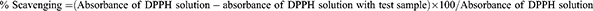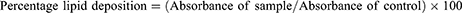Back to Journals » Journal of Experimental Pharmacology » Volume 15
Screening of Antioxidant, Antibacterial, Anti-Adipogenic, and Anti-Inflammatory Activities of Five Selected Medicinal Plants of Nepal
Authors Lamichhane G , Sharma G, Sapkota B , Adhikari M , Ghimire S, Poudel P , Jung HJ
Received 12 September 2022
Accepted for publication 11 January 2023
Published 2 March 2023 Volume 2023:15 Pages 93—106
DOI https://doi.org/10.2147/JEP.S388968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Gopal Lamichhane,1,2,* Grinsun Sharma,1,3,* Biswash Sapkota,1,4,* Mahendra Adhikari,1,5,* Sandhaya Ghimire,1,* Prakash Poudel,1,6 Hyun-Ju Jung2
1School of Health and Allied Sciences, Faculty of Health Sciences, Pokhara University, Pokhara, 33700, Nepal; 2Department of Oriental Pharmacy and Wonkwang-Oriental Medicines Research Institute, Wonkwang University, Iksan, Jeollabuk-do, 570-749, South Korea; 3Institute of Pharmaceutical Research and Development, College of Pharmacy, Wonkwang University, Iksan, 570-749, South Korea; 4Department of Pharmacy and Clinical Pharmacology, Madan Bhandari Academy of Health Sciences, Hetauda, 44107, Nepal; 5Department of Pharmacy, Institute of Pharmaceutics and Biopharmaceutics, Heinrich Heine University Düsseldorf, Düsseldorf, 40225, Germany; 6Pharmacy Program, Gandaki University, Pokhara, 33700, Nepal
*These authors contributed equally to this work
Correspondence: Prakash Poudel; Hyun Ju Jung, Email [email protected]; [email protected]
Introduction: Herbal products have been widely used for the treatment of diseases throughout the ages. In this research, we investigated antioxidant, antibacterial, anti-adipogenic, and anti-inflammatory activities of methanolic extracts of five ethnomedicinally important plants; namely, Alnus nepalensis, Dryopteris sparsa, Artocarpus lacucha, Litsea monopetala, and Lyonia ovalifolia.
Methods: We investigated the DPPH free radical scavenging potential, sensitivity of selected bacterial strains towards the extracts using a disc diffusion assay, anti-inflammatory activity in RAW-264.7 cells, and anti-adipogenic activity by the ORO assay in 3T3-L1 preadipocytes.
Results and discussion: The extract of A. nepalensis showed significant antioxidant activity (IC50=4.838 μg/mL), followed by A. lacucha, L. monopetala, and L. ovalifolia, exhibiting comparable IC50 values to that of ascorbic acid (IC50=5.063 μg/mL). Alnus nepalensis also showed good antibacterial activity in disc diffusion methods, with remarkable zones of inhibition in A. baumannii (14.66 mm) and P. mirabilis (15.50 mm) bacterial species. In addition, A. nepalensis was found to increase adipogenesis in 3T3-L1 cells, evidenced by increased lipid deposition in differentiated 3T3-L1 cells. A similar pattern of increased adipogenesis was observed on treatment with L. ovalifolia extracts. On the other hand, A. lacucha effectively reduced lipid deposition in 3T3-L1 cells at 100 μg/mL (75.18± 6.42%) by inhibiting adipogenesis, showing its potential use in the management of obesity. Furthermore, A. lacucha 100 μg/mL (15.91± 0.277 μM) and L. monopetala 75 μg/mL (12.52± 0.05 μM) and 100 μg/mL (11.77± 0.33 μM) significantly inhibited LPS-induced nitric oxide production in RAW 264.7 cells. Also, A. nepalensis and L. ovalifolia inhibited NO production significantly, endorsing their anti-inflammatory potential.
Conclusion: The findings from these in-vitro studies suggest that the selected five plants possess remarkable antioxidant, antibacterial, anti-adipogenic, and anti-inflammatory activities. This study opens the door to conduct further advanced in-vivo experiments to find possible lead compounds for the development of valuable therapeutic agents for common health problems.
