Back to Journals » Medical Devices: Evidence and Research » Volume 7
Sealing vessels up to 7 mm in diameter solely with ultrasonic technology
Authors Timm R, Asher R, Tellio K, Welling AL, Clymer J , Amaral J
Received 26 April 2014
Accepted for publication 27 May 2014
Published 30 July 2014 Volume 2014:7 Pages 263—271
DOI https://doi.org/10.2147/MDER.S66848
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Richard W Timm, Ryan M Asher, Karalyn R Tellio, Alissa L Welling, Jeffrey W Clymer, Joseph F Amaral
Ethicon Inc., Cincinnati, OH, USA
Introduction: Ultrasonic energy is a mainstay in the armamentarium of surgeons, providing multifunctionality, precision, and control when dissecting and sealing vessels up to 5 mm in diameter. Historically, the inability to seal vessels in the 5–7 mm range has been perceived as an inherent limitation of ultrasonic technology. The purpose of this study was to evaluate sealing of vessels up to 7 mm in diameter with an ultrasonic device that modulates energy delivery during the sealing period.
Methods: In ex vivo benchtop and in vivo acute and survival preclinical models, a new ultrasonic device, Harmonic ACE®+7 Shears (Harmonic 7), was compared with advanced bipolar devices in sealing vessels 1–7 mm in diameter with respect of burst pressure, seal reliability, and seal durability. Lateral thermal damage and transection time were also evaluated.
Results: Ex vivo tests of Harmonic 7 demonstrated significantly greater median burst pressures than an advanced bipolar device both for vessels <5 mm in diameter (1,078 mmHg and 836 mmHg, respectively, P=0.046) and for those in the range of 5–7 mm (1,419 mmHg and 591 mmHg, P<0.001). In vivo tests in porcine and caprine models demonstrated similar rates of hemostasis between Harmonic 7 and advanced bipolar devices, with high success rates at initial transection and seal durability of 100% after a 30-day survival period.
Conclusion: Sealing 5–7 mm vessels is not a limitation of the type of energy used but of how energy is delivered to tissue. These studies document the ability of ultrasonic energy alone to reliably seal large vessels 5–7 mm in diameter, with significantly greater burst pressure observed in in vitro studies than those observed with an advanced bipolar technology when energy delivery is modulated during the sealing cycle. Furthermore, the seals created in 5–7 mm vessels are shown to be reliable and durable in in vivo preclinical studies.
Keywords: ultrasonic, Harmonic, vessel sealing, burst pressure, 7 mm
Introduction
Ultrasonic technology for cutting and sealing soft tissue was introduced >20 years ago as a safer alternative to monopolar electrosurgery.1 During the ensuing period, the advantages of ultrasonic sealing and cutting devices over conventional electrosurgery, such as excellent hemostasis with minimal thermal damage,2 reduced risk of nerve damage via the strictly mechanical mechanism of action of ultrasound,3 and low visual obstruction from mist or smoke,4 have become well documented. Furthermore, its adoption as a mainstay in the armamentarium of surgeons was solidified by improvements in ergonomics, delivery mechanisms, and dissection capabilities, which resulted in a large portfolio of tools used by surgeons in all areas of surgery for their multifunctionality, precision, and control.5
Early versions of the 5 mm ultrasonic shears were limited to sealing vessels up to 3 mm in outer diameter.6 Later versions, notably Harmonic® ACE (Harmonic ACE®+7 Shears; Ethicon Inc., Cincinnati, OH, USA) (Harmonic 7) extended the range of reliable sealing capability to 5 mm.7 Advanced bipolar devices introduced in the mid to late 1990s, such as LigaSure™ (LigaSure™ 5 mm Blunt Tip LF1537 Laparoscopic Instrument; Covidien, Mansfield, MA, USA), Gyrus (Olympus Corporation, Tokyo, Japan), and Enseal (Ethicon Inc.)®, were developed and cleared by the US Food and Drug Administration (FDA) to seal vessels up to 7 mm in diameter. Until now, no tissue sealer using ultrasonic technology alone has been approved to seal vessels of this size, giving users the impression that there is a 5 mm “glass ceiling” for ultrasonic vessel-sealing capability. Even the recent combination of advanced bipolar energy with ultrasonic technology relies on bipolar energy to produce vessel seals.8
Sealing vessels with ultrasonic technology, like all thermal sealing, is under the control of three critical and interdependent variables: compression, heat, and time.9–11 Furthermore, because cutting occurs simultaneously with coagulation, unlike with bipolar technologies, the frequency, geometry, and compression of the device are critical determinants of seal strength as well as cutting ability.1,12 Recent advances in the control of ultrasonic energy delivery through adaptive tissue technology furnished the possibility of sealing vessels up to 7 mm in diameter by precise modulation of energy delivery and time without needing to alter device geometry. This was supported by evidence that vessel seals in the 3–5 mm diameter range demonstrated increased burst pressures and decreased thermal damage when adaptive tissue technology was included in the device.13
Adaptive tissue technology is a platform capability within the Harmonic system that actively monitors the instrument during use and enables the system to sense and respond appropriately to changes in tissue conditions using a set of proprietary algorithms. These algorithms have been designed to deliver improved hemostasis, thermal management, and precision, depending on the settings. Harmonic 7 (Figure 1) is a recently FDA-cleared device that leverages adaptive tissue technology with the addition of predictive analytics to modulate energy delivery during the sealing cycle.
