Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Screening of long non-coding RNA and TUG1 inhibits proliferation with TGF-β induction in patients with COPD
Authors Tang WX, Shen ZY, Guo J, Sun SH
Received 30 March 2016
Accepted for publication 20 September 2016
Published 28 November 2016 Volume 2016:11(1) Pages 2951—2964
DOI https://doi.org/10.2147/COPD.S109570
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Richard Russell
Wenxiang Tang,1 Zhenyu Shen,2 Jiang Guo,2 Shenghua Sun1
1Department of Respiratory Medicine, The Third Xiangya Hospital of Central South University, 2Department of Respiratory Medicine, Xiangtan Central Hospital, Hunan, People’s Republic of China
Objective: To evaluate differentially expressed long noncoding RNAs (lncRNAs) and the potential role of lncRNA TUG1 in patients with chronic obstructive pulmonary disease (COPD).
Methods: Total RNA was extracted from both COPD and non-COPD lung tissues, and microarray analysis was performed with 25,628 lncRNA probes and 20,106 mRNA probes. In addition, five up-regulated and five down-regulated lncRNAs were selected for identification using quantitative real-time polymerase chain reaction. COPD cell model was established by transforming growth factor β (TGF-β) treatment. Cell Counting Kit-8 assay was used to detect BEAS-2B and HFL1 cell proliferation after TUG-siRNA transfection with TGF-β treatment. In addition, the expression levels of α-SMA and fibronectin proteins were determined using Western blot in BEAS-2B and HFL1 cells after TUG-siRNA transfection with TGF-β treatment.
Results: There were 8,376 (32.7%) differentially expressed lncRNAs and 5,094 (25.3%) differentially expressed mRNAs in COPD lung tissues compared with non-COPD lung tissues. Five of the analyzed lncRNAs (BC038205, BC130595, TUG1, MEG3, and LOC646329) were markedly increased, while five lncRNAs (LOC729178, PLAC2, LOC339529, LINC00229, and SNHG5) were significantly decreased in COPD lung tissues compared with non-COPD lung tissues (n=20) (***P<0.001). Knockdown of lncRNA TUG1 promotes BEAS-2B and HFL1 cell proliferation after TGF-β treatment through inhibiting the expression levels of α-SMA and fibronectin.
Conclusion: Abundant, differentially expressed lncRNAs and mRNAs were identified by microarray analysis and these might play a partial or key role in the diagnosis of patients with COPD. LncRNA TUG1 may become a very important class of biomarker and may act as a potential diagnostic and therapeutic target for patients with COPD.
Keywords: COPD, microarray analysis, long noncoding RNA, lncRNA TUG1, TGF-β
Introduction
Lung diseases mainly include chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), asthma, cystic fibrosis (CF), and non-small-cell lung cancer (NSCLC). COPD is an abnormal inflammatory reaction of lungs to harmful particles or gases (primarily cigarette smoke).1 COPD has become a primary public health problem and is the fourth main reason of chronic morbidity and mortality in the US.2 It has been the cause of more than 2,500,000 deaths every year worldwide, according to the 2002 report of the World Bank/World Health Organization.3 There are several reasons for the development of COPD, including the inspiration of nocuous particles such as cigarette smoke, the destruction of the balance between proteolytic and antiproteolytic molecules,4 and oxidative stress.5 However, there are difficulties in selecting an appropriate therapeutic method for COPD, especially with regard to the lack of classical studies.6 Therefore, it is also urgent to find specific and effective markers for the diagnosis of COPD. IPF presents as a common, chronic, and progressive lung disease with poor prognosis.7 Asthma is characterized by periods of reversible airflow obstruction. CF presents as an autosomal recessive disease and is characterized by chronic endobronchial infection, progressive airway obstruction, and abnormal airway secretions.8
Long noncoding RNAs (lncRNAs), a group of noncoding RNAs (ncRNAs) which are non-protein coding transcripts transcribed by RNA polymerase II,9 have a length of more than 200 nucleotides.10,11 With regard to their biological functions, lncRNAs play a significant role in chromatin structure and function,12 promoting numerous biological functions including gene transcription, regulating splicing and participating in epigenetics,13,14 genomic rearrangement,15 etc. Studies indicated that lncRNAs were connected with various diseases9 and resulted in abnormal expression of abundant gene products which may, in turn, generate biological processes involving growth,16 differentiation,17 and carcinogenesis.18 They have also been implicated in numerous human diseases19–21 such as neurodegeneration diseases,22 cardiovascular diseases,23 and cancers.24 Previously, differentially expressed lncRNAs and mRNAs between fibrotic lung and normal lung tissues were selected for micro-array analysis. Among them, AJ005396 and S69206 were found to be increased in the fibrotic lung tissues.25 MALAT-1 is highly expressed in NSCLC and is involved in poor prognosis.26 One study has reported the aberrant expression of lncRNAs in the CF bronchial epithelium.27 However, the lncRNAs involved in the development of COPD or asthma have not been studied.