Keywords: Alnus nepalensis, Dryopteris sparsa, Artocarpus lacucha, Litsea monopetala, Lyonia ovalifolia
Introduction
Medicinal plants have been used to manage health ailments for ages and are still a critical intervention for many people in developing countries. Herbal resources have also been exploited to discover lead compounds and/or their intermediates, which can be developed into potent therapeutic agents by suitable modification. Given that many plants have not been scientifically explored for their pharmacological activities, nature undoubtedly holds great potential for the possible discovery of bioactive herbs and compounds.1–6
The health-protective effect of fruits and vegetables for humans is mainly attributed to their antioxidant potential. Secondary metabolites derived from plants, in particular polyphenols, are the major contributors acting against oxidative stress. These polyphenols are distributed widely throughout the plant kingdom. So far, over 8000 different structures are known to have aromatic rings and one or more hydroxyl substituents as characteristic structural properties. Among them, flavonoids are commonly distributed phenolic phytochemicals. They are good antioxidants, known for their potential for metal chelation and free radical scavenging capacity.7 Besides their antioxidant properties, polyphenols possess anti-inflammatory, antitumor, pro-apoptotic, anti-angiogenic, cardioprotective, and anti-obesity activities.8 They have also been shown to inhibit low-density lipoprotein (LDL) oxidation and DNA damage, and to have antithrombotic and antimicrobial effects.7 Other activities include the modulation of cholesterol levels, anti-epileptic effects, inhibition of adipogenesis, and effects on glutamate metabolism.9 The literature also shows that several plants, such as grapes, cinnamon, Streblus asper, and Sida cordifolia, contain a range of polyphenols, with diverse bioactivities.10–14 Therefore, estimation of the total phenolic, flavonoid, and antioxidant potential of plant extracts can provide valuable information about their pharmacological and nutraceutical potential. Also, chemical and pharmacological screening of plants can provide supportive data enabling them to be developed into nutraceuticals by incorporating health claims and/or nutritional claims.15
Nepal is rich in medicinal and aromatic plants, constituting about 1515 to 2331 medicinally useful plants. This country is rich in biodiversity due to variaion in landscapes and physiographic regions. More than a hundred ethnic groups living in Nepal uses plants for the treatment of diseases, making the country rich in ethnomedicinal knowledge.16 If this information is used properly in drug discovery, it can increase the chances of discovering promising drug candidates compared to random screening.17 Several drug discoveries have been successfully correlated with the ethnomedicinal uses of medicinal plants.18–21 Also, several bioactive medicinal plants can be combined to prepare pharmacologically active herbal formulations with reduced toxicity and increased efficacy.22–25 For this reason, we selected five ethnomedicinally important medicinal plants for our study. Alnus nepalensis D. Don is traditionally used to manage diarrhea, dysentery, stomach ache, cuts, and wounds.26 Artocarpus lacucha Bunch.-Ham. bark is another popular ethnomedicinal plant, which is used to treat boils and pimples, to eliminate purulent matter, and to treat worm infections.27 The edible young shoots of Dryopteris sparsa (D. Don) Kuntze are used in treating helminthic infections.28 Litsea monopetala (Roxb.) Pers. is used in cattle diarrhea, stomach ache, gastric ulcers, and bone fractures.29,30 Lyonia ovalifolia (Wall.) Drude is traditionally used for scabies, wound healing, allergy, and parasitic infection, and as an insecticidal agent.31–33
Although all five selected plants are reported to have valuable medicinal properties in the folk medicinal system of Nepal, there is little scientific evidence for their therapeutic potential. Previous research demonstrated the presence of antifilarial activity of diarylheptanoids, reported in a crude methanolic extract of A. nepalensis.34 The natural stilbene oxyresveratrol, isolated from the heartwood of A. lacucha, has received a lot of attention because of its huge therapeutic potential. Antioxidant, anti-inflammatory, neuroprotective, hepatoprotective, antimicrobial, and anticancer activities have already been found in oxyresveratrol.35 The biological activity of crude extracts of the genus Litsea, and the phytochemicals isolated from these extracts, has also been reported. Arfan et al evaluated the use of phenolic compounds isolated from the bark extract of L. monopetala for their antioxidant activity.36 Potential neuroprotective compounds, including secorhodomollolides A and D, and lyonin A, have been isolated from L. ovalifolia.37 However, bioactive molecules in D. sparsa, and their pharmacological uses, remain unclear. Therefore, there is a strong rationale to validate the ethnomedicinal uses of such medicinal plants of Nepal. In this research, the anti-adipogenic, anti-inflammatory, antioxidant, and antibacterial activities of selected five medicinal plants were screened scientifically using well-accepted in-vitro test methods.
Materials and Methods
Plant Materials
Plant samples were collected from different places in the Kaski district of Nepal, with an elevation ranging from 900 to 1200 m. Collected plant samples were dedusted and chopped into fine pieces measuring 1–1.5 cm. The chopped plant parts were shade dried at room temperature and stored in air-tight containers. The selected plant species were identified with the help of a taxonomist from the National Herbarium Laboratories (KATH), Godawari, Kathmandu, and preserved in Pokhara University Herbarium, Pokhara University, Pokhara, Nepal. Details of the collection site, parts used, herbarium numbers, and local name of the selected plants are presented in Table 1. Photographic images of the dried plant samples are shown in Figure 1.
 |
Table 1 List of Selected Medicinal Plants |
 |
Figure 1 Photographs of the selected medicinal plants. |
Cells, Bacteria, Reagents, and Chemicals
Aluminum chloride (AlCl3) and Folin–Ciocalteu phenol reagent were purchased from Fisher Scientific, India. Ascorbic acid was purchased from Qualigens Fine Chemicals, India. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was from Wako Pure Chemicals, Japan. Ferric chloride (FeCl3) was from Rasayan Laboratories, India. 3T3-L1 preadipocyte and RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC). Bacteria were isolated from clinical samples, identified and supplied by Manipal Teaching Hospital, Phulbari Pokhara. Growth medium (Dulbecco’s modified Eagle’s medium [DMEM]), penicillin and streptomycin antibiotic solution, phenol red-free DMEM, fetal bovine serum (FBS), and newborn calf serum (NCS) were from Life Technologies Corporation, Waltham, MA, USA. Components of the differentiation media (3-isobutyl-1methylxanthine [IBMX], dexamethasone [DXM], and insulin) were from Sigma-Aldrich, Darmstadt, Germany. Isopropanol, 10% formalin, and Oil Red O (ORO) were from Sigma-Aldrich, Darmstadt, Germany. The cell viability assay kit (thiazolyl blue tetrazolium bromide [MTT]) was from Alfa Aesar, Heysham, UK. Dimethyl sulfoxide (DMSO) was from Junsei Chemicals Co Ltd, Japan. Lipopolysaccharide (LPS) from Escherichia coli 0111:B4 and Griess reagent were obtained from Sigma-Aldrich, Darmstadt, Germany.