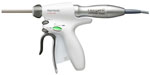  | Figure 1 Handle of the Harmonic ACE®+7 with advanced hemostasis button (green). |
Herein we report on the successful development of an ultrasonic device, Harmonic 7, which is able to seal vessels up to 7 mm in diameter with burst pressures significantly greater than those observed with advanced bipolar technologies. Using benchtop testing, the ultrasonic device was compared with advanced bipolar devices for both rate of hemostatic sealing and burst pressure, and the impact of the Harmonic 7 activation time on seal strength was evaluated. In addition, the ultrasonic and advanced bipolar devices were tested in preclinical porcine and caprine studies to examine initial hemostasis and seal durability during simulated hypertensive crisis (blood pressure challenge), both acutely and after a 30-day survival period. Histological evaluation was also performed to ensure that the historically low thermal damage of ultrasonic devices was not significantly altered in a clinically meaningful way by the changes in energy delivery.
Methods
The devices tested were Harmonic 7 and LigaSure.
Benchtop
Ultrasonic and bipolar devices were used to seal and transect porcine carotid arteries (Animal Biotech Industries, Inc., Danboro, PA, USA) distributed into two groups by outer diameter: small (3–5 mm) and large (>5–7 mm). Porcine carotid arteries are believed to better represent human arteries than arteries of other locations in pigs.14 Harmonic 7 was operated at power level 3 only for vessels ≤5 mm in diameter, and in the advanced hemostasis (AH) mode for vessel sizes ≤7 mm. LigaSure was operated in the default mode indicated by the instructions for use (two green bars). Diameter was measured without compressing the vessels to simulate their natural round state. Sealing of all vessels was performed under an axial tension of 50 g on the vessel, which was filled with saline at a pressure of 60–120 mmHg. The test devices were applied and activated and the transection sites were observed visually for any signs of leakage at transection. Sealed vessels were tested by filling with saline solution at a rate of 47 mL/min while monitoring pressure until a fall in pressure occurred or the maximum pressure (~2,000 mmHg) was reached. Both sides of the seal underwent burst pressure testing. If a vessel leaked at transection, it was assigned a burst pressure of 0 mmHg. Time to transection was recorded as the amount of time from initial activation to complete transection of the vessel. In the case of Harmonic 7, vessel transection occurred as a direct result of the activation. In the case of LigaSure, vessel transection included the time to throw the knife blade after the completion tone was registered.
The effect of time on Harmonic 7 seal strength and the influence of time on vessel seal were evaluated in the in vitro porcine carotid arteries by activating each device 14 times: twice at each of 2, 4, 6, 8, 10, and 12 second durations, as well as two full transections. Each time duration was evaluated in 40 vessels for a total of 280 experimental data points. Vessels were defined as “recannulated” if no pressure was required to observe saline flow through the vessel – in other words, the lumen of the vessel opened back up spontaneously with no pressure required to do so. “Recannulated at pressure” was defined as saline flowing through the seal only when pressure was applied. The amount of pressure required for recannulation was noted. Either type of recannulation could be observed only if the transection was not completed. A “partial transection” was defined as a vessel that was not completely divided along the seal area and separated like a full transection but some of the vessel had begun to separate into a full transection after the device was activated for a period of time and then pulled away. “No transection” is an event defined as the vessel not being separated after device removal when the device was stopped at a prespecified time. Despite no transection, it is conceivable that a vessel was sealed well enough to have a burst pressure. “Burst” is an event defined as bursting upon pressurization after complete transection of the vessel with an intact seal.
