
D. Time Interval Between Primary Vitrectomy and Presentation of MH
The time between vitrectomy and detection of FTMH varies considerably. Some studies eliminated early-onset MHs for the simple reason that iatrogenic trauma could not be excluded in such cases. Kumagai et al excluded eyes in which MH developed within one month of primary surgery for the reason stated above.18 Some other studies did not exclude early-onset (short interval) MHs as in our study. Therefore, we recorded a mean time of MH presentation of 1.04 months (range 0.25 to 3 months). Similarly, others have reported short intervals, including Lee et al 0.6months and Schlenker et al two days.
E. Size of Macular Hole
The choice of MH surgical technique is affected by MH size. Also, the anatomical success of idiopathic MH surgery has been closely associated with MH size. Macular holes less than 400 microns have been shown to have considerably higher anatomical success with conventional surgery compared to those > 500 microns. In the current study, the size of the MH was defined as the minimum hole diameter drawing a line with the “inbuilt” OCT caliper parallel to the retinal pigment epithelium. The mean MH size in this current study is 566 microns (Table 2). Other reports in this area of research have not considered MH size. We observed that the rapidity of occurrence and the large size of the MHs was unique to this series compared to other idiopathic MH. For example, case 1 developed an 840-micron size MH within one week of surgery, case 2 a 700-micron MH within three weeks, and case 5 a 450 micron MH within one week. These three cases had an entirely attached macula before primary surgery (ie, no retinal detachment involving the macula). The acuteness and rapid progression to a large MH contrast with the evolution of idiopathic MHs, known to follow a stage-wise development proposed by Gass (ie, stages 1 to 4). This observed rapid progression could negatively affect the visual outcome of these secondary MHs.
F. Primary Diagnosis Before Initial Vitrectomy
Several authors have reported rhegmatogenous RD as the commonest primary indication for vitrectomy for this complication. In our study, three eyes (50%) had primary vitrectomy for RRD, the commonest indication. Rhegmatogenous RD was noted as the commonest primary indication for surgery in 7 out of the eight publications reviewed by Kang et al. Furthermore, in Lee’s publication, RRD accounted for 50% of the cases, the most number of cases. Other studies have considered and reported their findings on the occurrence of FTMH in an all-RD group of patients. The largest of these series on FTMH occurring after RD repair was reported by Khurana et al, who studied 25 eyes, Garcia – Arumi studied 20 eyes, and Schlenker reported his findings in 18 RD eyes. Khurana established the significance of ERM in causing the MH after RD surgery, while Schlenker suggested the role of CME in the pathogenesis of MH. He also reported on the association of post RD repair MH with macular off RD and multiple surgeries to reattach the retina. Medina et al reported similar findings as Schlenker and described findings of an association with ERM and myopia. However, that RD is the commonest primary indication for post vitrectomy MHs, may be related to previous reports of RD being the commonest indication for vitrectomy and the occurrence of ERM in several cases of RD post vitrectomy.19
Other reported indications for primary vitrectomy resulting in FTMH include vitreous hemorrhage from different causes, idiopathic and secondary ERM, proliferative diabetic retinopathy (PDR), proliferative vitreoretinopathy (PVR), submacular hemorrhage, related to silicone oil removal (SOR), lamellar macular hole, macular edema, and vitreomacular traction.
In the current study, case 1 developed an FTMH after vitrectomy before inserting an Ahmed Glaucoma Drainage Device (GDD) into the anterior vitreous cavity. The MH in this patient (case 1) required two attempts to close it using the inverted ILM flap technique. We also reported that a vitrectomy for removing sub-ILM hemorrhage secondary to a ruptured retinal arterial macroaneurysm (RAM) was a cause for FTMH. RAM has been associated with the occurrence of early-onset and delayed MH formation. One case of FTMH following vitrectomy for a sub-macular hemorrhage from RAM was reported in the series by Kang.6 Sato et al presented three cases of RAM associated with early-onset MHs.20 Case 3 in Sato’s series had a sub ILM hemorrhage which was removed using vitrectomy. They hypothesized that early-onset MHs might arise from micro laceration occurring at the fovea due to a gap between the intraocular pressure (IOP) and very elevated pressure in the subretinal space derived from systolic blood pressure immediately after the rupture of the macroaneurysm and tangential retinal traction related to the hydrodynamics of arterial hemorrhage, or both. The MH was noticed three weeks after the vitrectomy in our patient but could have occurred earlier. It was, therefore, an early onset MH. ERM was present in this eye (Figure 3) and could have contributed to the occurrence of the MH, as has been suggested to happen in delay onset cases of MH due to RAM. Our case was an 85-year-old woman, similar in age to the 87-year-old woman reported by Sato.
