Back to Journals » Clinical Ophthalmology » Volume 16
Screening and Risk Factors for Retinopathy of Prematurity in a Tertiary Care Hospital in Cairo, Egypt
Authors Noor MS , Elbarbary M, Embabi SN, Zaki MA, Awad H, Al-Feky M
Received 2 August 2022
Accepted for publication 21 September 2022
Published 1 October 2022 Volume 2022:16 Pages 3257—3267
DOI https://doi.org/10.2147/OPTH.S383493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohamed Salaheldeen Noor,1 Magdy Elbarbary,2 Sherif N Embabi,1 Mohamed A Zaki,1 Hisham Awad,1 Mariam Al-Feky1
1Ophthalmology Department, Ain Shams University, Cairo, Egypt; 2Paediatrics Department, Ain Shams University, Cairo, Egypt
Correspondence: Mohamed Salaheldeen Noor, Ophthalmology Department, Ain Shams University, 22 Abdullah Ibn Eltaher street, Makram Edeid, Nasr city, Cairo, Egypt, Tel +201093769896, Email [email protected]
Purpose: To evaluate the retinopathy of prematurity (ROP) prevalence, risk factors and screening outcome in a tertiary hospital in Cairo, Egypt.
Methods: A prospective observational study was done in Neonatal Intensive Care Unit in Ain Shams University Hospital. A total of 159 premature infants were screened for ROP based on the most inclusive criteria reported to date. Screening included premature infants with gestational age (GA) of ≤ 34 weeks or birth weight (BW) of ≤ 2000 grams, or GA > 34 weeks or BW > 2000 grams, with multiple co-morbidities. The prevalence of ROP, plus disease and their correlation with risk factors of interest were studied.
Results: The GA of the included infants ranged from 27 to 36 weeks, mean (SD) 31.87 (± 1.81) weeks. The BW ranged from 640 to 3900 grams, mean (SD) 1784.71 (± 560.30) grams. The prevalence of ROP more than stage 0 was 25.8% (41 infants), 7.3% of the cases (11 infants) showed plus disease and 6.3% (10 infants) showed severe ROP requiring treatment. Of those, 2 cases (20%) fell outside the British Guideline’s criteria for Screening. There was a highly significant (p < 0.0001) correlation between ROP more than stage 0 and low GA, low BW, mechanical ventilation, respiratory distress syndrome, necrotizing enterocolitis, intraventricular haemorrhage, and blood transfusion. No significant correlation was found between appearance of ROP more than stage 0 and gender (p = 0.911), patent ductus arteriosus (p =0.187), or sepsis (p =0.998).
Conclusion: ROP is a significant problem in the premature infants in Egypt. Extremely premature infants with lower BW are more prone to develop ROP. However, cases with higher GA and BW than mentioned in the British guidelines screening criteria especially with multiple comorbidities showed severe ROP requiring intervention, which implies the need to develop a screening guideline for the Egyptian population.