Preclinical
All in vivo procedures were reviewed and the animals approved for use in the studies by the Ethicon Endo-surgery Institutional Animal Care and Use Committee. The Harmonic 7 ultrasonic devices were evaluated in multiple in vivo porcine studies and compared with advanced bipolar devices in several in vivo caprine subjects.
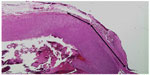
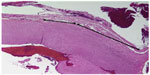
For the acute porcine studies, after standard induction of anesthesia, the animal was placed in dorsal recumbency, and a ventral midline neck incision and laparotomy were performed. The devices were then applied to transect the vessels, which included the gastroepiploic veins and artery (isolated or as a pedicle), carotid artery (unilateral), short gastric pedicles, and splenic vein and artery, completing a splenectomy. For the acute study, hemostasis was evaluated intraoperatively after initial transection and again after a simulated hypertensive crisis (overstress test) post-transection in which a vasopressor agent (phenylephrine) was titrated intravenously to increase the systolic blood pressure to at least 200 mmHg for a period of at least 10 minutes. After euthanasia via intravenous injection of pharmaceutical grade potassium choride while under a surgical plane of anesthesia, transection seals with 1–2 cm of adjacent untreated artery were nonthermally excised and fixed in 10% neutral buffered formalin. The tissue was processed and lateral thermal damage was assessed via histological staining with hematoxylin and eosin (H&E) by a blinded pathologist.
The survival porcine study followed the same surgical preparation and procedures for splenectomy and unilateral carotid artery transection, and hemostasis was evaluated intraoperatively after initial transection and observed for 10 minutes before the incisions were closed using a standard surgical technique (sutures/tissue glue). At ≥30 days postoperatively, vessel seal integrity was challenged with the pig under general anesthesia by simulating an acute hypertensive crisis as described. Following completion of the blood pressure challenge period, the animal was euthanized as described. At necropsy all vessel transection sites were observed for hemorrhage.
For the caprine studies, similar surgical preparation and a ventral midline neck incision were performed. For the acute study, following a bilateral carotid artery transection with the device, hemostasis was evaluated intraoperatively at initial transection and again after a simulated hypertensive crisis (overstress test). A vasopressor agent (epinephrine or vasopressin) was used for the blood pressure challenge. After each challenge period, the vasopressor agent was discontinued to allow the blood pressure to return to baseline. Each goat underwent four rounds of bilateral carotid artery transection and subsequent blood pressure challenges. At the completion of the study, the carotid vessels were collected for histological assessment of lateral thermal damage.
The survival caprine surgical procedure consisted of a unilateral carotid artery transection. Hemostasis was evaluated intraoperatively after initial transection and observed for 10 minutes before the incision was closed. At ≥30 days postoperatively, vessel seal integrity was challenged with the goat under general anesthesia by simulating an acute hypertensive crisis as described. Following completion of the blood pressure challenge period, the animal was euthanized as described. At necropsy all vessel transection sites were observed for hemorrhage.
Continuous variables such as burst pressure or thermal damage were compared using Student’s t-test, analysis of covariance, or the Mann–Whitney test, as appropriate, and binomial variables such as hemostasis and adhesions were compared using Fisher’s exact test.
Results
Benchtop studies
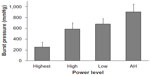
During development of Harmonic 7, the strength of the vessel seal was evaluated over a range of power levels in 5–7 mm porcine carotid arteries, with the AH mode producing the highest burst pressures (Figure 2). In this series of experiments, the high level was similar to power level 5, and the low level was similar to power level 3, which is indicated for sealing vessels up to 5 mm in diameter. The highest power level used is not available on generators and is evaluated only for the purpose of these experiments. The results demonstrated that modulated power delivery with the AH mode created the strongest seals in vessels of diameters up to 7 mm. Transection time at the highest power level averaged 2.6 seconds, whereas in AH mode transection time was variable due to energy modulation but up to 17.1 seconds in duration.
