Back to Journals » International Journal of Nanomedicine » Volume 18
Screening, Expression and Identification of Nanobody Against Monkeypox Virus A35R
Authors Meng N , Cheng X , Sun M , Zhang Y , Sun X, Liu X, Chen J
Received 20 July 2023
Accepted for publication 24 November 2023
Published 5 December 2023 Volume 2023:18 Pages 7173—7181
DOI https://doi.org/10.2147/IJN.S431619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Ni Meng, Xiaolong Cheng, Mengyao Sun, Yushan Zhang, Xueke Sun, Xifu Liu, Jing Chen
Ministry of Education Key Laboratory of Molecular and Cellular Biology; Hebei Anti-Tumor Molecular Target Technology Innovation Center; Hebei Research Center of the Basic Discipline of Cell Biology; College of Life Science, Hebei Normal University, Shijiazhuang, 050024, People’s Republic of China
Correspondence: Jing Chen; Xifu Liu, Email [email protected]; [email protected]
Introduction: The monkeypox (Mpox) virus epidemic presents a significant risk to global public health security. A35R, a crucial constituent of EEV, plays a pivotal role in virus transmission, serves as a vital target for vaccine development, and has potential for serological detection. Currently, there is a dearth of research on nanobodies targeting A35R. The purpose of this study is to identify specific nanobodies target A35R, so as to provide new antibody candidates for Mpox vaccine development and diagnostic kit development.
Methods: Three nanobodies specific to the monkeypox virus protein A35R were screened from a naïve phage display library. After four rounds of panning, positive phage clones were identified by enzyme-linked immunosorbent assay (ELISA). Further, the nanobody fusion protein was constructed in pNFCG1-IgG1-Fc vector and expressed in HEK293F cells and purified by affinity chromatography. The specificity and affinity of the nanobodies were identified by ELISA. The binding kinetics of the VHH antibody to A35R were assessed via employment of a bio-layer interferometry (BLI) apparatus, thereby determining the nanobodies affinity.
Results: The three purified nanobodies showed specific high-affinity binding MPXV A35R, of them, VHH-1 had the best antigen binding affinity (EC50 = 0.010 ug/mL). In addition, VHH-1 on Protein A biosensor can bind Mpox virus A35R, with an affinity constant of 54 nM as determined in BLI assay.
Conclusion: In sum, we has obtained three nanobody strains against Mpox virus A35R with significant affinity and specificity, therefore laying an essential foundation for further research as well as the applications of diagnostic and therapeutic tools of Mpox virus.
Keywords: monkeypox virus, A35R, nanobody, phage display library
Introduction
Monkeypox (Mpox) is a zoonotic infectious disease, whose virus is a member of the Orthopoxvirus genus.1 The recent Mpox virus outbreak is the largest recorded outbreak in non-endemic countries to date. As of October, 2023, with a cumulative total of more than 91,000 confirmed cases and about 160 deaths, among which the United States has reported more than 30,600 monkeypox cases, ranking first in the world (https://ourworldindata.org/explorers/monkeypox). The re‐emergence of Mpox virus and the rising incidence in non‐endemic countries is turning into an upcoming threat to global health.2 Detecting Mpox virus nucleic acids through polymerase chain reaction (PCR) is the primary method of diagnosing Mpox virus infection.3
Orthopoxvirus infection or immunity may confer immunity to other viruses of the genus. As a result, following the monkeypox outbreak, the FDA has approved two vaccines to prevent Mpox virus: ACAM2000 (a second-generation live VACV vaccine) and JYNNEOS (a third-generation attenuated vaccine based on modified Ankara Vaccinia (MVA)). The two VAVC-based smallpox vaccines have cross-protective effects against Mpox virus.4 According to the recent study, the smallpox vaccine does not fully protect against MPOX during the current outbreak.5 In addition, ACAM2000 is a highly regenerative VACV vaccine that can cause serious adverse events: systemic vaccinia, vaccinia eczema, progressive vaccinia, myocarditis, and death.6 On the other hand, JYNNEOS, a replication-deficient vaccine, is expected to have the lowest rate of serious adverse events.7 However, the immune targets of JYNNEOS and ACAM2000 are unclear, and the effects of their gene products on immunity and adverse reactions are unknown. Therefore, it is imperative to develop new vaccines specific to Mpox virus to ensure full protection and safety.