Keywords: retinopathy of prematurity, screening, risk factors, epidemiology, prematurity
Introduction
Retinopathy of prematurity (ROP), initially described as retrolental fibroplasia by Terry in 1942, is a retinal vascular disease that affects premature infants1 and is characterised by abnormal retinal fibrovascular proliferation which can lead to total retinal detachment and blindness if no intervention was done in the critical treatment period. Under normal conditions, the relatively hypoxic intrauterine environment allows normal retinal vascularization. However, after birth, the hyperoxic environment—including room air—suppresses the growth of retinal vessels and results in incomplete retinal vascularization (ROP Phase 1). With the increased metabolic demand by the growing retina and incomplete retinal vascularisation, further retinal hypoxia occurs which potentiates ROP development (ROP Phase 2).2 With increased survival of preterm infants, ROP has become the leading cause of preventable blindness in children, especially in developed countries.3
Reported risk factors for ROP include low gestational age (GA), low birth weight (BW), high level of supplemental oxygen, and the presence of comorbidities such as patent ductus arteriosus (PDA), necrotising enterocolitis (NEC), intraventricular haemorrhage (IVH), neonatal sepsis, anaemia, and low energy intake and low weight gain in early life.4,5 Therefore, several guidelines were developed to determine the infants to be screened for ROP, including the British Royal College of Paediatrics and Child Health in 2008 which recommended screening premature infants with GA of <32 weeks or BW of <1501 g.6 On the other hand, the American Academy of Pediatrics (AAP) in 2013 recommended screening for ROP if GA was ≤30 weeks or BW ≤1500 g. with screening of selected infants with BW of 1500 to 2000 g or GA of >30 weeks with an unstable clinical course after assessment by the attending neonatologist.7
Studies conducted in developing countries have demonstrated that the average birth weight and gestational age for infants with ROP was higher than what is reported in the literature for infants with ROP in developed countries, which implies the need for different screening criteria in developing countries.8–10 ROP was classified into five stages depending on the severity of retinal fibrovascular proliferation and into three zones depending on the location of disease according to the most recent International Classification of Retinopathy of Prematurity, 3rd edition (ICROP3).12 Plus disease was determined as the sign of severity of the disease, in which there is severe retinal venous dilatation and arteriolar tortuosity, iris vascular engorgement, poor pupillary dilatation, and vitreous haze.11 Aggressive posterior ROP, which is a severe form of ROP, is characterised by posterior vascularisation, prominent plus disease, and rapid progression.11 With the most recent International Classification of Retinopathy of Prematurity, 3rd edition (ICROP3), the term aggressive ROP (A-ROP) replaced aggressive-posterior ROP (AP-ROP). This decision was made “because of increasing recognition that aggressive disease may occur in larger preterm infants and beyond the posterior retina, particularly in regions of the world with limited resources.”12
There is a narrow window for intervention in cases of severe ROP to prevent retinal detachment and vision loss. Early Treatment for Retinopathy of Prematurity (ETROP) developed guidelines that suggest intervention if the ROP is type 1 pre-threshold, which is 1) zone I, any stage of ROP, with plus disease; 2) zone I, stage 3 ROP, with or without plus disease; or 3) zone II, stage 2 or 3 ROP, with plus disease,13 and close follow-up if ROP is type 2 pre-threshold, which is 1) zone I, stage 1 or 2 without plus; 2) zone II, Stage 3 without plus.13 Interventions include laser photocoagulation, intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF), and/or vitreoretinal surgery for advanced cases.14
Many studies were done to assess the prevalence and risk factors for ROP in different countries with various outcomes which suggest the need for development of local guidelines, with the reported prevalence rates ranging from 12.6% to 44.5%,15–18 and that of treatment-requiring ROP ranging from 1.5% to 11.7% in other countries.15–18 The aim of this study was to evaluate the ROP prevalence, screening, and risk factors in a tertiary hospital in Cairo, Egypt.
Methods
Study Design and Setting
A prospective observational study was carried out in Neonatal Intensive Care Unit (NICU) in Ain Shams University Hospital. In this study, 159 premature born in the period between 1 September 2018 and 1 September 2021 were screened for retinopathy of prematurity (ROP).
Approval for the study was obtained from the ethical committee at the faculty of medicine, Ain Shams university (Approval Number: FWA: 000017585). Informed consent was taken from the parents of the study participants after explanation of the study benefits and methods of examination. Also, data were de-identified following encoding to ensure confidentiality of study participants. The study complied with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Screening was based on the most inclusive criteria reported to date. All premature infants with gestational age (GA) of ≤34 weeks or birth weight (BW) of ≤2000g were included. Infants were also included if GA >34 weeks or BW >2000 g but multiple co-morbidities existed, such as RDS, PDA, sepsis, NEC, IVH, repeated blood transfusion or unstable clinical course determined by neonatologists. All infants who were lost before sufficient number of ocular examinations could be done either to rule out ROP or assess the progression/regression of the established ROP were excluded from this study.