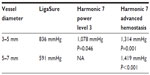
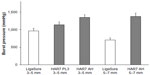
Based on these initial studies, the AH mode was chosen as the best means to achieve optimal blood vessel seals without changing instrument geometry and was therefore compared with existing technologies of ultrasonics and advanced bipolar for efficacy. Results for median burst pressures for Harmonic and LigaSure in both 3–5 mm and 5–7 mm porcine carotid arteries are given in Table 1 and for mean burst pressures in Figure 3. Harmonic 7 in AH mode had significantly higher median burst pressures than LigaSure for both 3–5 mm (P=0.001) and 5–7 mm (P<0.001) vessels. Harmonic 7 at power level 3 also had significantly higher median burst pressure than LigaSure for 3–5 mm vessels (P=0.046) but less than that observed with the AH mode (P=0.031). Conversely, Harmonic 7 in AH mode delivered longer transection times than LigaSure for vessels 5–7 mm in diameter. Mean transection time for Harmonic 7 was 12.7 seconds (range 7.8–18.5 seconds) versus 5.0 seconds (range 3.4–6.5 seconds) for LigaSure, depending upon tissue type and thickness. In general, longer sealing times corresponded to higher burst pressures and more reliable sealing.
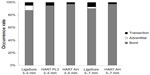
Both ultrasonic and advanced bipolar sealing modalities achieved a high rate of complete seals that failed only by bursting under pressure (Figure 4). Harmonic 7 in AH mode had a lower rate of leaks at transection for 5–7 mm vessels. Unlike LigaSure, Harmonic 7 in either power level 3 or AH mode did not experience any adventitial leaks.
As some surgeons may attempt to seal a vessel prior to transection using the so-called “ultrasonic clip” method that seals not to complete vessel transection but to a specific amount of time or visual cue, the security of such seals was evaluated by applying energy for defined periods of time to 5–7 mm porcine carotid arteries using the Harmonic 7 AH mode (Figure 5). The rate of vessel recannulation decreased as the time interval of sealing increased and was highly correlated with time. No vessel was reliably sealed at 2 seconds. In contrast, 97.4% were completely sealed when the device was allowed to reach full transection. That is, although there was a visual seal seen with shorter durations of activation, these “seals”, when placed under pressure, allowed fluid to pass: ie, the compressed lumen recannulated. No vessel was fully transected with a seal that would withstand a burst pressure similar to that of full activation unless at least 8 seconds of activation was applied. Furthermore, recannulation with pressure was observed only after a minimum of 4 seconds of activation and demonstrated a burst pressure that although in many cases was greater than physiologic pressures was always less than half that observed when vessels were sealed enough to burst. There was only one partial transection observed in this study. Transection otherwise was an all or nothing event dependent upon the time of activation. Burst pressure was also dependent upon transection time, with the highest burst pressures achieved when energy was activated through the complete vessel transection.
In vivo preclinical studies
Evaluation of Harmonic 7 in an in vivo porcine study (Table 2) showed high rates of hemostasis at initial transection that remained secure during a simulated hypertensive crisis for both acute and survival studies. In the AH mode, all seals were hemostatic both initially and upon challenge.
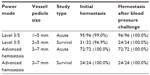  | Table 2 Hemostasis results for initial vessel sealing and at blood pressure challenge for power level 3/5 and advanced hemostasis mode in acute and survival porcine studies |
In an acute in vivo caprine study, Harmonic 7 and LigaSure had similar rates of hemostasis (Table 3), but Harmonic 7 displayed significantly less thermal damage than LigaSure (P=0.003), as evaluated by H&E histological staining (Figures 6 and 7). In a caprine 30-day survival study of Harmonic 7 using AH mode, carotid artery vessel seals in all five animals were hemostatic at initial sealing (100%) and after a simulated hypertensive crisis at the completion of the survival period (100%). Survival studies were not performed with LigaSure.