The gene of the Mpox virus is approximately 197,205 base pairs in length, featuring variable regions at both termini and a highly conserved central genomic region in the middle.8 The central genomic regions harbor crucial enzymes, including DNA polymerases and capping enzyme, as well as structural proteins such as M1R, E8L, H3L, A29L, A35R, and B6R. There are two different forms of Mpox virus infection during replication: extracellular enveloped virus (EEV) and intracellular mature virus (IMV). EEV form that is dominant during inter-host transmission, which expresses approximately 25 membrane proteins on the mature virion, including A35R and B6R; IMV form that is dominant during intra-host transmission, additional 6 proteins on the enveloped virion, including M1R and A29L proteins.9,10 A35R is homologous to VACV outer membrane-specific protein A33R, with viral nucleotide sequence similarity of 95.03%.8 As information about A35R is scarce, we draw parallels from VACV A33R. There is a hypothetical C-type lectin domain in A33R protein, which makes it a type II membrane glycoprotein.11 A33R is an envelope virus-specific protein that is expressed early and late after infection, which is necessary for effective cell-to-cell transmission.9,12 The formation of the A33R, A34R, and B5R complex plays a crucial role in the liberation of viral particles, and the lack of A33R significantly reduces the infectivity of VACV.13 A33R is a potential target for antibody neutralization of vaccinia virus, and experiments in animal models have confirmed that A33R vaccine has a protective effect and can produce neutralizing antibodies after inoculation.14,15 A35R is an important component of EEV and plays an important role in Mpox virus transmission. mRNA vaccines targeting A35R also showed good results. The quadrivalent mRNA vaccine targeting A29L, A35R, M1R and B6R induces immune response and has a good protective effect against vaccinia virus. In addition, lipid nanoparticle encapsulated mRNA vaccines (M1R, E8L, A29L, A35R, B6R) and other viral antigen combination candidate vaccines have shown superior ability to induce neutralizing antibodies and protect mice from VACV attack.12,16–20 In conclusion, the Mpox virus A35R is an important target for vaccine development and a potential target for serological detection.
A unique type of antibody composed completely of heavy chains without light chains is expressed by camelids (dromedaries, llamas, alpacas, etc.), called heavy-chain antibodies (HCAbs).21 Nanobody refers to the variable domain of heavy-chain antibody (VHH), which is the smallest complete functional structure of heavy-chain antibody. The nanobody has the same antigen-binding activity and stability as the original heavy-chain antibody, and its molecular weight is only 1/10 of that of monoclonal antibody, which is the smallest unit known to bind the target antigen.22 Nanobodies have a longer complementarity determining region 3 (CDR3) than that of humans, which can form a finger-like structure and bind to epitopes that traditional antibodies are not accessible.23 Nanobodies have demonstrated their benefit for more sensitive diagnostic tests.
As research on nanobodies progresses, their potential applications are expanding. In addition to, their demonstrated efficacy in tumor treatment and prevention, nanobodies have shown promise in the prevention and treatment of virulent viruses.24–26 It has been found that nanobodies screened specific for the receptor-binding domain of MERS-CoV spike protein can effectively cross-neutralize the infection of multiple MERS-CoV strains isolated from human and camel.27 In addition, for the novel influenza A virus, a novel approach has been developed using nano-antibodies: nano-antibodies targeting exposed viral antigens can recruit innate immune cells to the viral site for replication, and such nanobodies can trigger protective antiviral activity rather than directly neutralizing the virus.28 Due to the uniqueness and superiority of the structure and function of nano-antibodies, it is widely concerned that new interventions can be developed against other emerging pathogens, and nanobodies have good application prospects in immune detection such as ELISA, fluorescence immunoassay, immunochromatography, and electrochemical luminescence immunoassay.29
To screen for unknown anti-A35R antibodies, we used a naïve phage display VHH library. After four rounds of panning, preliminarily screened three Mpox virus protein A35R nanobodies with different gene sequences. The three nanobodies identified by the enzyme-linked immunosorbent assay (ELISA) had excellent specificity and affinity. The results of this study can facilitate the clinical detection, diagnosis, and treatment of Mpox virus, as well as related downstream research.