Extraction, Phytochemical Screening, and Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) Determination
Dried samples (50 g) of all selected plant parts were extracted in 100% methanol (300 mL) by cold maceration method. All of the extracts were concentrated using a vacuum pump rotatory evaporator from Buchi, New Castle, USA. The concentrated extracts were dried using a freeze dryer from IIShin Lab Co (South Korea) to obtain the lyophilized extract. Phytochemical screening was performed according to the method reported by Bhatnagar et al.38 Qualitative phytochemical screening of major groups of secondary metabolites, such as alkaloids, anthraquinones, flavonoids, glycosides, phenolics, reducing sugars, saponins, tannins, and terpenoids, was performed.
The Folin–Ciocalteu method was used to estimate the TPC in crude extract samples.39 In brief, 1 mL of 2 N Folin–Ciocalteu phenol reagent was mixed with 1 mL of 1 mg/mL plant extract (prepared by dissolving it in methanol), and then the mixture was diluted by the addition of 5 mL distilled water. After incubating for 5 minutes, 1 mL of 10% Na2CO3 solution was added, and then the mixture was incubated for 1 hour in the dark at room temperature. The absorbance of the final mixture was measured at 725 nm using a UV-visible spectrophotometer (Shimadzu, Japan). TPC was expressed as micrograms gallic acid equivalent per milligram of extract (µg GAE/mg), obtained by calibration curves of gallic acid at 500, 400, 300, 200, 100, and 50 µg/mL concentrations.
The aluminum chloride chelation method was used to approximate the amount of flavonoids in all plant extracts.40 First, a stock solution (1 mg/mL) of each plant extract in methanol was diluted with water in a 1:5 ratio and mixed with 0.3 mL of 5% sodium nitrite solution. Then, the mixture was incubated for 5 minutes and 0.3 mL of 10% of AlCl3 was added to it. This was followed by the addition of 2 mL of 1 M sodium hydroxide. The absorbance of the final mixture was taken at 510 nm using a UV-visible spectrophotometer. Total flavonoid was expressed as micrograms of quercetin equivalent per milligram (µg QE/mg) of the plant extract, obtained by calibration curves of quercetin at 500, 400, 300, 200, 100, and 50 µg/mL concentrations.
Antioxidant Activity
The antioxidant activity of the extracts was evaluated using DPPH free radical scavenging methods.41 The stock solution of 1 mg/mL of each plant extract and ascorbic acid was prepared by dissolving in methanol. Different concentrations of the test samples (0.1, 1, 10, and 100 µg/mL) were prepared by serial dilution using methanol. Then, 1 mL of these diluted samples at different concentrations was mixed with 1 mL of DPPH (60 µM) solution. The mixture was incubated for 30 minutes at room temperature, and the absorbance was measured at 517 nm using a UV-visible spectrophotometer. Each assay was performed in triplicate. The percentage radical scavenging activity of each plant extract at different dilutions was calculated using the following equation:
Antibacterial Activity
Antibacterial activity was assessed against Acinetobacter baumannii, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Salmonella enterica enterica typhi by the disc diffusion method.42 Sterile agar (20 mL) medium was transferred into Petri dishes under aseptic conditions, and allowed to solidify. Bacterial suspensions with a density equivalent to 0.5 McFarland were prepared and inoculated into the agar with cotton swabs under aseptic conditions. Sterile paper discs of 6 mm diameter were impregnated with 10 μL of 100 mg/mL stock solution to make 1 mg/disc. Each extract and the standard antibiotic disc were gently placed on top of the agar layer to give better contact with the agar plates preinoculated with bacteria. A disc with 10% DMSO was used as a negative control. Reference antibiotics (azithromycin, cefoxitin, and ciprofloxacin) were used as positive controls. The plant extract discs and standard antibiotic discs were allowed to diffuse into the plates for 1 hour, and then they were incubated at 37°C for 24 hours in an inverted position. The zone of inhibition was measured using digital Vernier calipers. Each assay was performed in triplicate.
Anti-Adipogenic Activity
The anti-adipogenic activity was assessed in the 3T3-L1 cell line using the methods explained by Pandeya et al22 3T3-L1 preadipocytes were cultured and subcultured in 10% NCS supplemented with DMEM growth medium with 1% antibiotics (penicillin–streptomycin). Cells were maintained under a humidified atmosphere of 5% CO2 at 37°C. Then, the obtained cells were seeded in a 96-well plate at a cell density of 7×103 cells per well and left overnight. The next day, the cells were treated with different concentrations of plant extracts in 10% FBS-supplemented DMEM. After 48 hours, the MTT assay was performed to calculate cell viability at different concentrations. Thus, a safe dose was used to evaluate the anti-adipogenic activity.
The Oil Red O (ORO) staining assay was used to quantify the amount of lipid deposited in differentiated 3T3-L1 cells. 3T3-L1 preadipocytes were seeded into the 24-well plate at a cell density of 5×103 cells per well in 10% FBS-supplemented DMEM culture medium. The cultured plates were kept in an incubator until cells reached full confluency, and the cell medium was changed every 2 days. After that, cells were co-treated with non-toxic concentrations of extracts (obtained by the MTT assay) in cell differentiation media for 2 days. The cell differentiation media contained dexamethasone (1 µM), insulin (5 µg/mL), and 3-isobutyl-2-methylxanthine (IBMX 0.5 mM) in 10% FBS-supplemented DMEM. Differentiation media with the tested concentrations of extracts were replaced after 2 days by adipocyte maintenance medium (10% FBS-supplemented DMEM with 5 µg/mL of insulin; without extracts). After 2 days, the adipocyte maintenance medium was replaced by 10% FBS-supplemented DMEM. On the eighth day of the experiment, cells were washed with phosphate-buffered saline and fixed in 10% formalin. Fixed cells were stained with ORO and viewed under an EVOS XL (Life Technologies, USA) microscope. The deposited stain was dissolved in isopropanol and quantified using a microplate reader at 520 nm.