Discussion
The benefits of ultrasonic cutting and coagulation in comparison with conventional electrosurgery are well documented in numerous studies. Based on lower heat production in the tissue, incisions made with ultrasonic devices result in less inflammation than those made with electrosurgery,15 along with enhanced revascularization and faster fibrosis remodeling.16 The purely mechanical action of ultrasonic devices, without the flow of electrical current that occurs in electrosurgery, allows usage of the devices in the vicinity of nerves, with greatly reduced risk of injury.3 Recently, the differences between ultrasonic and electrosurgery have been examined at a molecular level via transcriptomics and proteomics.17 These studies demonstrated that in the acute phase of wound healing following incisions in subcutaneous tissue, ultrasonic energy produced lower levels of immunological and inflammatory mediators, indicating a reduced amount of iatrogenic trauma. In addition, there was an early shift to wound-healing proteins with ultrasonic energy observed in these studies.
Adaptive tissue technology is a transformative capability of the Harmonic ultrasonic platform that actively monitors the instrument during use and enables the system to sense and respond appropriately to changes in tissue conditions using a set of proprietary algorithms.18 These algorithms can be designed and modified to deliver improved hemostasis, thermal management, and precision, depending on how they are set. The introduction of adaptive tissue technology to ultrasonic devices has notably improved the performance of these devices. For example, the addition of adaptive tissue technology to existing devices is associated with lower peak device temperatures, increased seal strength, faster cutting, and improved visualization from reduction in mist.13 The results observed with adaptive tissue technology are consistent with those observed in piezoelectric surgery in which modulation of energy delivery results in reduced heat and improves the effectiveness of cutting in bone surgery.19
Harmonic 7 introduces the use of predictive analytics within the adaptive tissue technology algorithms to modulate the delivery of energy to tissue. Based on data obtained from over 18,000 seals performed on vessels up to 7.5 mm in diameter, the system was trained to optimize energy delivery using numerous inputs received by the generator in the AH mode. As shown in Figure 8, the device is monitoring during the three phases of preheating, vessel sealing (compression), and transection. Based on monitoring of multiple parameters, the amount and length of time energy is delivered is altered to result in the best-predicted seal.
  | Figure 8 Three phases of vessel sealing are modulated based on predictive analytics to produce optimal seals for the tissue conditions encountered. |
The AH mode of Harmonic 7, used under the conditions specified in benchtop testing, produced a higher success rate and statistically higher burst pressures than an advanced bipolar device for vessels up to 7 mm in diameter. The benchtop data also demonstrate the importance of using the AH mode for large vessels (>5–7 mm), because seals were not as reliable and did not reach the same burst pressures when other power settings were used. In in vivo preclinical testing, Harmonic 7 at power level 3 had a complete seal success rate that was equivalent to advanced bipolar devices in sealing vessels up to 5 mm in diameter, and in the AH mode for vessels and pedicles up to 7 mm in diameter. Most importantly, there was no evidence of hemorrhage at any vessel seal for Harmonic 7 at the completion of a 30-day survival period and after simulated hypertensive crisis.
Temperature and lateral thermal damage are important considerations for surgeons when using energy-based technologies. First, and most importantly, surgeons are concerned about injury to nearby structures either by direct contact or by the visually unrecognizable transmission of energy. Second, surgeons are concerned about the potential impact of tissue damage on the inflammatory response and overall recovery of the patient. Both Harmonic 7 modes (power level 3 and AH mode) have demonstrated histologic thermal damage that was statistically less compared with advanced bipolar devices, with no differences in tissue sticking or adhesions between the tissue-sealing methods. However, these differences were small and the potential clinical impact unknown.
The generation of heat by an advanced energy device is a critical component to vessel sealing and tissue transection. The temperature the instrument reaches is dependent on multiple variables, including tissue type, tissue thickness, energy used, and power setting. Most, if not all, currently marketed advanced energy devices reach instrument temperatures of at least 100°C during activation on tissue. There are, however, situations when the temperature of an ultrasonic device can reach well beyond the 100°C range due to tissue conditions, sticking of tissue to the blade, and excessive activation times beyond those needed to actually seal and transect the tissue. In this regard, it is important to recognize that the temperature of the instrument (ultrasonic or advanced bipolar) is not the same as the temperature of the tissue. In the case of ultrasonic devices, the tissue temperature is usually much lower than the device because heat flows from the device to the tissue following a thermal gradient.20 In the case of bipolar devices, the temperature is often higher in the tissue because of the ohmic heating effect immediately adjacent to the jaws of the device.21 In large part, the increase in temperature observed with ultrasonic devices occurs after transection but before the tissue is completely separated from the device. Tissue separates at about 100°C if compression is provided with a relatively sharp blade. The rapid increase in temperature using an ultrasonic device is largely a function of the active blade coming into contact and running on the nonactive tissue pad, especially when there is a tissue tag left or partial transection.20 Adaptive tissue technology monitors blade conditions through proprietary algorithms and provides surgeons with a secondary auditory tone when transection is near completion. Although blade temperature is not directly measured, changes in the blade temperature result in very small changes in the resonant frequency of the device that can be monitored. Specific changes in frequency result in generation of a tone to notify the surgeon that additional surgical action, such as increasing tension of the tissue on the active blade, may be required to complete the transection. The secondary tone also indicates that adaptive tissue technology has reduced the power level to deliver enhanced thermal management of the blade.