Genomic screening techniques revealed that taurine treatment of mouse retinal cells resulted in the up-regulation of lncRNA TUG1 (7.1 kb in length).28 LncRNA TUG1 is highly conservative, and its inactivation results in damage to the formation of photoreceptors in the developing rodent retina.28 Recently, researchers have found that TUG1 was significantly decreased in NSCLC.29 However, some studies showed that TUG1 was increased in gastric cancer, osteosarcoma, and bladder cancer,30–32 probably because lncRNAs showed predominantly tissue-specific expression,33,34 whereas the function and potential mechanism of TUG1 in patients with COPD are still unknown. Therefore, in our study, we explored the possible function and mechanism of TUG1 in patients with COPD.
In our study, we detected differentially expressed lncRNAs and mRNAs in COPD and non-COPD lung tissues using human microarray analysis. We conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and gene ontology (GO) enrichment analysis of the differentially expressed mRNAs. Then, we selected five up-regulated and five down-regulated lncRNAs, which were identified using quantitative real-time polymerase chain reaction (qRT-PCR), from 20 COPD and 20 non-COPD lung tissues. Furthermore, we selected lncRNA TUG1 and analyzed its effect on proliferation and expression levels of α-SMA and fibronectin proteins by knocking out lncRNA TUG1 through transforming growth factor β (TGF-β) treatment. The knocking out of lncRNA TUG1 inhibited the expression levels of α-SMA and fibronectin in BEAS-2B and HFL1 cells. Our results manifested that differentially expressed lncRNAs can act as potential molecular biomarkers for the diagnosis of COPD and that lncRNA TUG1, in particular, may play an important role as a diagnostic marker in the COPD patients.
Materials and methods
Patients and clinical specimens
The study was approved by the Institutional Review Board of Third Xiangya Hospital, Central South University. Lung tissues from human COPD and non-COPD patients were collected from the Third Xiangya Hospital in 2015. Written informed consent to participate in the study was acquired from all the patients. The tissue samples were immediately placed in the refrigerator at −80°C until further analysis.
Cell culture and transfection
BEAS-2B (human lung epithelial cells) and HFL1 (human lung fibroblasts) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). BEAS-2B was cultured in LHC-8 medium without gentamicin (Thermo Fisher Scientific, Waltham, MA, USA) and HFL1 was cultured in F-12K medium (Thermo Fisher Scientific). Penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% fetal bovine serum (FBS; Thermo Fisher Scientific) were added to all the culture media. Cell lines were cultured with 5% CO2 at 37°C. For cell treatments, we used 2 ng/mL TGF-β (R&D Systems, Inc., Minneapolis, MN, USA) for 48 h. Transient transfection was assayed using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. NC (negative control) siRNA and TUG1-siRNA were synthesized. The siRNA sequences used to target TUG1 were 5′-GCU UGG CUU CUA UUC UGA AUC CUU U-3′ (sense) and 5′-AAA GGA UUC AGA AUA GAA GCC AAG C-3′ (antisense).29 BEAS-2B and HFL1 cells were transfected with Lipofectamine 3000 (Thermo Fisher Scientific). Cells were cultured for 48 h after transfection and then were analyzed by cell viability assay and Western blot analysis.