The final results were expressed as percentage deposition of lipid in differentiated cells compared to control, as follows:
Anti-Inflammatory Activity
RAW 264.7 cells were thawed and subcultured in 10% FBS and 1% antibiotic (penicillin and streptomycin)-supplemented DMEM culture medium. Cultured cells were kept in an incubator in a humidified atmosphere at 37°C and 5% CO2. For the cell viability assay, subcultured stock cells at around 90% confluency were collected and seeded into a 98-well plate with a cell density of 2.5×104 cells per well in DMEM supplemented with 10% FBS. Cells were left overnight and then treated with different concentration of extracts in DMEM supplemented with 10% FBS. Eighteen hours after treatment, cell viability was measured using the MTT assay kit following the manufacturer’s protocol.
LPS-induced nitric oxide (NO) production in RAW 264.7 cells is a highly exploited method in research, as a cellular inflammatory model. NO produced in this way can be quantified in a cell culture medium in the form of nitrite (NO2-), a stable degradation product of NO. For anti-inflammatory activity, the nitrite concentration in the medium was quantified by Griess reagent using the modified methods explained by Alhallaf and Perkins.43 In brief, the subcultured cells were seeded at a cell density of 15×104 cells per well in a 48-well plate in DMEM supplemented with 10% FBS, and incubated overnight. Then, treatment was carried out at a non-toxic concentration in phenol red-free DMEM supplemented with 10% FBS and incubated at 37°C. One hour after incubation, inflammation was induced by adding LPS solution to make 1 µg/mL concentration in the culture medium. After 18 hours of treatment, the nitrite concentration was measured in the culture medium using Griess reagent (equal amounts of 1% sulfanilamide in 5% phosphoric acid + 0.1% naphthyl ethylenediamine di-hydrochloride in water). Then, 100 µL of culture medium was mixed with 100 µL of Griess reagent, and absorbance was measured after 10 minutes in a microplate reader at 540 nm wavelength. The quantity of nitrite in the cell supernatant was determined by comparison with the standard curve of sodium nitrite at different concentrations.
Statistical Analysis
Data are presented as a mean of three independent experiments ± standard deviation. Statistical significance between the groups was analyzed using a two-tailed Student’s t-test in Microsoft Excel 2010. Statistical significance was set at p˂0.05.
Results
Extraction Yield Value, Phytochemical Screening, and TPC and TFC Quantification
Measurement of the extraction yield value revealed the highest amounts in L. ovalifolia leaves (16.73%) and D. sparsa (16.73%), followed by A. lacucha (15.12%), L. monopetala (12.64%), and A. nepalensis (8.69%). The secondary metabolite screening tests revealed the presence of flavonoids, phenols, tannins, and terpenoids, but none of the samples showed the presence of alkaloids (Table 2). The quantification of TPC using Folin–Ciocalteu methods revealed variation from 423.49±0.89 µg GAE/mg of extract (A. nepalensis) to 46.38 µg GAE/mg of extract (D. sparsa) among the selected plants. Also, A. lacucha (331.47±0.21 µg GAE/mg), L. monopetala (167.08±2.25 µg GAE/mg), and L. ovalifolia (105.77±5.56 µg GAE/mg) showed significant phenolic content in their extracts. Furthermore, A. nepalensis had the highest amount of TFC (408.88±2.08 µg QE/mg of extract) among the selected plant extracts, as obtained by the aluminum chloride colorimetric method. This was followed by L. monopetala (371.55±12.99 µg QE/mg), and A. lacucha (360.88±3.54 µg QE/mg); D. sparsa (195.05±3.12 µg QE/mg) and L. ovalifolia (178.21±3.01 µg QE/mg of extract) had the lowest amounts of TFC, as shown in Figure 2.
 |
Table 2 Presence and Absence of Phytochemicals in Selected Plant Extracts Obtained by Different Phytochemical Screening Methods |
 |
Figure 2 Total phenol (µg GAE/mg of extract) content (TPC) and total flavonoid (µg QE/mg of extract) content (TFC) of methanolic extract of five selected medicinal plants. |
Antioxidant Activity
The DPPH free radical scavenging activity of the extracts showed that A. nepalensis extract inhibited DPPH free radical even at a low concentration, with an IC50 value of 4.838 µg/mL, which is slightly lower than that of the ascorbic acid standard (5.063 µg/mL). In addition, A. lacucha, L. monopetala, and L. ovalifolia had comparable IC50 values of 6.18 , 8.79 , and 12.86 µg/mL, respectively, as shown in Figure 3.
 |
Figure 3 IC50 values of selected plant extracts. |
Antimicrobial Activity
Bacterial susceptibility to the extracts was assessed by measuring the zone of inhibition (mm), which is shown in Figure 4. Only A. nepalensis showed antibacterial properties, represented by the zones of inhibition against A. baumannii (14.66 mm) and P. mirabilis (15.50 mm), among the selected plants. The positive controls, azithromycin, cefoxitin, and ciprofloxacin, inhibited A. baumannii with zones of inhibition of 29, 0, and 33.66 mm, and P. mirabilis with zones of inhibition of 15, 17.66, and 27.66 mm, respectively. This indicates that A. nepalensis extract has better antimicrobial properties than cefoxitin on A. baumannii, and was as effective as azithromycin and cefoxitin on P. mirabilis.