The addition of adaptive tissue technology has been shown to reduce the peak temperatures observed with Harmonic devices in previous studies.13 Studies also demonstrate that despite increases in blade temperature, minimal conductive heat is transmitted to surrounding tissue, as evidenced by the low amount of lateral thermal damage measured for all Harmonic devices and the ability to operate close to nerves (2 mm) without impacting the electrophysiological function of the nerve.3 Nevertheless, surgeons must always be mindful that the instrument is hot following activation, and heat mitigation techniques should be employed prior to touching or grasping tissue.
The Harmonic 7 creates strong and durable vessel seals by modulating the delivery of energy and extending the compression and thermal sealing time. This is consistent with the current understanding of how thermal seals are formed, namely via compression, heat, and time, but also expands our understanding of the process by simplifying the relationship to two components: heat over time and compression over time. Harmonic 7 handles these differently by modulating the energy delivery so that the amount of heat is not the same throughout the sealing cycle (Figure 8). These studies further document the importance of time in achieving the optimal seal by varying the activation duration without achieving transection. Reliable and durable sealing occurred only when energy delivery continued through the complete cycle to vessel transection, which raises serious doubt as to the validity of “ultrasonic clips”. The ability of the device to modulate energy delivery was furthermore substantiated by the variability of time required from vessel to vessel.
The concept of extending the length of time to produce better seals is in direct juxtaposition to the desire of surgeons to experience the fastest seal formation possible. Although surgeons first and foremost want hemostatic security and safety, they also want this to occur as fast as possible. In fact, all available technologies to date have placed a premium on the fastest sealing time. Despite this desire, previous investigators, as well as our own testing, have shown the positive impact of time on obtaining optimal seals both with ultrasonics and electrosurgery. For example, using bipolar energy, longer periods of energy activation resulted in better seals, with periods of 15–20 seconds being ideal for traditional bipolar technologies.21–24 Similar findings have been shown with advanced bipolar technologies.10,11 We, as well as others, have shown the importance of seal time on vessel-sealing security. For example, a significant difference has been shown in burst pressures with Harmonic ACE at power level 3 versus power level 5 for vessels in the 5 mm range, which correlates with length of the transection time.25 Furthermore, recent advances in the understanding of device tissue interactions with surgical staplers have documented the importance of extending compression time in order to deliver better hemostasis and more consistent staple formation.26
The ability to seal 7 mm diameter vessels is not the only important aspect of this device, as surgeons rarely, if ever, seal 6–7 mm vessels with any energy device. Even though the inferior mesenteric artery is often cited as the reason for 7 mm vessel-sealing capacity, the inferior mesenteric artery is, on average, only 3–4 mm in size.27 In fact, no human vessel in the abdomen is >5 mm in diameter other than the renal, splenic, and iliac arteries, and arteries >5 mm are usually divided with a belt and suspenders approach using multiple ties, clips, and/or staples. The key aspect of the ability to seal 7 mm vessels may therefore rest in the confidence, safety margin, and control it provides the surgeon. Indeed, burst pressures in the AH mode were greater than those observed with bipolar devices for vessels from 3 mm to 7 mm in diameter. In that regard, it is noteworthy that burst pressures in the AH mode were greater than those observed at power level 3. Thus, the AH mode can also be used under specific situations where the surgeon wants greater confidence in the security of the seal. For example, surgeons faced with sealing a uterine artery or an inflamed vessel can choose to use the AH mode to further optimize the sealing cycle, even if the vessels are ≤5 mm in diameter. The AH mode may also be useful when vessels are sealed at an angle. Burst pressure studies show that a 5 mm vessel divided at a 45° angle has an equivalent burst pressure to that of a 7 mm vessel divided perpendicularly.28 In other words, even though the vessel is 5 mm in diameter, the area of seal is equivalent to a 7 mm vessel. As a result, the need for 7 mm vessel sealing is as much about size as the angle of sealing when it comes to vessel security.