Screening and Data Recording
Ocular examination was performed using binocular indirect ophthalmoscope, +28 Dioptre lens and a paediatric lid speculum. An indenting instrument was also used to gently rotate or indent the globe for assessment of retinal periphery up to Ora Serrata. Pupil dilatation was done using a combination of Tropicamide 0.5% and phenylephrine 1% eye drops, instilled 4 times (once every 15 minutes) starting 1 hour before ocular examination.
The severity of ROP was identified according to the most recent International Classification of Retinopathy of Prematurity, 3rd edition (ICROP3).12 The first screening was initiated at 4 weeks postnatal age for all infants. After initial examination, infants were divided either they needed treatment within 48 to 72 hours or follow-up according to the Early Treatment of Retinopathy Of Prematurity study (ETROP study).13 The schedule of follow-up visits was determined according to the severity of retinal findings recorded on each visit following the ETROP study protocol.13
Data recording included GA, BW, presence of respiratory distress syndrome (RDS) and its grade based on chest x-ray findings according to the four-stage radiographic scale,19 mechanical ventilation (MV) and its duration, continuous positive airway pressure (CPAP) therapy and its duration, patent ductus arteriosus (PDA), sepsis, necrotizing enterocolitis (NEC) and intraventricular haemorrhage (IVH). In cases that needed blood transfusion, the number of blood transfusions was recorded.
At the end of follow-up period, data recording included the most severe ROP stage during the follow-up period for each examined eye. For the cases that required treatment, postmenstrual age (PMA) at which treatment was initiated was recorded. For all eyes, PMA of full retinal vascularization in close proximity to Ora Serrata was recorded if <60 weeks, otherwise considered persistent avascular retina according to Chiang et al.12
Statistical Analysis
Data were collected, revised, coded, and entered to the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges when parametric and presented as median, interquartile range (IQR) when non-parametric. Also, qualitative variables were presented as number and percentages.
The comparison between groups with qualitative data were done by using Chi-square test. The comparison between two groups with quantitative data and parametric distribution was done by using the independent t-test, while the comparison between two groups with quantitative data and non-parametric distribution was done by using Mann–Whitney test.
The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant if <0.05, and highly significant if <0.01.
Results
Demographic Data
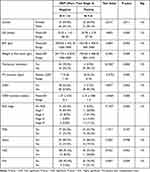
The demographics of the screened infants are shown in Table 1. One-hundred and fifty-nine patients were included in this study, 88 males (55.3%) and 71 females (44.7%). The GA of the included infants ranged from 27 to 36 weeks, with a mean (SD) of 31.87 (± 1.81) weeks. Infants had BW ranging from 640 to 3900 grams, with a mean (SD) of 1784.71 (± 560.30) grams.
 |
Table 1 Distribution of Gender, Gestational Age (GA), Birth Weight (BW), Weight at First Exam and Postmenstrual Age (PMA) at First Exam in the Studied Infants |
Prevalence of Retinopathy of Prematurity
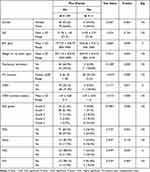
The prevalence of ROP changes more than stage 0 in the screened infants was 25.8% (41 infants). Nine screened infants (5.7%) had complete retinal vascularization at the first examination, while 109 infants (68.6%) had incomplete retinal vascularization at their first examination (stage 0 ROP). Nineteen infants (11.9%) had stage 1 ROP while 12 infants (7.5%) had stage 2 ROP, and 4 infants (2.5%) had stage 3 ROP. Six infants (3.8%) had aggressive ROP (AROP). Nine infants (6%) had ROP changes in zone 1, while 80 infants (53.3%) had ROP changes in zone 2 and 61 infants (40.7%) had ROP changes in zone 3 (Table 2).