Another cause of vitrectomy reported in the current study is vitreous hemorrhage secondary to active polypoidal choroidal vasculopathy (PCV) with submacular hemorrhage. There was one case of FTMH formation in PCV with vitreous hemorrhage reported in the series by Kang et al. There are few reports of FTMH occurring after PCV treatment with intravitreal antiVEGF. The occurrence of an FTMH is a rare complication of PCV. Our case developed MH after recurrent subretinal and then vitreous hemorrhage. The MH developed after one month of vitrectomy. Macular OCT evaluation performed soon after the vitrectomy showed a relatively thin retina with significant subretinal fluid. Tangential foveomacular tractional forces acting within the outer and inner retina are likely responsible for the MH formation.
Garcia- Arumi et al reported 14 cases after RD which was repaired with vitrectomy and encircling band. In the current report, case 4 had a combined encircling band, vitrectomy, and silicone oil for a total retinal detachment involving the macula. Her retina was reattached with a single surgery. At the time of removal of silicone oil (ROSO), an MH was noticed by the surgeon. An ILM flap was mobilized and inverted over the MH. It is possible that this MH was present before the ROSO but was missed. However, the MH was not present before the primary vitrectomy since a preoperative OCT showed an intact detached foveomacular area, Figure 5. It is also possible that a partial thickness hole or a pseudo-macular hole was mistaken for an FTMH. Intraoperative OCT (iOCT) would have helped confirm a diagnosis of FTMH during the ROSO, but iOCT was not available. However, a post-ROSO OCT showed the presence of an ILM flap over a discontinuous inner retina, with an intact outer retina, Figure 6. Outer retinal reconstruction has been shown to occur after the inverted ILM flap technique used to treat FTMH.21 Apart from case 4, an FTMH also occurred one month after the ROSO in case 3. MH after ROSO occurred in 5 cases reported by Kang et al.6 Medina et al16 reported two cases of MH formation while silicone oil was still in the eye. In our opinion, ROSO may be associated with ERM formation, contraction of preexisting ERM, foveomacular traction and resultant FTMH. The convexity of silicone oil meniscus at the fovea does not make it an ideal tamponade for MH. ROSO could favor the tangential forces induced by residual vitreous cortex or ERM and result in FTMH formation.
G. Macular Hole Treatment and Outcome
Published literature on this topic has reported on the treatment and outcomes of this form of secondary MHs. Generally, the reporting of results shows that high rates of anatomical success can be achieved with repeat vitrectomy, ILM peeling, and various forms of tamponade (including gas and silicone oil). However, the visual outcome is variable and depends on the primary pathology. Lee, Kang, and Khurana have reported good visual results after MH closure. Kang observed that poor visual outcome was associated with long eyeballs (high myopia) and poorer preoperative BCVA. Medina et al observed that visual recovery was limited even when MH closure was achieved; they also noticed a significant association between macula “off” RD and high myopia on the outcome. This finding is supported by the conclusion derived by Garcia- Arumi et al, who reported that the limited visual outcome depended on macula “off” status. In the current study, three eyes had a macula “on” status and were not myopic but had limited improvement in vision. Case 4 had a primary macula “of” RD and had better vision. In case 4, the MH was identified during ROSO and addressed promptly. Perhaps the time before repairing the MH could be an important factor, influencing visual outcome. Case 5, who had a macula “on” RD but delayed MH repair, had limited improvement in BCVA. Therefore, it is safe to suggest that factors which influence the visual outcome of this group of MHs are still open to research and could be dependent on the underlying or preexisting condition and MH-related factors, including MH size and time interval before repairing the MH.
In our study, three eyes were repaired using an inverted ILM flap and air tamponade, while one eye had ILM peel and gas. The inverted ILM flap technique for closure of large MH has become famous for large MHs,21–23 but has not been previously reported in any series on MH after vitrectomy. The average size of the 6 MHs (minimum diameter) shown in Table 2 is 566 microns (range 400–840 microns). The inverted ILM flap effectively repaired all the MHs, and all remained closed after a mean follow-up of 7.6 months. We noticed an improvement in BCVA in two out of the four surgically closed MHs (both eyes had a 2 Snellen line improvement in BCVA), while in two eyes, BCVA remained the same as preoperative vision.