Arraystar lncRNA array
Tissues were cryopulverized using the BioPulverizer™ (Biospec Products, Inc., Bartlesville, OK, USA) and homogenated using the Mini-Bead-Beater-16 (Biospec). The homogenized samples were separated. RNA was precipitated from the aqueous phase by mixing with isopropyl alcohol, and the precipitate was washed with 75% ethanol and redissolved with RNase-free water. Total RNA was extracted and RNA quality was determined. Labeling reaction was carried out using Quick Amp Labeling Kit, One-Color (Agilent p/n 5190-0442), then the labeled/amplified RNA was purified and labeled cRNA quality was determined. Hybridization was performed using Agilent Gene Expression Hybridization Kit (Agilent p/n 5188-5242) according to manufacturer’s instructions and the microarray was washed. Agilent microarray scanner (Agilent p/n G2565BA) was used and data were extracted using Agilent Feature Extraction software (version 11.0.1.1).
RNA preparation, reverse transcription, and qRT-PCR analysis
Total RNA was extracted from human lung cells and tissues by using TRIzol reagent according to the manufacturer’s instructions. RevertAid™ First Strand cDNA Synthesis Kit (no K1622; Thermo Fisher Scientific) was used to synthesize the cDNA with random primers and 1.0 μg RNA was included in each reverse transcription reaction. qPCR was performed with SYBR Premix Ex Taq (Takara Bio Inc., Kusatsu, Japan). The primers of objective genes and GAPDH (internal loading control) were designed and are shown in Table 1. The ABI 7500 Real-Time PCR System (Applied Biosystems, Warrington, UK) was used for PCR reactions. All the experiments were repeated at least three times and the results are displayed as the mean ± standard deviation.
  | Table 1 Sequence of the primers used for validation of selected lncRNAs |
Cell viability assay
First, 50 μM NC siRNAs or TUG1 siRNAs (si-TUG1) were transfected into BEAS-2B and HFL1 cells for 48 h. Second, the cells were treated with TGF-β (2 ng/mL) for 48 h. BEAS-2B, BEAS-2B/TGF-β, BEAS-2B/TGF-β/NC-siRNA, and BEAS-2B/TGF-β/TUG1-siRNA cells were seeded in 96-well plates at 1×104 cells per well at 37°C. At 24, 48, and 72 h, 10 μL of cholecystokinin octapeptide (CCK-8; Dojindo Laboratories, Kumamoto, Japan) was added to each well and incubated for 4 h. The absorbance was detected at a wavelength of 450 nm. The methods used for the treatment and detection of HFL1 cells were identical with those used for BEAS-2B cells.
Western blot analysis
The total proteins of BEAS-2B and HFL1 cells were extracted using a lysis buffer including 10% protease inhibitor. BCA Protein Assay kit (QCbio Science Technologies Co., Ltd., Shanghai, People’s Republic of China) was used to detect the concentrations of total protein. Approximately, 30 μg of heat-denatured proteins were added to sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis, the proteins were transferred to polyvinylidene difluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA), blocked with 5% skim milk (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), and incubated with primary antibody overnight at 4°C. Next day, the PVDF membranes with proteins were incubated with the secondary antibody for 2 h. Special antibodies were detected by using enhanced chemiluminescence (ECL) reagents (Amersham Biosciences, Inc., Little Chalfont, UK). The ImageJ software program was used to analyze the expression levels of special proteins. The primary antibodies, anti-α-SMA and anti-fibronectin, were purchased from Abcam (Cambridge, UK).
Statistical analysis
Statistical Product and Service Solutions (SPSS) software 21 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA USA) were used to analyze the data of all experiments by Student’s t-tests. P-values less than 0.05 were considered to be statistically significant.