 |
Table 2 Distribution of ROP Stages and Aggressive ROP (AROP) Among the Screened Infants |
The postmenstrual age for appearance of ROP changes more than stage 0 (stage 1 or more) ranged from 32 to 40 weeks. Plus disease appeared in 11 cases (7.3%) and pre-plus appeared in 9 infants (6.2%). Ten of the screened infants (6.3%) had severe ROP changes that needed treatment with 8 infants receiving treatment in both eye (5%) and 2 infants receiving treatment in one eye (1.3%). Of those, 2 cases (20%) fell outside the British Guidelines for Screening. The first case was a male premature infant with GA 34 weeks and birth weight 2000 grams, with 28 days of exposure to mechanical ventilation, 2 weeks of CPAP exposure, RDS grade 4, IVH, 4 times blood transfusion. This infant showed AROP that required bilateral intravitreal anti-VEGF injection. The second case was a male premature infant with GA 34 weeks and BW 1960 g. with RDS grade 4, 21 days of exposure to mechanical ventilation, neonatal sepsis, 3 times blood transfusion, NEC. The infant showed bilateral stage 3 ROP in zone 2 with plus disease that improved after bilateral anti-VEGF injection.
The PMA of receiving treatment in the screened infants ranged from 33 to 41 weeks. Three of the screened infants (1.9%) did not achieve full retinal vascularization with persistent avascular area in the retina more than 2-disc diameters from Ora Serrata. One hundred and fifty-six infants (98.1%) achieved full retinal vascularization at PMA ranging from 37 to 51 weeks (Table 3). The PMA of achieving full retinal vascularization in the cases that had stage 0 ROP ranged from 37 to 41 weeks, while the PMA of achieving full retinal vascularization in the cases that had ROP changes more than stage 0 ROP ranged from 38 to 51 weeks (Table 4). Three cases that had ROP changes more than stage 0 did not achieve full retinal vascularization.
Risk Factors for Development of Retinopathy of Prematurity
In the screened infants, there was a highly significant (p < 0.0001) correlation between appearance of ROP changes more than stage 0 and GA, BW, and weight at first examination of the screened infants (4 weeks after delivery), receiving mechanical ventilation, mechanical ventilation duration, receiving CPAP, CPAP duration, RDS grade, presence of NEC, IVH, blood transfusion, number of blood transfusions (Table 5). On the other hand, no significant correlation was found between appearance of ROP changes more than stage 0 and gender (p = 0.911), presence of PDA (p = 0.187), or sepsis (p =0.998) (Table 5).
Also, there was a highly significant correlation (p < 0.0001) in the screened cases between the development of plus disease (which is the sign of severity) and GA, BW, and weight at first examination of the screened infants (4 weeks after delivery), receiving mechanical ventilation, mechanical ventilation duration, RDS stage, blood transfusion, and number of blood transfusions. There was a significant correlation between plus disease and both receiving CPAP (p = 0.041) and NEC (p = 0.020) (Table 6). However, no significant correlation was found between appearance of plus disease and gender (p = 0.464), PDA (p = 0.054), IVH (p =0.524) or sepsis (p =0.115) (Table 6).
Discussion
In this work, we demonstrate that the prevalence of ROP according to the most inclusive screening criteria in a tertiary care hospital in Egypt is 25.8%. The most important risk factors for developing ROP are low GA, low BW receiving mechanical ventilation especially for long duration, receiving CPAP, high grade of RDS, presence of NEC, IVH, blood transfusion and increased number of blood transfusions
Different guidelines were developed to determine the infants to be screened for ROP. These guidelines showed variation among developed and developing countries; In 2008, The United Kingdom guideline development group recommended screening all infants with GA <32 weeks or with birth weights of less than 1501 g.20 The American guidelines published by the American Academy of Paediatrics in 2013 recommended screening for ROP if GA was ≤30 weeks or BW ≤1500 g, with selected infants with higher BW or GA who had unstable clinical course determined by the attending neonatologist.21 More recent guidelines developed in the United States recommended screening infants with even less GA and BW.22 On the other hand, other studies conducted in developing countries demonstrated that the average birth weight and gestational age for infants with ROP was higher than what is reported in the literature for infants with ROP in developed countries, which implies the need for different screening criteria in developing countries.23 As our tertiary hospital lies in a developing country, we opted for including all infants with GA ≤ 34 weeks or BW ≤ 2000g, with screening of selected infants with higher BW or GA who had unstable clinical course determined by the attending neonatologist. We also assessed the contribution of different risk factors to the development of ROP and plus disease.