The occurrence and closure of MH from PCV after intravitreal VEGF has been reported previously.24,25 Interestingly in the one eye with an MH following hemorrhage from active PCV, the MH closed spontaneously following a session of PDT. Our case is the first report of MH closure after PDT following a detailed search on PubMed to the best of our knowledge. After PDT, MH closure was associated with resolution of subretinal and intraretinal fluid and reattachment of the retina, as seen in Figure 12. Resolution of the subretinal fluid after PDT resulted in the near re-apposition of the MH edges. There was a type 2 closure, with RPE exposed.26 As reported for idiopathic MH, this type of closure is associated with a poor visual outcome.26 Therefore, not surprising, vision in this eye remained the same as before PDT.
Case 3 in our series declined surgery to close the MH. The MH increased in size in her eye, but the vision remained the same. Observation of MH and monitoring hoping for self-closure of the MH is a strategy, and there have been reports of spontaneous closure of the MH with improvement in BCVA.8 In our study, case 5 demonstrated OCT features of attempted spontaneous hole closure while waiting for MH surgery, as seen in Figure 9. Lastly, we should mention that topical treatment of secondary MH has been reported with anatomical success and improvement in vision.27
The retrospective nature of this study and small sample size are significant limitations. The small sample size did not allow for any statistically significant analysis. However, our reporting of this series suggests that visual recovery after the repair of post vitrectomy MH is limited and not as reported after idiopathic MH. This finding implies that these secondary macular holes should be prevented when possible. Since ERM presence and tangential traction have been associated as a causative factor, prophylactic ERM and internal limiting membrane peeling should be considered in at-risk eyes during the primary vitrectomy. Such eyes will include cases in which there is preoperative ERM in RRD and other causes of retinal and vitreous hemorrhage. One must consider the risk of complications of ILM peeling against the low incidence of secondary MH. ILM peeling can itself induce MHs. Also, since delaying surgical repair of MH was associated with poor visual outcomes in our study, immediate repair of the MH as soon as diagnosed is advised.
Conclusion
Though rare, occurrence of an FTMH should be considered a possible complication of vitrectomy for RRD and other indications. This group of secondary MHs differs considerably from idiopathic MHs. Despite the association in several cases to contraction of ERM, more research is required to understand their pathogenesis, preferred treatment, and factors influencing treatment outcome since these have shown to be different from idiopathic MHs. Various forms of hemorrhage, including pre retinal, subretinal, sub internal limiting membrane, and vitreous hemorrhage as seen with RAMA, PCV, PDR, and other retinovascular diseases, are important predispositions of FTMHs. The interval between the formation of FTMH and the repair may be an essential factor influencing the visual outcome. The inverted ILM flap technique provides a good option for successful anatomical closure in large diameter holes. We also reported hole closure after PDT for MHs related to PCV.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–639.
2. Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752–759. doi:10.1016/S0002-9394(14)72781-3
3. Hassenstein A, Scholz F, Richard G. OCT bei makulaforamen [OCT in macular holes]. Ophthalmologe. 2004;101:777–784. German. doi:10.1007/s00347-004-1053-x
4. Kumawat D, Venkatesh P, Brar AS, et al. Atypical macular holes. Retina. 2019;39:1236–1264. doi:10.1097/IAE.0000000000002448
5. Lee SH, Park KH, Kim JH, et al. Secondary macular hole formation after vitrectomy. Retina. 2010;30:1072–1077. doi:10.1097/IAE.0b013e3181cd4819
6. Kang HG, Han JY, Choi EY, et al. Clinical characteristics, risk factors, and surgical outcomes of secondary macular hole after vitrectomy. Sci Rep. 2019;9(1):19535. doi:10.1038/s41598-019-55828-x