Surrounding tissue also plays a role in the visibility of the vessel being fully placed in the jaws of the instrument. An important surgical technique is to ensure that the tips of the device can be seen and that the vessel being transected is fully captured within the jaws. Regardless of the modality, advanced bipolar or ultrasonic, a partially transected vessel will not deliver the same vessel security as a fully sealed and transected vessel. Therefore, there may be times when skeletonizing the vessel is appropriate to ensure adequate visibility of the vessel and tips of the instrument prior to energy activation and subsequent transection.
These studies have also begun to explore an important area in respect of ultrasonic energy, namely the creation of so-called ultrasonic clips. In this process, surgeons apply energy to the vessel proximal and distal to the intended transection site, but do not activate the device long enough to produce transection. The device is then activated between these two “clips” to a complete transection. The belief is that this will strengthen the overall seal by providing two sites of closure, as one might do with two metallic clips. The recannulation data presented in this study show that for vessels 5–7 mm, an ultrasonic clip provides no additional security, as almost all vessels were recannulated or recannulated at pressure if the complete seal cycle to transection did not occur. In fact, one could argue that it provides the surgeon with a false sense of security and could potentially reduce the reliability of the seal by weakening the vessel by thermal damage in the area proximal to the transection.
Conclusion
These studies document the ability of ultrasonic energy alone to reliably seal large vessels 5–7 mm in diameter with significantly higher initial burst pressure than with an advanced bipolar technology in benchtop studies. Seals created in AH mode are demonstrated to be long-lasting and resistant to fluctuations in blood pressure. These studies also add to the existing body of literature regarding seal formation and support the importance of time in the seal formation process and the resulting security of the seal. Using a longer period of compression and heating leads to superior results in respect of burst pressures that were consistently observed with the ultrasonic device. These results, however, should not be interpreted to prove that longer sealing times are always necessary under all conditions. Clearly, other factors, such as the type of energy used, the time pattern of tissue heating, end effector geometry, compression force applied, and tissue characteristics, must also be considered. These remain areas for future research.
Disclosure
All the authors are employed by Ethicon Inc., the manufacturer and distributor of Harmonic technology.
References
Amaral JF. Laparoscopic application of an ultrasonically activated scalpel. Gastrointest Endosc Clin North Am. 1993;3(2):381–391. | |
Phillips CK, Hruby GW, Durak E, et al. Tissue response to surgical energy devices. Urology. 2008;71(4):744–748. | |
Chen C, Kallakuri S, Vedpathak A, et al. The effects of ultrasonic and electrosurgery devices on nerve physiology. Br J Neurosurgery. 2012;26(6):856–863. | |
Schmidbauer S, Hallfeldt KK, Sitzmann G, Kantelhardt T, Trupka A. Experience with ultrasound scissors and blades (UltraCision) in open and laparoscopic liver resection. Ann Surg. 2002;235(1):27–30. | |
Kanehira E, Kinoshita T, Omura K. Ultrasonically-activated devices for endoscopic surgery. Minim Invasive Ther Allied Technol. 1999;8(2):89–94. | |
Landman J, Kerbl K, Rehman J, et al. Evaluation of a vessel sealing system, bipolar electrosurgery, harmonic scalpel, titanium clips, endoscopic gastrointestinal anastomosis vascular staples and sutures for arterial and venous ligation in a porcine model. J Urol. 2003;169(2):697–700. | |