Materials and Methods
Reagents and Materials
The Mpox virus A35R protein was purchased from ACROBiosystems (Beijing, China). Monkeypox virus (strain Zaire-96-I-16) A35R, His Tag is expressed from human 293 cells. It contains AA Arg 58 - Thr 181 (Accession # Q8V4U4). The phage nanobody library was constructed by Shenzhen KangTi Biopharm Technology Co., Ltd., which was a natural nanobody library composed of 105 alpaca peripheral blood. Mouse anti-M13 Bacteriophage Antibody with HRP was obtained from Sino Biological (Beijing, China). Isopropyl-beta-D-thiogalactopyranoside (IPTG), tetracycline, ampicillin, kanamycin, PEG8000, trypsin, TMB and 10% AEBSF were purchased from Sangon Biotech (Shanghai, China).
Screening VHH Nanobodies for the Anti-MPV A35R Protein
MPV-A35 protein in carbonate buffer (25μg/mL) was coated on Nunc Immuno MaxiSorbTubes (Thermo Fisher Scientific), overnight at 4°C. The next day, washing with PBS 3 times, then blocking buffer (3% BSA) was added to the tube and incubated at room temperature for 2 h. Washing with PBS 3 times, added the phage library solution and incubated for 1h at room temperature. After washing with PBS with 0.1% Tween-20 (PBST) 20 times to remove the non-specific phage. Bound phages were eluted by 30 min of digestion using 500 μL of 0.25 mg/mL trypsin at room temperature with rotating. The reaction was stopped with 10 μL 10% AEBSF. The eluted phages were used to infect E. coli SS320 for titer determination. The amount of input phage of the next four rounds was 1 × 1012 pfu, and the washing time was 20 times. The output phage titer of every round of panning was measured.
Phage ELISA
Seventy-two phage clones were randomly selected during the final round of panning, and their antigen-binding ability was analyzed by ELISA. A MaxiSorb 96-well plate was coated with 100 μL of 1 μg/mL antigen at 4°C overnight. The next day, the plate was washed 3 times with PBS buffer and blocked with 3% BSA buffer at room temperature for 1 h. Washing with PBST 5 times, 25 μL of soluble fragment supernatant in 75 μL of PBS supplemented with 3% (w/v) bovine serum albumin (BSA) was added to each well of the ELISA plate, and the plate was incubated at room temperature for 1 h with gentle agitation. The wells were washed with 5 times with PBST, and 100 μL of 1:10,000 anti-M13-HRP antibody in PBS supplemented with 3% (w/v) BSA was added to each well. The plate was then incubated at room temperature for 1 h with gentle agitation. The wells were washed 5 times with PBST and 1 time with PBS, and 100 μL of TMB solution was added to each well. The reaction was stopped with 50 μL of 1 M sulfuric acid, and the absorbance was read at 450–650 nm in a UV–Vis plate reader.
Construction, Expression, and Purification of the Nanobody
After phagemid extraction, the VHH was amplified via PCR using specific primers (Forward: 5′-CGGAATTCGGCGGTGCAGCTGGTGGAGT-3′, Reverse: 5′- CGGGA TCCATGGTGATGGTGATGATGGCGTGCGCCTGAGGAGACGGT-3′). The VHH gene was cloned into pNFCG1-IgG1-Fc vectors using the EcoRI/NheI restriction site. The construct was transferred into HEK293F. Soluble Fc-tagged nanobodies were purified on a protein A Superflow Sepharose column in binding buffer and was eluted with elution buffer. The purity of the eluted proteins was assessed by 12% SDS-PAGE and Western blot.
Affinity Measurements
The affinity of VHH-A35R was assessed by sandwich ELISA on 96-well plates coated with commercial A35R protein (experimental group) or BSA protein (negative control). Optical density measurement indicated that VHH-A35R bound specifically to the A35R but not BSA protein. In addition, the specificity of the nanobodies which have sufficient quantity for subsequent studies was further detected with one irrelevant protein TIM3 by monoclonal phage ELISA, and the concentration for coating was 1 μg/mL. For detection of the sensitivity of the nanobodies, nanobodies at different concentration (10−6 μg/mL−10 μg/mL) was added to an ELISA plate and monoclonal phage ELISA performed as described above.