Anti-Adipogenic Activity
The cell viability of 3T3-L1 using the MTT assay showed that all plant extracts were safe for treatment in cells at concentrations up to 150 µg/mL, except for A. nepalensis (Figure 5). Slightly higher cell viability compared to the control group was observed at lower concentrations of A. lacucha, L. monopetala, D. sparsa, and L. ovalifolia. The difference was significant with L. ovalifolia and L. monopetala compared to the control. A safe dose was used for the evaluation of anti-adipogenic activity in cells.
Inhibition of adipogenesis in cells, indicated by reduced lipid deposition in differentiated adipocytes, was evaluated using the ORO assay. The results showed that A. lacucha significantly inhibited the lipid deposition in differentiated 3T3-L1 cells at a concentration of 100 µg/mL (75.18±6.42%) (Figure 6). This result is supported by an ORO-stained image showing fewer red-stained fat droplets in this group compared to the control (Figure 7). Significantly increased deposition of lipid was observed at the lower dose of D. sparsa (50 µg/mL; 111.90±3.76%), the higher dose of A. nepalensis (100 µg/mL; 115.57±5.31%), and both doses of L. ovalifolia (50 µg/mL: 122.67±1.81%; 100 µg/mL: 134.68±14.01% dose-dependent) compared to the control.
Anti-Inflammatory Activity
The cell viability of RAW 264.7 cells using the MTT assay showed that all plant extracts were safe for treatment in cells up to 150 µg/mL, except for A. nepalensis, which showed a significant reduction of cell viability (74.82±3.94%) at 150 µg/mL (Figure 8). Slight toxicity was seen for A. lacucha at higher concentrations: 80 µg/mL (84.51±4.106%), 100 µg/mL (90.366±1.07%), and 150 µg/mL (82.62±9.65%). The difference was significant only for 80 µg/mL. The rest of the plant extracts were found to be safe at the selected doses for treatment in cells. The obtained safe dose was used for the evaluation of anti-inflammatory activity in the LPS-induced RAW 264.7 cell model.
Quantification of a stable breakdown product of the NO free radical, NO2−, accumulating in the cell media, was performed using Griess reagent, which demonstrated a significant increase in the amount in LPS-treated cells compared to control (Figure 9). All of the plant extracts, at all concentrations, reduced the LPS-induced NO production. However, significant differences in production compared to the LPS control were seen at the highest dose (100 µg/mL: 15.91±0.277 µM) of A. lacucha, and at doses of 75 µg/mL (12.52±0.05 µM) and 100 µg/mL (11.77±0.33 µM) of L. monopetala, 75 µg/mL (15.21±0.01 µM) of D. sparsa, and 75 µg/mL (15.668±1.04 µM) and 100 µg/mL (15.886±1.18 µM) of A. nepalensis (Figure 9).
Discussion
Among all of the studied extracts, A. lacucha significantly inhibited adipogenesis in 3T3-L1 cells. A significant reduction in lipid droplets can be observed in Figure 7. This extract also resulted in dose-dependent inhibition of NO production in RAW 264.7 cells. Similarly, extract of A. lacucha was found to have good antioxidant potential, with an IC50 of 6.18 µg/mL observed in the DPPH free radical scavenging activity test. In previous research, oxyresveratrol, a natural polyphenol with strong anti-adipogenic potential, was isolated from the heartwood of A. lacucha. This compound possesses anti-obesity, neuroprotective, antioxidative, antiviral, and anti-inflammatory activity.35,44 Furthermore, A. lacucha produced several flavonoids and phenolic compounds upon chemical isolation.45 These secondary metabolites may be responsible for the observed bioactivities. With its good anti-adipogenic and antioxidant activity, this plant has great potential for the isolation of lead compounds and to become a nutraceutical with multiple health benefits.
In the present study, L. monopetala was found to have good antioxidant potential in the DPPH free radical scavenging assay and anti-inflammatory activity in the LPS-induced RAW 264.7 macrophage model. A significant reduction in NO was observed at both treatment doses. Previous studies have also shown the good antioxidant activity and high phenolic content of L. monopetala.46 Further evaluation of the molecular mechanism of anti-inflammatory activity is necessary to determine the mechanism of NO inhibition. However, the genus Litsea has been reported to possess a wide range of secondary metabolites, including alkaloids, sesquiterpenes, flavonoids, lactones, lignans, and essential oil. Owing to these diverse secondary metabolites, promising bioactivities were observed in L. monopetala..47
The extract from D. sparsa resulted in a significant NO reduction at a lower dose when evaluated for anti-inflammatory activity. Except for its anti-inflammatory activity, D. sparsa did not show significant activity in any of the performed assays. This plant was also found to have lower phenolic and flavonoid contents than the other tested plants. Antioxidant activity was also found to be lower, having a higher IC50 value of 22.24 µg/mL. The lower amounts of phenolics and flavonoids may explain the higher IC50 of this plant. Although this plant showed positive test results for the presence of anthraquinones, glycosides, and saponins, no literature was found supporting the pharmacological properties of this plant. Therefore, further scientific evaluation is needed to justify its ethnomedicinal use in helminthic infection.