Newcomb WL, Hope WW, Schmelzer TM, et al. Comparison of blood vessel sealing among new electrosurgical and ultrasonic devices. Surg Endosc. 2009;23(1):90–96. | |
Milsom J, Trencheva K, Monette S, et al. Evaluation of the safety, efficacy, and versatility of a new surgical energy device (THUNDERBEAT) in comparison with Harmonic ACE, LigaSure V, and EnSeal devices in a porcine model. J Laparoendosc Adv Surg Tech A. 2012; 22(4):378–386. | |
Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121(4):823–831. | |
Wallwiener CW, Rajab TK, Zubke W, et al. Thermal conduction, compression, and electrical current: an evaluation of major parameters of electrosurgical vessel sealing in a porcine in vitro model. J Minim Invasive Gynecol. 2008;15(5):605–610. | |
Reyes DA, Brown SI, Cochrane L, Motta LS, Cuschieri A. Thermal fusion: effects and interactions of temperature, compression, and duration variables. Surg Endosc. 2012;26(12):3626–3633. | |
Foschi D, Cellerino P, Corsi F, et al. The mechanisms of blood vessel closure in humans by the application of ultrasonic energy. Surg Endosc. 2002;16(5):814–819. | |
Broughton D, Welling AL, Monroe EH, Pirozzi K, Schulte JB, Clymer JW. Tissue effects in vessel sealing and transection from an ultrasonic device with more intelligent control of energy delivery. Med Devices (Auckl). 2013;6:151–154. | |
Latimer CA, Nelson M, Moore CM, Martin KE. Effect of collagen and elastin content on the burst pressure of human blood vessel seals formed with a bipolar tissue sealing system. J Surg Res. 2014;186(1):73–80. | |
Rubino LJ, Konstantakos EK, Stills HF Jr, et al. Healing of iatrogenic skeletal muscle wounds is affected by incision device. Surg Innov. 2010;17:85–91. | |
Usas A, Usaite D, Gao X, Huard J, Clymer JW, Malaviya P. Use of an ultrasonic blade facilitates muscle repair after incision injury. J Surg Res. 2011;167:e177–e184. | |
Nanduri B, Pendarvis K, Shack LA, et al. Ultrasonic incisions produce less inflammatory mediator response during early healing than electrosurgical incisions. PLoS ONE. 2013;8(9):e73032. | |
Raghavan D, Howington JA, Broughton D, Henderson CE, Clymer JW. Comparison of two ultrasonic coagulating shears in sealing pulmonary vessels. Open Access Surgery. 2013;6:15–21. | |
Labanca M, Azzola F, Vinci R, Rodella L. Piezoelectric surgery: Twenty years of use. Br J Oral Maxillofac Surg. 2008;46(4):265–269. | |
Koch C, Friedrich T, Metternich F, Tannapfel A, Reimann HP, Eichfeld U. Determination of temperature elevation in tissue during the application of the harmonic scalpel. Ultrasound Med Biol. 2003;29(2):301–309. | |
Habermann W, Müller W. Tissue penetration of bipolar electrosurgical currents: Joule overheating beyond the surface layer. Head Neck. 2013;35(4):535–540. | |
Harrison JD, Morris DL. Does bipolar electrocoagulation time affect vessel weld strength? Gut. 1991;32(2):188–190. | |
Laine L, Long GL, Bakos GJ, Vakharia OJ, Cunningham C. Optimizing bipolar electrocoagulation for endoscopic hemostasis: assessment of factors influencing energy delivery and coagulation. Gastrointest Endosc. 2008;67(3):502–508. | |
Cezo JD, Passernig AC, Ferguson VL, Taylor KD, Rentschler ME. Evaluating temperature and duration in arterial tissue fusion to maximize bond strength. J Mech Behav Biomed Mater. 2014;30(2014):41–49. | |
Mantke R, Halangk W, Habermann A, et al. Efficacy and safety of 5-mm-diameter bipolar and ultrasonic shears for cutting carotid arteries of the hybrid pig. Surg Endosc. 2011;25(2):577–585. | |
Morita K, Maeda N, Kawaoka T, et al. Effects of the time interval between clamping and linear stapling for resection of porcine small intestine. Surg Endosc. 2008;22:750–756. | |
Milnerowicz S, Milnerowicz A, Tabola R. A middle mesenteric artery. Surg Radiol Anat. 2012;34(10):973–975. | |
Voegele AC, Korvick DL, Gutierrez M, Clymer JW, Amaral JF. Perpendicular blood vessel seals are stronger than those made at an angle. J Laparoendosc Adv Surg Tech A. 2013;23(8):669–672. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.