Results
Expression of lncRNAs and mRNAs in COPD lung tissues
Six patient samples with or without COPD were randomly selected for this study. Histopathology was assessed by a pathologist and patient characteristics are shown in Table 2 LncRNA array (Kangcheng, Shanghai, People’s Republic of China) was employed to measure the differently expressed lncRNAs in COPD and non-COPD lung tissues. We detected the expression of 25,628 lncRNAs and 20,106 mRNAs using microarray analysis. According to fold-change (COPD vs non-COPD lung tissues) and P-value, as shown in Table 3, we screened out significantly differentially expressed lncRNAs (8,376 [32.7%]) and mRNAs (5,049 [25.1%]) in three COPD lung tissue samples and compared with non-COPD lung tissue samples (fold-change >2.0 or <0.5; P<0.05). Among the differentially expressed lncRNAs, 3,939 (15.4%) were identified to be up-regulated, whereas 4,437 (17.3%) were identified to be down-regulated in the COPD lung tissue groups. Furthermore, 396 (1.6%) lncRNAs were up-regulated, whereas 438 (1.7%) were down-regulated when the fold-change was >5 (Figure 1A). Among the differentially expressed mRNAs, 3,623 (18.0%) were up-regulated, whereas 1,471 (7.3%) were down-regulated in the COPD lung tissue groups. Also, 305 (1.5%) mRNAs were up-regulated, whereas 71 (0.4%) mRNAs were down-regulated when the fold-change was >5 (Figure 1B). Hierarchical clustering also displayed variations in the expression of lncRNAs (Figure 1C) and mRNAs (Figure 1D) between COPD and non-COPD lung tissues.
  | Table 3 Analysis of differentially expressed lncRNAs and mRNAs between COPD and non-COPD lung tissues |
Analysis of genomic locus among differentially expressed lncRNAs
According to the relationship of lncRNA with its nearby coding gene and the coordinate of the coding gene, as shown in Table 4, among the up-regulated lncRNAs, 785 (19.9%) were exon sense-overlapping lncRNAs (the lncRNA’s exon overlaps a coding transcript exon on the same genomic strand), 215 (5.5%) intron sense-overlapping lncRNAs (the lncRNA overlaps the intron of a coding transcript on the same genomic strand), 972 (24.7%) natural antisense lncRNAs (the lncRNA is transcribed from the antisense strand and overlaps with a coding transcript), 195 (5.0%) bidirectional lncRNAs (the lncRNA is oriented head to head to a coding transcript within 1,000 bp), 1,701 (43.2%) intergenic lncRNAs (there are no overlapping or bidirectional coding transcripts nearby the lncRNA), and 71 (1.8%) nonoverlapping antisense lncRNAs (the lncRNA is transcribed from the antisense strand without sharing overlapping exons). Among the down-regulated lncRNAs, 274 (6.2%) were exon sense-overlapping lncRNAs, 160 (3.6%) intron sense-overlapping lncRNAs, 1,267 (28.6%) natural antisense lncRNAs, 176 (4.0%) bidirectional lncRNAs, 1,548 (34.9%) intergenic lncRNAs, 974 (22.0%) intronic antisense lncRNAs, and 38 (0.9%) nonoverlapping antisense lncRNAs.
  | Table 4 The proportion of differentially expressed lncRNAs in COPD lung tissues based on genomic locus position |
KEGG pathway analysis of the differentially expressed mRNAs
Pathway analysis is a functional analysis mapping genes to KEGG pathways.35 KEGG pathway analysis demonstrated numerous enrichment-related pathways including 46 up-regulated and 8 down-regulated pathways. Among up-regulated pathways, the top 10 pathways in homosapiens (humans) are listed in Figure 2A, which include lysosome, Staphylococcus aureus infection, osteoclast differentiation, leishmaniasis, B-cell receptor signaling pathway, rheumatoid arthritis, tuberculosis, other glycan degradation, inflammatory bowel disease, and hematopoietic cell lineage. Simultaneously, the down-regulated pathways include dilated cardiomyopathy, rap1 signaling pathway, hypertrophic cardiomyopathy, PPAR signaling pathway, bladder cancer, bile secretion, regulation of actin cytoskeleton, and adrenergic signaling in cardiomyocytes (Figure 2B).