Methanolic extract of the leaves of A. nepalensis showed the highest antioxidant activity among the extracts of all plant samples, with an IC50 value of 4.83 µg/mL, which is lower than that of standard ascorbic acid (5.063 µg/mL). The amounts of total phenols (423.49±0.89 µg GAE/mg extract) and total flavonoids (408.88±2.08 µg QE/mg extract) were the highest of all the samples. Anti-inflammatory activity, as indicated by the inhibition of NO production, was significant at both concentrations. Furthermore, potent antibacterial activity against A. baumannii and P. mirabilis, with zones of inhibition of 14.7±0.6 mm and 15.5±0.6 mm, respectively, was observed in bacterial susceptibility tests. The A. nepalensis extract was found to increase adipogenesis in 3T3-L1 cells, and the difference was significant at a higher dose. Recent studies have shown that this plant contains diarylheptanoids with anti-inflammatory and antifilarial activity.48,49 These diarylheptanoids, especially platyphylloside, inhibited the production of pro-inflammatory cytokines (mainly TNF-α and IL-6). Similar affirmative activity of this compound was observed in an in-vivo model of mice.50 A diarylheptanoid-rich fraction of this plant sample also seemed to attenuate pathogenesis in malaria, as evidenced by significant antiplasmodial activity in enzyme assays. This was further supported by the reduction of parasitemia and increased survival in an in-vivo study.51 All of these studies validate the anti-inflammatory and antibacterial activity of methanolic extract of A. nepalensis leaves found in this study. Since mature adipocytes are actively involved in glucose uptake, storage, and metabolism, an increase in adipogenesis is used as an approach to treat diabetic complications.52,53 Likewise, a significant increase in adipogenesis in 3T3-L1 cells during the treatment with A. nepalensis methanolic extract shows its high potential in diabetes management.
In this study, L. ovalifolia showed anti-inflammatory activity at a higher dose in RAW 264.7 cells. The plant also demonstrated mild antioxidant activity (IC50 value of 15.99±0.013 µg/mL) and significant amounts of total phenols (105.77±5.56 µg GAE/mg extract) and total flavonoids (178.21±3.01 µg QE/mg extract). The presence of triterpenes, diterpenoids, sesquiterpenoids, phenylpropanoids, iridoids, and lignans has already been reported in this plant.54,55 These secondary metabolites may be responsible for the anti-inflammatory and antioxidant activity of the extract. Also, the methanolic extract of this plant showed a dose-dependent increase in adipogenesis in 3T3-L1 cells, and the difference was significant compared to the control. Deposition of more lipids in treated cells compared to the control can also be observed in Figure 7. In previous research, lyonofolin B iridoids with insulin secretagogue activity were discovered by Hussain et al.56 This plant could thus be further studied and used to discover lead compounds to develop anti-hyperglycemic agents or manage diabetes-related metabolic disorder. Although the antimicrobial tests in this study did not demonstrate any antibacterial properties against the tested bacterial stains, antiviral and anti-inflammatory agents were isolated from L. ovalifolia in previous studies. The reported results also endorse the ethnomedicinal value of this plant as an anti-scabies, analgesic, and anti-allergic agent.31,57,58
This study provides good scientific justification for ethnomedicinally useful plants with regard to their antioxidant, antibacterial, anti-adipogenic, and anti-inflammatory activities, based on limited chemical and cell-based assays. More sophisticated analytical methods, such as liquid chromatography and mass spectroscopy (LC-MS), nuclear magnetic resonance (NMR) imaging, or animal model studies, should be conducted to obtain more pragmatic results. Therefore, this study provides a strong scientific basis for conducting more intensive research on the selected medicinal plants.
Conclusion
This study examined the antioxidant, antibacterial, anti-adipogenic, and anti-inflammatory activities of five ethnomedicinally important plants of Nepal. Among these five plants, A. nepalensis and A. lacucha exhibited significant TPC, TFC, and antioxidant capacity. The results also showed that high levels of polyphenolic compounds in plant extracts contribute to their antioxidant activities. A similar trend, although less prominent, was also observed between polyphenolic content and anti-inflammatory, antibacterial, and anti-adipogenic activities. Furthermore, A. nepalensis, A. lacucha, and L. monopetala extracts showed promising antimicrobial, anti-adipogenic, and anti-inflammatory activities. Data from these preliminary in-vitro tests provide a scientific basis for the traditional uses of these plants and demonstrate a need to perform further bioassay-guided fractionation to isolate potent bioactive molecules and validate their pharmacological activities.
Acknowledgments
This paper was suported by Wonkwang University in (2021) and Pokhara University. We would like to acknowledge Pokhara University, School of Health and Allied Sciences, Nepal, and Wonkwang University (2021), Department of Oriental Pharmacy, Republic of Korea, for providing research facilities for conducting this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. World Health Organization; 2013.
2. Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi:10.3390/molecules21050559
3. Samy RP, Gopalakrishnakone P. Current status of herbal and their future perspectives. Nat Preced. 2007;2007:1 doi: 10.1038/npre.2007.1176.1.
4. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi:10.1021/acs.jnatprod.9b01285
5. Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–1614. doi:10.1016/j.biotechadv.2015.08.001
6. Lamichhane G, Pandeya PR, Lamichhane R, et al. Anti-obesity potential of ponciri fructus: effects of extracts, fractions and compounds on adipogenesis in 3T3-L1 preadipocytes. Molecules. 2022;27(3):676. doi:10.3390/molecules27030676
7. Urquiaga I, Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol Res. 2000;33(2):55–64. doi:10.4067/S0716-97602000000200004
8. Enaru B, Socaci S, Farcas A, et al. Novel delivery systems of polyphenols and their potential health benefits. Pharmaceuticals. 2022;14(10):946. doi:10.3390/ph14100946
9. Rasouli H, Farzaei MH, Khodarahmi R. Polyphenols and their benefits: a review. Int J Food Prop. 2017;20(sup2):1700–1741 doi: 10.1080/10942912.2017.1354017.