BLI Cross-Competitive Binding Assay for Nanobodies
Affinity determination by bio-layer interferometry (BLI) affinity assays were performed on a ForteBio Octet2 biolayer interferometry instrument (Molecular Devices ForteBio LLC, Fremont, CA) at 25°C with shaking at 1000 rpm. To measure the affinity of Nanobodies with A35R, anti-human Fc (AHC) biosensors (cat.# 18-5060, Fortebio) were hydrated in water for 30 min prior to 60s (sec) incubation in a kinetic buffer (PBS, 0.02% (v/v) Tween-20, pH 7.0). Purified Nb-Fcs were loaded in a kinetic buffer for 200s prior to baseline equilibration for 200s in a kinetic buffer. Association of Mpox virus A35R in a two-fold dilution series from 0.625ug/mL to 10 ug/mL was performed prior to dissociation for 180s. Association of nanobodies in a serial dilution was performed prior to dissociation for 180s. After each cycle, the biosensors were regenerated via 3 short pulses of 5s each of 100 mM pH 2.7 glycine-HCl followed by running buffer. The data were baseline subtracted before fitting performed using a 1:1 binding model and the ForteBio data analysis software. KD, Ka and Kd values were evaluated with a global fit applied to all data.
Structure Determination
Homology modeling is extensively used to generate a valid structure of protein utilizing the amino acid sequence. We used the SWISS-MODEL (https://swissmodel.expasy.org/) server for the generation of the homology model of the VHH-A35R Nanobodies. We selected the PDB ID of 3K7B to be the antigen of the three-dimensional x-ray crystallographic structure of A35R. The protein sequence of nanobodies was given in the SWISS-MODEL server. After preparing the 3D structure of the protein by SWISS-MODEL server, we downloaded the PDB file, and it was used for the molecular docking. Rigid protein–protein docking (ZDOCK) was performed between A35R and VHH to study the relationships. We developed our complex structural model of the VHH-A35R nanobodies interactions on the antibody–antigen docking program ZDOCK by using default parameters and evaluating shape complementarity and desolvation energy to obtain VHH-A35R nanobody complexes. To analyze the epitope of A35R which the VHH nanobodies interacted, we used a Pymol program to analysis the VHH nanobodies complexes.
Competition Enzyme-Linked Immunosorbent Assay
The predicted epitope of A35R was synthesized via a chemical approach. Competitive ELISA assays were utilized to verify the accuracy of the predicted epitopes. A35R protein was individually coated on a 96-well microplate at 4°C overnight. Different concentrations (0.01ng/mL to 10,000 ng/mL) of TTWLIDYVE and HEKESCN-GLYYQ were added to 0.005μg/mL VHH-1 and VHH-2 for incubation, respectively. After blocking with 2% MPBS (0.2% Milk), mixtures of peptide and antibody were added in the 96-well microplate. Next, goat anti-human IgG Fc cross-adsorbed secondary antibody. HRP (Invitrogen, 1:10,000) was used to bind biotin-conjugated peptides for detection. Finally, a TMB substrate was added for development, and the reaction was stopped by adding 1 M H2SO4. The absorbance was measured at 450 nm.
Statistical Analysis
All data were expressed as mean ± standard deviation (S.D.). Statistical analysis was conducted using one-way analysis of variance (ANOVA) among the treatment and control groups. p < 0.01 was considered statistically significant.
Results
Screening of Special VHH Against A35R
To isolate antibody clones that bind to the Mpox virus A35R, a Naïve VHH library was used for the panning against the A35R protein. The panning was performed for a total of four rounds with enrichment of VHH clones against A35R protein. Successful enrichment of the antibody binders is evidenced by the apparent increase of phage recovery at fourth panning (Figure 1A). Seventy-two individual clones were randomly picked after the fourth round of panning. The individual clones were grown and nanobodies were induced and expressed with IPTG, and periplasmic extracts were tested in ELISA against recombinant A35R protein. Eighteen individual clones were considered positive colonies and sent for sequencing since the ratio between the signal in wells with coated antigen and background signal was more than 10-fold (Figure 1B). A framework and complementarity determining region for nanobodies was identified using the international immunogenetics information system (IMGT). Among 18 positive clones, 3 distinct VHH fingerprints were identified, based on their unique CDR3 (Figure 1C). The amino acid sequences of CDR1, CDR2, and FRs were also different among different groups.