7. Schlenker MB, Lam WC, Devenyi RG, et al. Understanding macular holes that develop after repair of retinal detachment. Can J Ophthalmol. 2012;47:435–441. doi:10.1016/j.jcjo.2012.05.001
8. Gurung RL. Spontaneous formation and closure of full thickness macular hole in a vitrecto-mised eye: a case report. Nepal J Ophthalmol. 2017;9:199–202. doi:10.3126/nepjoph.v9i2.19270
9. Khurana RN, Wykoff CC, Bansal AS, et al. The association of epiretinal membrane with macular hole formation after rhegmatogenous retinal detachment repair. Retina. 2017;37:1073–1078. doi:10.1097/IAE.0000000000001307
10. Sokol JT, Ferenchak K, Rosen DT, et al. Macular hole formation after pars plana vitrectomy for diabetic tractional retinal detachment. Ophthalmic Surg Lasers Imaging Retina. 2018;49(12):e256–e262. doi:10.3928/23258160-20181203-16
11. Brown GC. Macular hole following rhegmatogenous retinal detachment repair. Arch Ophthalmol. 1988;106:765–766. doi:10.1001/archopht.1988.01060130835034
12. Benzerroug M, Genevois O, Siahmed K, et al. Results of surgery on macular holes that develop after rhegmatogenous retinal detachment. Br J Ophthalmol. 2008;92:217–219. doi:10.1136/bjo.2007.122796
13. Rush RB, Simunovic MP, Aragon AV, et al. Postoperative macular hole formation after vitrectomy with internal limiting membrane peeling for the treatment of epiretinal membrane. Retina. 2014;34:890–896. doi:10.1097/IAE.0000000000000034
14. Garcia-Arumi J, Boixadera A, Martinez-Castillo V, et al. Macular holes after rhegmatogenous retinal detachment repair: surgical management and functional outcome. Retina. 2011;31:1777–1782. doi:10.1097/IAE.0b013e31820a69c3
15. Takashina H, Watanabe A, Tsuneoka H. Full-thickness macular hole formation in the postoperative period after initial vitrectomy for rhegmatogenous retinal detachment. Case Rep Ophthalmol. 2017;8:595–601. doi:10.1159/000479727
16. Medina CA, Ortiz AG, Relhan N, et al. Macular hole after pars plana vitrectomy for rhegmatogenous retinal detachment. Retina. 2017;37:1065–1072. doi:10.1097/IAE.0000000000001351
17. Brouzas D, Dettoraki M, Lavaris A, et al. Postoperative eccentric macular holes after vitrectomy and internal limiting membrane peeling. Int Ophthalmol. 2017;37:643–648. doi:10.1007/s10792-016-0320-6
18. Kumagai K, Ogino N, Furukawa M, et al. Surgical outcomes for patients who develop macular holes after pars plana vitrectomy. Am J Ophthalmol. 2008;145:1077–1080.
19. Okonkwo ON, Lewis K, Hassan AO, et al. Indications and outcomes of vitrectomy surgery in a series of 1000 black African eyes. BMJ Open Ophthalmol. 2019;4:e000083. doi:10.1136/bmjophth-2017-000083
20. Sato R, Yasukawa T, Hirano Y, et al. Early-onset macular holes following ruptured retinal arterial macroaneurysms. Graefes Arch Clin Exp Ophthalmol. 2008;246:1779–1782. doi:10.1007/s00417-008-0930-4
21. Okonkwo ON, Hassan AO, Gyasi ME, et al. Outer retina reconstruction following inverted internal limiting membrane flap technique for large macular holes. Saudi J Ophthalmol. 2021;34:160–166. doi:10.4103/1319-4534.310408
22. Silva N, Ferreira A, Nawrocka Vel Michalewska ZA, et al. Inverted internal limiting membrane flap technique: is it the best option for macular holes? Clin Ophthalmol. 2021;15:3295–3303. doi:10.2147/OPTH.S284614
23. Okonkwo ON, Hassan AO, Oderinlo O. Inverted internal limiting membrane flap technique for extra large macular holes. Current Trends Ophthalmol. 2018;1:07–13. doi:10.18314/ctoy.v1i1.371
24. Sethia A, Sheth J, Gopalakrishnan M, et al. Spontaneous formation and closure of full thickness macular hole after treatment with anti-vascular endothelial growth factor therapy in polypoidal choroidal vasculopathy. Indian J Ophthalmol. 2019;67:1756–1758. doi:10.4103/ijo.IJO_1597_18
25. Cho JH, Park SE, Han JR, et al. Macular hole after intravitreal ranibizumab injection for polypoidal choroidal vasculopathy. Clin Exp Optom. 2011;94:586–588. doi:10.1111/j.1444-0938.2011.00614.x
26. Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87:1015–1019. doi:10.1136/bjo.87.8.1015
27. Niffenegger JH, Fong DS, Wong KL, Modjtahedi BS. Treatment of secondary full-thickness macular holes with topical therapy. Ophthalmol Retina. 2020;4:695–699. doi:10.1016/j.oret.2020.01.014
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.