10. Xia EQ, Deng GF, Guo YJ, Li HB. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11(2):622–646. doi:10.3390/ijms11020622
11. De Giani A, Pagliari S, Zampolli J, et al. Characterization of the biological activities of a new polyphenol-rich extract from cinnamon bark on a probiotic consortium and its action after enzymatic and microbial fermentation on colorectal cell lines. Foods. 2022;11(20):3202. doi:10.3390/foods11203202
12. Shahed-Al-Mahmud M, Shawon M, Ahmed J, Islam T, Rahman M. In vivo anti-diarrheal activity of methanolic extract of Streblus asper leaves stimulating the Na+/K+-ATPase in Swiss albino rats. Indian J Clin Biochem. 2020;35(1):72–79. doi:10.1007/s12291-018-0781-7
13. Shahed-Al-Mahmud M, Jahan T, Islam T. Antidiarrheal activities of hydroalcoholic extract of Sida cordifolia roots in Wister albino rats. Orient Pharm Exp Med. 2018;18(1):51–58. doi:10.1007/s13596-017-0295-5
14. Denev P, Kratchanova M, Ciz M, et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014;157:37–44. doi:10.1016/j.foodchem.2014.02.022
15. Lamichhane G, Pandey PR. Regulatory aspects of nutraceuticals and functional foods in Nepal. Int J Nutraceuticals Funct Foods Novel Food. 2020. doi:10.17470/NF-020-0025
16. Kunwar RM, Baral B, Luintel S, et al. Ethnomedicinal landscape: distribution of used medicinal plant species in Nepal. J Ethnobiol Ethnomed. 2022;18(1):1–11. doi:10.1186/s13002-022-00531-x
17. Farnsworth NR. Ethnopharmacology and drug development. In: Ethnobotany and the Search for New Drugs. Vol. 185. John Wiley & Sons, Ltd.; 1994:42–51.
18. Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 2020;19(5):1199–1209. doi:10.1007/s11101-019-09629-9
19. Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. Medicinal plants: past history and future perspective. J HerbMed Pharmacol. 2018;7(1):1–7. doi:10.15171/jhp.2018.01
20. Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(suppl 1):69–75. doi:10.1289/ehp.01109s169
21. Singh YD, Panda MK, Satapathy KB. Ethnomedicine for drug discovery. In: Advances in Pharmaceutical Biotechnology. Springer; 2020:15–28.
22. Pandeya PR, Lamichhane R, Lamichhane G, et al. 18KHT01, a potent anti-obesity polyherbal formulation. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.807081
23. Pandeya PR, Lamichhane G, Lamichhane R, et al. Antiobesity activity of two polyherbal formulations in high-fat diet-induced obese C57BL/6J mice. Biomed Res Int. 2022;2022:1–12. doi:10.1155/2022/9120259
24. Pandeya PR, Lamichhane R, Lee K-H, Lamichhane G, Kim S-G, Jung H-J. Efficacy of a novel herbal formulation (F2) on the management of obesity: in vitro and in vivo study. Evid Based Complement Alternat Med. 2021;2021:1–14. doi:10.1155/2021/8854915
25. Pandeya PR, Lee K-H, Lamichhane R, Lamichhane G, Poudel A, Jung H-J. Evaluation of anti-obesity activity of an herbal formulation (F2) in DIO mice model and validation of UPLC-DAD method for quality control. Appl Sci. 2021;11(16):7404. doi:10.3390/app11167404
26. Sati SC, Sati N, Sati OP. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn Rev. 2011;5(10):174. doi:10.4103/0973-7847.91115
27. Kumar MBS, Kumar MCR, Bharath AC, et al. Screening of selected biological activities of Artocarpus lakoocha Roxb (Moraceae) fruit pericarp. J Basic Clin Pharm. 2010;1(4):239.
28. Sathiyaraj G, Muthukumar T, Ravindran KC. Ethnomedicinal importance of fern and fern allies traditionally used by tribal people of Palani Hills (Kodaikanal), Western Ghats, South India. J Med Herbs Ethnomed. 2015;1:4–9. doi:10.5455/jmhe.2015-07-08
29. Pande PC, Tiwari L, Pande HC. Ethnoveterinary Plants of Uttaranchal- A Review. CSIR; 2007.
30. Ferdous MR, Ashrafudolla M, Hossain MS, Bellah SF. Evaluation of antioxidant, analgesic and antidiarrheal activities of methanolic extract of Litsea monopetala (roxb.) leaves. Clin Pharmacol Biopharm. 2018;7(3):185. doi:10.4172/2167-065X.1000185
31. Adhikari M, Thapa R, Kunwar RM, Devkota HP, Poudel P. Ethnomedicinal uses of plant resources in the Machhapuchchhre rural municipality of Kaski District, Nepal. Medicines. 2019;6(2):69. doi:10.3390/medicines6020069
32. Subba B, Basnet P. Antimicrobial activity of some medicinal plants from east and central part of Nepal. Int J Appl Sci Biotechnol. 2014;2(1):88–92. doi:10.3126/ijasbt.v2i1.9697
33. Joshi AR, Joshi K. Indigenous knowledge and uses of medicinal plants by local communities of the Kali Gandaki Watershed Area, Nepal. J Ethnopharmacol. 2000;73(1–2):175–183. doi:10.1016/S0378-8741(00)00301-9
34. Yadav D, Kushwaha V, Saxena K, Verma R, Murthy PK, Gupta MM. Diarylheptanoid compounds from Alnus nepalensis express in vitro and in vivo antifilarial activity. Acta Trop. 2013;128(3):509–517. doi:10.1016/j.actatropica.2013.07.015
35. Likhitwitayawuid K. Oxyresveratrol: sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules. 2021;26(14):4212. doi:10.3390/molecules26144212
36. Arfan M, Amin H, Kosinska A, Karamac M, Amarowicz R. Antioxidant activity of phenolic fractions of Litsea monopetala [Persimon-leaved Litsea] bark extract. Pol J Food Nutr Sci. 2008;58(2):1.