Expression and Specificity of the Nanobodies
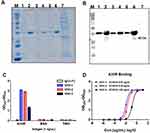
All the nanobodies were expressed with a C-terminal human Fc-tag for purification. Three of the nanobodies with different amino acid sequences of CDR3 were expressed successfully by the eukaryotic expression system with pNFCG1-Fc. The vectors were transfected into HEK293F cells using the FectoPRO transfection reagent (Polyplus-transfection) as per the manufacturer’s instructions. After 5 days of culture, supernatants of cell culture were harvested, and antibodies were purified by Protein A column chromatography. The SDS-PAGE and Western blot showed that the recombinant proteins had single bands with high purity. The molecular weights of nanobodies were 40 kDa (Figure 2A and B). The results showed that three nanobodies had a specific binding with the tag antibody.
The specificities of the three nanobodies were determined via ELISA by comparison with other proteins, including BSA and TIM3. The results showed a specific binding activity with Mpox virus A35R with no other cross-activity detected in the ELISA results (Figure 2C). The affinity of three recombinant proteins to A35R was detected by ELISA. VHH-1 and VHH-2 showed a high absorbance value at 450 nm, and the EC50 of nanobodies was lower than 0.126 ng/uL, demonstrating that the selected nanobodies had a high affinity to Mpox virus A35R (Figure 2D). Among the three nanobody strains, VHH-1 had the highest binding affinity to MPOX-A35R whose EC50 = 0.01 ng/uL.
The Affinity and Binding Epitopes of Nanobodies
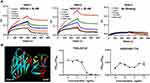
Due to the potency of nanobodies being greatly affected by their affinity to antigen. The binding kinetics of the three nanobodies were measured using SPR assays with the purified A35R. The results showed that the VHH-1 and VHH-2 have affinities to A35R with KD values ranging from 50 nM to 120 nM (Figure 3A).
To characterize the binding epitopes between the nanobodies and A35R, we performed molecular docking between nanobodies and A35R using the computer molecular simulation software ZDOCK to predict possible positions where VHH-1 region binding may occur on A35R proteins. The yellow portion of the A35R sequence in the prediction model suggests the presence of a potential binding epitope between VHH-1 and A35R. In order to further confirm the binding of VHH-1 to the predicted key sites, two peptides were synthesized based on the corresponding amino acid sequence to mimic the epitope on A35R molecules. These peptides were then tested for their binding with VHH-1. The competitive ELISA assay demonstrated that the binding of VHH-1 antibody to A35R was inhibited by the peptide TTWLIDYVE, while other peptides did not show any inhibitory effects (Figure 3B). These experimental findings indicate that TTWLIDYVE may serve as the antigen epitope for VHH-1. The binding sites of VHH-2 and VHH-3 antibodies were simulated; however, the competitive ELISA detection results indicated inaccuracies in the predicted binding sites. Subsequently, future investigations will persist in the pursuit of identifying the binding sites of antigens and antibodies.
Discussion
The emergence of Mpox virus has posed unprecedented threats to human health, leading to a global concern. Recently, more cases of Mpox virus infection were found in China.30 No effective therapy has been established for the management of Mpox virus infection in humans, aside from supportive treatment. Consequently, it is imperative to develop novel therapeutic drugs to combat Mpox virus. Numerous orthopoxvirus-specific monoclonal antibodies (mAbs) and mRNA vaccines have been created, primarily targeting M1R, E8L, H3L, A29L, A35R, and B6R.17–19,31,32 Nanobodies are generally regarded as a substitute for conventional antibodies because of their unique structure and chemical stability and become an emerging force in therapeutic drugs and clinical diagnostic reagents. However, as of now, the existence of nanobodies specifically targeting monkeypox virus antigens remains unreported.