Gene ontology enrichment analysis of the differentially expressed mRNAs
The Gene Ontology project provides a controlled vocabulary to describe gene and gene product attributes in any organism (http://www.geneontology.org). There are three domains in ontology: biological process, cellular component, and molecular function. Fisher’s exact test was used to find out if, by chance, there was more overlap between the differently expressed list and the GO annotation list than would be expected. The P-value denotes the significance of GO terms enrichment in the differently expressed genes. The lower the P-value, the more significant is the GO term (P-value ≤0.05 is recommended). According to the GO analysis results, there were 1,115 up-regulated and 346 down-regulated GO terms in the biological process (COPD vs non-COPD lung tissues). Figures 3 and 4 show the GO classification for the biological process of down-regulated (Figure 3A) and up-regulated mRNAs (Figure 4A). The bar plot shows the top 10 enrichment score values of the significant enrichment terms. Biological processes of down-regulated mRNAs (Figure 3D) mainly include synaptic transmission, regulation of biological quality, growth, etc., while those of up-regulated mRNAs (Figure 4D) mainly include cellular response to chemical stimulus, regulation of response to stimulus, cell death, etc. Similarly, there were 34 down-regulated (Figure 3B and E) and 127 up-regulated GO terms (Figure 4B and E) in the cellular component (COPD vs non-COPD lung tissues). There were 53 down-regulated (Figure 3C and F) and 115 up-regulated GO terms (Figure 4C and F) in molecular function (COPD vs non-COPD lung tissues).
Identification of 10 differentially expressed lncRNAs
Based on the previously mentioned studies, we selected five up-regulated and five down-regulated lncRNAs. According to the qRT-PCR results, lncRNAs BC038205, BC130595, TUG1, MEG3, and LOC646329 were markedly increased in COPD lung tissues compared to non-COPD lung tissues (n=20) (***P<0.001; Figure 5A). Simultaneously, lncRNAs LOC729178, PLAC2, LOC339529, LINC00229, and SNHG5 were significantly decreased in COPD lung tissues compared to non-COPD lung tissues (n=20) (***P<0.001; Figure 5B). Table 5 presents detailed information about significant differentially expressed lncRNAs in different diseases.
  | Table 5 Detailed information of significant differentially expressed lncRNAs in diseases |
Silencing of TUG1 affects the proliferation ability in BEAS-2B and HFL1 cells after TGF-β pretreatment
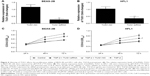
To determine whether silencing of TUG1 expression by siRNA affected the proliferation of human lung cells, the cells were cultured with 50 μM NC siRNAs or TUG1 siRNAs (si-TUG1) for 48 h. Transfection effect was detected by qRT-PCR. The expression level of lncRNA TUG1 decreased in BEAS-2B (Figure 6A) and HFL1 cells (Figure 6B) with TUG1 siRNAs. Additionally, to testify whether TGF-β affected the proliferation of human lung cells, cells were treated with 2 ng/mL TGF-β for 48 h. Then growth rates were determined by Cell Counting Kit-8 (CCK-8) assay. The proliferation of BEAS-2B cells with TGF-β and si-TUG1 was markedly increased relative to BEAS-2B cells with TGF-β (P<0.05). The proliferation of BEAS-2B cells with TGF-β was markedly suppressed compared with BEAS-2B cells (*P<0.05) (Figure 6C). Similar results were observed in HFL1 cells (Figure 6D).
Silencing of TUG1 suppresses the expression levels of α-SMA and fibronectin proteins in BEAS-2B and HFL1 cells
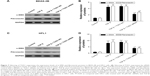
Finally, to explore the expression levels of α-SMA and fibronectin proteins, we cultured BEAS-2B and HFL1 cells. Some cells were treated with 50 μM NC siRNAs or TUG1 siRNAs (si-TUG1) and 2 ng/mL TGF-β for 48 h, while others were treated with 2 ng/mL TGF-β for 48 h. Western blot assay was performed to detect the expression of α-SMA and fibronectin proteins, which was increased in TGF-β BEAS-2B cells compared with BEAS-2B cells. When BEAS-2B cells were transfected with TUG1 siRNAs and TGF-β for 48 h, the expression of α-SMA and fibronectin proteins was decreased relative to BEAS-2B cells with TGF-β and NC siRNAs. TGF-β promoted the expression of α-SMA and fibronectin proteins in BEAS-2B cells (Figure 7A). Similar results were obtained with HFL1 cells (Figure 7C). Relative quantities were defined as the ratio of the grayscale of α-SMA and fibronectin to that of GAPDH (Figures 7B and 7D).