37. Wu Z-Y, Li H-Z, Wang W-G, et al. Lyonin A, a new 9, 10-secograyanotoxin from Lyonia ovalifolia. Chem Biodivers. 2011;8(6):1182–1187. doi:10.1002/cbdv.201000188
38. Bhatnagar S, Sahoo S, Mohapatra AK, Behera DR. Phytochemical analysis, antioxidant and cytotoxic activity of medicinal plant Combretum roxburghii (Family: Combretaceae). Int J Drug Dev Res. 2012;4(1):193–202.
39. Michiu D, Socaciu M-I, Fogarasi M, et al. Implementation of an analytical method for spectrophotometric evaluation of total phenolic content in essential oils. Molecules. 2022;27(4):1345. doi:10.3390/molecules27041345
40. Mammen D, Daniel M. A critical evaluation on the reliability of two aluminum chloride chelation methods for quantification of flavonoids. Food Chem. 2012;135(3):1365–1368. doi:10.1016/j.foodchem.2012.05.109
41. Rahman M, Islam M, Biswas M, Khurshid Alam AHM. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015;8(1):1–9. doi:10.1186/s13104-015-1618-6
42. Tekwu EM, Pieme AC, Beng VP. Investigations of antimicrobial activity of some Cameroonian medicinal plant extracts against bacteria and yeast with gastrointestinal relevance. J Ethnopharmacol. 2012;142(1):265–273. doi:10.1016/j.jep.2012.05.005
43. Alhallaf W, Perkins LB. The anti-inflammatory properties of chaga extracts obtained by different extraction methods against LPS-Induced RAW 264.7. Molecules. 2022;27(13):4207. doi:10.3390/molecules27134207
44. Tan HY, Tse IMY, Li ETS, Wang M. Oxyresveratrol supplementation to C57bl/6 mice fed with a high-fat diet ameliorates obesity-associated symptoms. Nutrients. 2017;9(2):147. doi:10.3390/nu9020147
45. Buddhisuharto AK, Pramastya H, Insanu M, Fidriann I. An updated review of phytochemical compounds and pharmacology activities of Artocarpus genus. Biointerface Res Appl Chem. 2021;11:14898–14905.
46. Khanal S, Bhatt BD. Study on biological activity of Litsea monopetala from Panchthar district of Nepal. J Inst Sci Technol. 2020;25(2):113–118. doi:10.3126/jist.v25i2.33747
47. Wang Y-S, Wen Z-Q, Li B-T, Zhang H-B, Yang J-H. Ethnobotany, phytochemistry, and pharmacology of the genus Litsea: an update. J Ethnopharmacol. 2016;181:66–107. doi:10.1016/j.jep.2016.01.032
48. Yadav D, Singh SC, Verma RK, et al. Antifilarial diarylheptanoids from Alnus nepalensis leaves growing in high altitude areas of Uttarakhand, India. Phytomedicine. 2013;20(2):124–132. doi:10.1016/j.phymed.2012.10.017
49. Vanucci-Bacqué C, Bedos-Belval F. Anti-inflammatory activity of naturally occuring diarylheptanoids- A review. Bioorg Med Chem. 2021;31:115971. doi:10.1016/j.bmc.2020.115971
50. Saxena A, Yadav D, Maurya AK, et al. Diarylheptanoids from Alnus nepalensis attenuates LPS-induced inflammation in macrophages and endotoxic shock in mice. Int Immunopharmacol. 2016;30:129–136. doi:10.1016/j.intimp.2015.12.002
51. Saxena A, Yadav D, Mohanty S, et al. Diarylheptanoids rich fraction of Alnus nepalensis attenuates malaria pathogenesis: in-vitro and in-vivo study. Phytother Res. 2016;30(6):940–948. doi:10.1002/ptr.5596
52. Sandouk T, Reda D, Hofmann C. Antidiabetic agent pioglitazone enhances adipocyte differentiation of 3T3-F442A cells. Am J Physiol Cell Physiol. 1993;264(6):C1600–C1608. doi:10.1152/ajpcell.1993.264.6.C1600
53. Shang W, Yang Y, Jiang B, et al. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ2 and C/EBPα gene expression. Life Sci. 2007;80(7):618–625. doi:10.1016/j.lfs.2006.10.021
54. Zhao D-R, Su L-H, Li R-T, Chen X-Q, Li H-M. Chemical constituents from the twigs and leaves of Lyonia ovalifolia. Biochem Syst Ecol. 2018;78:1–4. doi:10.1016/j.bse.2018.02.006
55. Zhang H, Zheng X, Zheng G, Teng Y, Zhou J, Yao G. Chemical constituents from the leaves of Lyonia ovalifolia var. hebecarpa. Biochem Syst Ecol. 2020;92:104129. doi:10.1016/j.bse.2020.104129
56. Hussain N, Hameed A, Ahmad MS, et al. New iridoids from Lyonia ovalifolia and their anti-hyperglycemic effects in mice pancreatic islets. Fitoterapia. 2018;131:168–173. doi:10.1016/j.fitote.2018.08.016
57. Negi R, Negi YK, Uniyal V, Saxena S, Bisht S. Phytochemical analysis and antibacterial activity of three indigenous plants of Garhwal Himalaya against some pathogenic microorganisms. J Pharm Res. 2012;5(3):1583–1586.
58. Lv X-J, Li Y, Ma S-G, et al. Bioactive megastigmane glucosides and monoterpenes from Lyonia ovalifolia. J Asian Nat Prod Res. 2019;21(6):559–572. doi:10.1080/10286020.2018.1509313
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.