The Mpox virus nucleotide sequence of the viral central genomic region shares 96.3% identity with that of VACV.8 A33R is a functional protein in the progression of orthopoxviruses, which is implicated in the rapid spread of the virus from cell to cell.13 When complement is present, A33R neutralizes antibody responses against EEV. A35R, which exhibits a viral nucleotide sequence similarity of 95.03% with VACV protein A33R, is identified as its homologous counterpart. Despite the significant homology between A33R in VACV and A35R in Mpox virus, these dissimilarities have the potential to impact the cross-protection of smallpox vaccines against Mpox virus. For example, the monoclonal antibody (1G10) demonstrates a strong binding affinity exclusively to VACV A33R while lacking the ability to bind to Mpox virus A35R.33 Hence, the acquisition of A35R antibodies is imperative as they hold potential as novel therapeutics or antiviral candidates. We have developed three nanobodies specific to the Mpox virus protein A35R and used the eukaryotic expression system to express them successfully. Moreover, we demonstrated that the nanobody of VHH-1 recognizes antigenic epitopes on monkeypox virus protein A35R, providing a new idea and scientific support for developing diagnostic methods of Mpox virus.
Limitation
Here, some limitations should be considered in this study. Firstly, the Mpox live virus neutralization assay is the gold standard for measuring antibody neutralization ability. However, it must be carried out in a biosafety level-3 laboratory. Secondly, the binding of the nanobodies to Chordopox virus A33R was not tested, and the epitope diversity and function of the nanobodies obtained need to be further explored. In addition, since nanobodies can be easily fused and modified by genetic engineering technology, we will develop nanobody complexes with Mpox A35R-specific nanobodies as the core of the technology.
Conclusion
In summary, our study developed three nanobodies against Mpox virus A35R from a non-immunized alpaca VHH library. Of them, VHH-1 exhibited the significant affinity and specificity against Mpox virus A35R. Therefore, laying an essential foundation for further research as well as the applications of diagnostic and therapeutic tools of Mpox virus.
Funding
This work was supported by the National Natural Science Foundation of China (No. 12204147), Natural Science Foundation of Hebei Province (C2023205003) and Science Foundation of Hebei Normal University (No. L2020B16).
Disclosure
Drs Jing Chen reports a patent 2023109114793 pending to Hebei Normal University. The authors declare no other conflicts of interest in this work.
References
1. Harris E. What to Know About Monkeypox. JAMA. 2022;327(23):2278–2279. doi:10.1001/jama.2022.9499
2. Saied AA, Metwally AA, Choudhary OP. Monkeypox: an extra burden on global health. Int J Surg. 2022;104:106745.
3. Nakhaie M, Arefinia N, Charostad J, et al. Monkeypox virus diagnosis and laboratory testing. Rev Med Virol. 2023;33(1):e2404. doi:10.1002/rmv.2404
4. Ahmed SF, Sohail MS, Quadeer AA, et al. Vaccinia-virus-based vaccines are expected to elicit highly cross-reactive immunity to the 2022 monkeypox virus. Viruses. 2022;14(9):1960. doi:10.3390/v14091960
5. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387(8):679–691. doi:10.1056/NEJMoa2207323
6. Meo SA, Al-Masri AA, Klonoff DC, et al. Comparison of biological, pharmacological characteristics, indications, contraindications and adverse effects of JYNNEOS and ACAM2000 monkeypox vaccines. Vaccines. 2022;10(11):1971. doi:10.3390/vaccines10111971
7. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(22):734–742. doi:10.15585/mmwr.mm7122e1
8. Wang Y, Yang KW, Zhou H. Immunogenic proteins and potential delivery platforms for mpox virus vaccine development: a rapid review. Int J Biol Macromol. 2023;245:125515.