Discussion
Currently, COPD is a primary cause of morbidity around the world and is associated with individualized development of chronic symptoms, airflow obstruction,36,37 and undermined health.38 Even worse, it presents with acute episodes of symptom exacerbation.39 Although studies related to clinical diagnoses, causes of development, and fundamental science have been conducted, the molecular mechanism underlying the development of COPD is still not fully known and effective treatment methods for COPD are urgently required. Therefore, in our study, the aim was to find potential and available biomarkers for the diagnosis and treatment of COPD.
LncRNAs are involved in various biological processes and participate in the process of RNA transcription, mRNA splicing, translation,11,40,41 and in the pathogenesis of numerous diseases.42–44 However, lncRNAs that regulate the pathogenetic mechanism of COPD are unclear at present. Microarray analysis has been suggested as an available and useful tool to detect molecular mechanisms and find potential biomarkers. Therefore, we studied the differential expression of lncRNAs between COPD and non-COPD lung tissues. According to the results of our microarray analysis, we detected the differential expression of 25,628 lncRNAs and 20,106 mRNAs between COPD and non-COPD lung tissues. Among them, the expression of 8,376 (32.7%) lncRNAs and 5,049 (25.1%) mRNAs was significant (fold-change >2.0 or <0.5; P<0.05).
Based on the analysis of genomic locus and the correlation with protein-coding genes among differentially expressed lncRNAs, we found 3,249 (38.8%) intergenic lncRNAs, 2,239 (26.7%) natural antisense lncRNAs, 1,059 (12.6%) exon sense-overlapping lncRNAs, 974 (11.6%) intronic antisense lncRNAs, 375 (4.5%) intron sense-overlapping lncRNAs, 371 (4.4%) bidirectional lncRNAs, and 109 (1.3%) nonoverlapping antisense lncRNAs. Therefore, most lncRNAs are intergenic (no overlapping or bidirectional coding transcripts nearby the lncRNA), and TUG1 also belongs to the class of intergenic lncRNAs.
Moreover, microarray assay also includes the pathway analysis and GO enrichment analysis based on differentially expressed mRNAs. Previous studies have shown that COPD is related to lysosome,45 Staphylococcus aureus infection,46 osteoclast differentiation,47 leishmaniasis,48 dilated cardiomyopathy,49 rap1 signaling pathway,50 and PPAR signaling pathway,51 which are concordant with our KEGG pathway results in patients with COPD. In addition, we found 1,115 up-regulated and 346 down-regulated GO terms in biological process, 127 up-regulated and 34 down-regulated GO terms in cellular component, and 115 up-regulated and 53 down-regulated GO terms in molecular function between COPD lung tissues and non-COPD lung tissues according to the GO analysis results. Data regarding other COPD-related pathways and genes correlated with biological process, cellular component, and molecular function can provide a lot of information for the study of COPD.
Furthermore, we selected up-regulated lncRNAs (BC038205, BC130595, TUG1, MEG3, and LOC646329) and down-regulated lncRNAs (LOC729178, PLAC2, LOC339529, LINC00229, and SNHG5) which were identified by qRT-PCR. The results were consistent with those obtained by microarray analysis. Specifically, studies have shown that lncRNA TUG1, induced by p53, inhibited specific genes related to cell-cycle regulation;52 silencing of lncRNA TUG1 suppressed retinal development in a developing mouse eye.28 Studies have shown that lncRNA TUG1 was highly expressed in hepatocellular carcinoma53 and urothelial carcinoma of the bladder.31 In our study, silencing of TUG1 by siRNA markedly increased the ability of proliferation in BEAS-2B and HFL1 cells. Results indicated that TUG1 deletion decreased the expression levels of α-SMA and fibronectin proteins which are mesenchymal markers in BEAS-2B and HFL1 cells.