The prevalence of ROP changes more than stage 0 in our screened group was 25.8%, with plus disease appearing in 7.3% of the cases. And, 6.3% of the screened infants needed treatment. Our prevalence was comparable to that reported in other studies conducted elsewhere, including Saudi Arabia (28%24), Iran (32%25) and Southern India (21.6%16). A study conducted in mainland China also similarly utilized the inclusive criteria of BW (up to 2000g) and GA (up to 34 weeks), and the reported prevalence of ROP and severe ROP requiring treatment were 17.8% and 6.8%, respectively.26 Previous studies to assess the prevalence and risk factors for ROP in Egypt were scarce and had variable results. In a large study of 402 preterm babies from neonatal ICUs in Mansoura city from March 2013 to March 2015, 237 (59%) infants developed ROP, among whom 24 (10.1%) developed plus disease.27 Another study included 240 infants in Itay Elbaroud City, Behera Province, Egypt, showed that the overall incidence of ROP was 34.1%, and the incidence of type 1 ROP was 26.3% of the infants.28 Other smaller scale studies reported that the prevalence of ROP ranged between 19.2%29 and 48.2%.30
ROP changes more than stage 0 were more prevalent in infants with lower GA and birth weight, with mean GA of affected infants around 31 weeks (30.78 ± 2.29) and mean BW around 1450 g (1452.90 ± 404.41). This was consistent with the United Kingdom guideline development group20 and the American Academy of Paediatrics guideline.21 However, ROP changes were also found in infants with GA up to 36 weeks and BW up to 2400 g, and even more; 2 cases (20% of treated cases) with GA and BW above the American and British guidelines showed severe ROP changes that required treatment. This was comparable to results found by studies carried out in China,26 India31 and Iran,32 which indicated the importance of developing new guidelines that include screening of infants with higher GA and BW in developing countries.
The PMA for appearance of any ROP changes more than stage 0 ranged from 32 to 40 weeks (mean 35.63 ± 1.76 weeks), while the PMA of appearance of severe ROP changes that needed treatment ranged from 33 to 41 weeks (mean 37.20 ± 2.24 weeks). No severe ROP changes were noticed before 6 weeks chronological age or before 33 weeks PMA. These findings are comparable with previous studies.33–35 This can be explained by understanding the pathophysiology of ROP, in which two phases of the disease occur. Phase I ROP leads to vaso-obliteration that starts immediately after birth due to a marked decrease in vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1). Phase II starts around 33 weeks PMA. During this phase, there is a rebound increase in VEGF levels, especially if there is retinal hypoxia with increasing retinal metabolism and demand for oxygen leading to abnormal vasoproliferation.36
The mean PMA for complete retinal vascularization in this study was 39.47 ± 0.83 weeks, which is comparable to what was found in previous studies.37,38 However, the mean PMA of complete retinal vascularization was higher in infants who had regressed ROP (41.29 ± 2.62 weeks). Furthermore, three cases in this study had persistent avascular retina with halted retinal vascularization in zone 3 more than 3-disc diameters from ora serrata. The three cases had severe ROP changes that needed intervention with intravitreal anti-VEGF (Ranibizumab) injection. Delayed retinal vascular development and persistent avascular retinal in cases of regressed ROP especially after intravitreal anti-VEGF injection was also reported in previous studies.39,40
There was no correlation between gender and appearance of ROP changes, which is consistent with the CRYO-ROP study41 and New York cohort study,42 but contradictory to other studies that found males to be more prone to ROP changes.42,43 Also, there was a significant correlation between appearance of ROP changes more than stage 0 and RDS stage, consistent with previous reports.44,45 Exposure to mechanical ventilation, CPAP especially for long durations, was associated with increased incidence of ROP changes more than grade 0 in this study. Similar results were reported by other studies.46,47