9. Yefet R, Friedel N, Tamir H, et al. Monkeypox infection elicits strong antibody and B cell response against A35R and H3L antigens. iScience. 2023;26(2):105957. doi:10.1016/j.isci.2023.105957
10. Shchelkunov SN, Totmenin AV, Safronov PF, et al. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–194. doi:10.1006/viro.2002.1446
11. Su H-P, Singh K, Gittis AG, et al. The structure of the poxvirus A33 protein reveals a dimer of unique C-type lectin-like domains. J Virol. 2010;84(5):2502–2510. doi:10.1128/JVI.02247-09
12. Hou F, Zhang Y, Liu X, et al. Novel mRNA vaccines encoding Monkeypox virus M1R and A35R protect mice from a lethal virus challenge. bioRxiv. 2022. doi:10.1101/2022.11.19.517190
13. Monticelli SR, Earley AK, Stone R, et al. Vaccinia virus glycoproteins A33, A34, and B5 form a complex for efficient endoplasmic reticulum to trans-golgi network transport. J Virol. 2020;94(7). doi:10.1128/JVI.02155-19
14. Fogg C, Lustig S, Whitbeck JC, et al. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78(19):10230–10237. doi:10.1128/JVI.78.19.10230-10237.2004
15. Hooper JW, Custer DM, Schmaljohn CS, et al. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266(2):329–339. doi:10.1006/viro.1999.0096
16. Zhang -R-R, Wang Z-J, Zhu Y-L, et al. Rational development of multicomponent mRNA vaccine candidates against mpox. Emerg Microbes Infect. 2023;12(1):2192815. doi:10.1080/22221751.2023.2192815
17. Zhang N, Cheng X, Zhu Y, et al. Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity. Sci China Life Sci. 2023;66:1–13.
18. Sang Y, Zhang Z, Liu F, et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct Target Ther. 2023;8(1):172. doi:10.1038/s41392-023-01432-5
19. Fang Z, Monteiro VS, Renauer PA, et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023;33(5):407–410. doi:10.1038/s41422-023-00792-5
20. Mucker EM, Thiele-Suess C, Baumhof P, et al. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol Ther Nucleic Acids. 2022;28:847–858. doi:10.1016/j.omtn.2022.05.025
21. Strack R. Nanobodies made versatile. Nat Methods. 2023;20(1):37. doi:10.1038/s41592-022-01757-z
22. Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82(1):775–797. doi:10.1146/annurev-biochem-063011-092449
23. Muyldermans S. Applications of nanobodies. Annu Rev Anim Biosci. 2021;9(1):401–421. doi:10.1146/annurev-animal-021419-083831
24. Saied AA, Metwally AA, Alobo M, et al. Bovine-derived antibodies and camelid-derived nanobodies as biotherapeutic weapons against SARS-CoV-2 and its variants: a review article. Int J Surg. 2022;98:106233. doi:10.1016/j.ijsu.2022.106233
25. Lu Y, Li Q, Fan H, et al. A multivalent and thermostable nanobody neutralizing SARS-CoV-2 Omicron (B.1.1.529). Int J Nanomedicine. 2023;18:353–367. doi:10.2147/IJN.S387160
26. Li M, Ren Y, Aw ZQ, et al. Broadly neutralizing and protective nanobodies against SARS-CoV-2 Omicron subvariants BA.1, BA.2, and BA.4/5 and diverse sarbecoviruses. Nat Commun. 2022;13(1):7957. doi:10.1038/s41467-022-35642-2
27. He L, Tai W, Li J, et al. Enhanced ability of oligomeric nanobodies targeting MERS coronavirus receptor-binding domain. Viruses. 2019;11(2):166. doi:10.3390/v11020166
28. De Vlieger D, Hoffmann K, Van Molle I, et al. Selective engagement of FcgammaRIV by a M2e-specific single domain antibody construct protects against influenza a virus infection. Front Immunol. 2019;10:2920. doi:10.3389/fimmu.2019.02920
29. Yong Joon Kim J, Sang Z, Xiang Y, et al. Nanobodies: robust miniprotein binders in biomedicine. Adv Drug Deliv Rev. 2023;195:114726. doi:10.1016/j.addr.2023.114726
30. Xu T, Zhang LL. Rising prevalence of mpox in China, Japan, and Republic of Korea. J Infect. 2023;87(4):e73–e74. doi:10.1016/j.jinf.2023.07.017
31. Li MJ, Ren Z, Wang Y, et al. Three neutralizing mAbs induced by MPXV A29L protein recognizing different epitopes act synergistically against orthopoxvirus. Emerging Microbes Infect. 2023;12(2). doi:10.1080/22221751.2023.2223669
32. Hou F, Zhang Y, Liu X, et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge. Nat Commun. 2023;14(1):5925. doi:10.1038/s41467-023-41628-5
33. Golden JW, Hooper JW. Heterogeneity in the A33 protein impacts the cross-protective efficacy of a candidate smallpox DNA vaccine. Virology. 2008;377(1):19–29. doi:10.1016/j.virol.2008.04.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.