TGF-β induces various cellular responses, including differentiation, inflammation, proliferation, and apoptosis, and plays a vital role in COPD, asthma, cancer, and other pathological processes.54 Study showed that TGF-β enhanced extracellular matrix synthesis to influence expression of the fibrogenic cytokine in numerous tissues.55 In our study, BEAS-2B and HFL1 cells were induced by TGF-β for 48 h. The ability of proliferation and expression of α-SMA and fibronectin proteins were analyzed. Results showed that TGF-β markedly inhibited the ability to proliferate and clearly promoted the expression of α-SMA and fibronectin proteins in BEAS-2B and HFL1 cells.
Conclusion
In summary, our study involving microarray analysis comprehensively indicated the expression levels of lncRNAs in COPD and non-COPD lung tissues. The target prediction of lncRNA and potential functional characteristics will help in exploring the potential effect in patients with COPD. KEGG pathway and GO enrichment analysis may help in exploring potential genomic regulatory networks and offer more new potential therapeutic targets. Furthermore, relevant research with regard to function and mechanism could make lncRNA TUG1 a potential diagnostic marker in patients with COPD.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7:53. | ||
Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(5):1256–1276. | ||
Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. 2004;364(9434):613–620. | ||
Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5(3):257–263. | ||
Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellularmechanisms. Eur Respir J. 2003;22(4):672–688. | ||
Lahzami S, Aubert JD. Lung transplantation for COPD-evidence-based. Swiss Med Wkly. 2009;139(1–2):4–8. | ||
American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. | ||
Mogayzel PJ Jr, Naureckas ET, Robinson KA, et al; Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–689. | ||
Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–227. | ||
Kong L, Zhang Y, Ye Z-Q, et al. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007;35(Web Server issue):W345–W349. | ||
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. | ||
Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. | ||
Kim A, Zhao H, Ifrim I, Dean A. Beta-globin intergenic transcription and histone acetylation dependent on an enhancer. Mol Cell Biol. 2007;27(8):2980–2986. | ||
Managadze D, Rogozin IB, Chernikova D, Shabalina SA, Koonin EV. Negative correlation between expression level and evolutionary rate of long intergenic noncoding RNAs. Genome Biol Evol. 2011;3:1390–1404. | ||
Krangel MS. T cell development: better living through chromatin. Nat Immunol. 2007;8(7):687–694. | ||
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. | ||
Guttman M, Donaghey J, Carey BW, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477(7364):295–300. | ||
Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. | ||
Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220(2):126–139. | ||
Klattenhoff CA, Scheuermann JC, Surface LE, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152(3):570–583. | ||
Gutschner T, Hämmerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73(3):1180–1189. | ||
Johnson R. Long non-coding RNAs in Huntington’s disease neurodegeneration. Neurobiol Dis. 2012;46(2):245–254. | ||
Congrains A, Kamide K, Oguro R, et al. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis. 2012;220(2):449–455. | ||
Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4587. | ||
Cao G, Zhang J, Wang M, et al. Differential expression of long non-coding RNAs in bleomycin-induced lung fibrosis. Int J Mol Med. 2013;32(2):355–364. | ||
Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. | ||
McKiernan PJ, Molloy K, Cryan SA, McElvaney NG, Greene CM. Long noncoding RNA are aberrantly expressed in vivo in the cystic fibrosis bronchial epithelium. Int J Biochem Cell Biol. 2014;52:184–191. | ||
Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15(6):501–512. | ||
Zhang EB, Yin DD, Sun M, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243. | ||
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP, Yao J. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013;14:2311–2315. | ||
Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107(5):555–559. | ||
Cao W-J, Wu H-L, He B-S, Zhang Y-S, Zhang Z-Y. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol. 2013;19(23):3658–3664. | ||
Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–1927. | ||
Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. | ||
Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010;38:D355–D360. | ||
Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–S121. | ||
Buist AS. Guidelines for the management of chronic obstructive pulmonary disease. Respir Med. 2002;96(Suppl C):S11–S16. | ||
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. | ||
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. | ||
Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15(Spec No 1):R17–R29. | ||
Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5(4):e1000459. | ||
Tufarelli C, Stanley JA, Garrick D, et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet. 2003;34(2):157–165. | ||
Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40(4):902–906. | ||
Lin R, Maeda S, Liu Ca, Karin M, Edgington TS. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene. 2007;26(6):851–858. | ||
Barreiro E, Sieck G. Muscle dysfunction in COPD. J Appl Physiol. 2013;114(9):1220–1221. | ||
Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36(11):1418–1423. | ||
Bai P, Sun Y, Jin J, et al. Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir Res. 2011;12:157. | ||
Dasgupta S, Saha M, Chakrabarti S, Chakraborty J. Visceral leishmaniasis with pleural effusion in an immunocompetent patient. Lung India. 2014;31(1):56–58. | ||
Poller W, Kühl U, Tschoepe C, Pauschinger M, Fechner H, Schultheiss HP. Genome–environment interactions in the molecular pathogenesis of dilated cardiomyopathy. J Mol Med(Berl). 2005;83(8):579–586. | ||
Lorenowicz MJ, Fernandez-Borja M, Hordijk PL. cAMP signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2007;27(5):1014–1022. | ||
Simone RE, Russo M, Catalano A, et al. Lycopene inhibits NF-kB-mediated IL-8 expression and changes redox and PPARγ signalling in cigarette smoke-stimulated macrophages. PLoS One. 2011;6(5):e19652. | ||
Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106(28):11667–11672. | ||
Huang MD, Chen WM, Qi FZ, et al. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165. | ||
Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. | ||
Epstein FH, Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331(19):1286–1292. | ||
Engström PG, Tommei D, Stricker SH, Ender C, Pollard SM, Bertone P. Digital transcriptome profiling of normal and glioblastoma-derived neural stem cells identifies genes associated with patient survival. Genome Med. 2012;4:76. | ||
Antúnez C, Boada M, González-Pérez A, et al. The membrane-spanning 4-domains, subfamily A (MS4A) gene cluster contains a common variant associated with Alzheimer’s disease. Genome Med. 2011;3(5):33. | ||
Gerhardinger A. Genomische und transkriptionelle Veränderungen im Pankreaskarzinom [dissertation]. Germany: Universitätsklinikum Ulm; 2012. [German]. | ||
Xu Y, Wang J, Qiu M, et al. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36(3):1643–1651. | ||
Braconi C, Kogure T, Valeri N, et al. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30(47):4750–4756. | ||
Qin R, Chen Z, Ding Y, Hao J, Hu J, Guo F. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma. 2013;60(5):486–492. | ||
Ying L, Huang Y, Chen H, et al. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol BioSyst. 2013;9(3):407–411. | ||
Wang P, Ren Z, Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem. 2012;113(6):1868–1874. | ||
Liu SJ, Nowakowski TJ, Pollen AA, et al. Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol. 2016;17:67. | ||
Li H, Yu B, Li J, et al. Characterization of differentially expressed genes involved in pathways associated with gastric cancer. PLoS One. 2015;10(4):e0125013. | ||
Kwak SH, Park KS. Genetic studies on diabetic microvascular complications: focusing on genome-wide association studies. Endocrinol Metab (Seoul). 2015;30(2):147–158. | ||
Zhao L, Guo H, Zhou B, et al. Long non-coding RNA SNHG5 suppresses gastric cancer progression by trapping MTA2 in the cytosol. Oncogene. Epub 2016 Apr 11. | ||
Ichigozaki Y, Fukushima S, Jinnin M, et al. Serum long non-coding RNA, snoRNA host gene 5 level as a new tumor marker of malignant melanoma. Exp Dermatol. 2016;25(1):67–69. | ||
Xin W, Liu X, Ding J, et al. Long non-coding RNA derived miR-205-5p modulates human endometrial cancer by targeting PTEN. Am J Transl Res. 2015;7(11):2433–2441. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.