In this study, there was no significant correlation between PDA and ROP or plus disease. Other studies, like this study, found no significant correlation between PDA and ROP.48,49 Also, no significant correlation was found in this study between sepsis and ROP or plus disease. However, sepsis was found to be independently associated with the development of severe ROP in premature infants.50 Meanwhile, other studies, like this study, found that sepsis status was not significantly different between premature infants with and without ROP.45,51
There was a highly significant correlation between necrotizing enterocolitis (NEC) and incidence of ROP in this study, with a significant correlation between NEC and plus disease. Perinatal inflammation, such as NEC, was reported to be a risk factor for ROP. The cellular mechanisms by which systemic inflammation may affect retinal vascular development and influence retinal neuronal function are still ill-defined. However, systemic inflammatory stress can perturb retinal angiogenesis and lead to vascular aberrations such as ROP.52
In this study, there was a highly significant correlation between intraventricular haemorrhage (IVH) and incidence or ROP more than stage 0. However, there was no significant correlation between IVH and plus disease. Association between IVH and ROP remains unclear. Previous studies have found a significant correlation between ROP and IVH.53–55 Other studies concluded that both IVH and ROP are complications of prematurity and there is no significant correlation between severity of ROP and IVH grade.56,57
There was a highly significant correlation between ROP and blood transfusion in this study, with a highly significant correlation between number of blood transfusions and ROP. Also, there was a highly significant correlation between plus disease and blood transfusion and number of blood transfusions. Previous studies showed that repeated blood transfusions can be a potential risk factor for ROP.58,59 However, other studies did not find a significant correlation between blood transfusion and ROP. As the most severely ill infants receive more RBC transfusions and are also more susceptible to develop ROP changes.60,61
Limitations of our work include the relatively small sample size of screened infants and the inclusion of one centre in the study. Not all risk factors reported to increase the risk for ROP were assessed in this study. However, the importance of our study lies in demonstrating that premature infants with higher GA and BW need screening for ROP in our local population, which carries public health implications.
In conclusion, our study demonstrates that the prevalence of ROP, according to the most inclusive screening criteria, in Ain Shams University, Cairo, Egypt, is 25.8%. The most important risk factors for developing ROP are low GA, low BW, receiving mechanical ventilation especially for long duration, receiving CPAP, high stage of RDS, presence of NEC, IVH, and blood transfusion. It is important to mention that severe ROP changes that needed intervention were found in infants with GA up to 34 weeks and BW up to 2000 g, which exceeds the British guidelines for screening for ROP and is similar to the Indian guidelines for ROP screening. This signifies the need for other national large-scale studies to develop an Egyptian guideline for screening for ROP.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jefferson E. Retrolental fibroplasia. Arch Dis Child. 1952;27(134):329. doi:10.1136/adc.27.134.329
2. Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122(1):200–210. doi:10.1016/j.ophtha.2014.07.050
3. Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J Am Assoc Pediatr Ophthalmol Strabismus. 2012;16(6):501–507. doi:10.1016/j.jaapos.2012.09.004
4. Thomas K, Shah PS, Canning R, Harrison A, Lee SK, Dow KE. Retinopathy of prematurity: risk factors and variability in Canadian neonatal intensive care units. J Neonatal Perinatal Med. 2015;8(3):207–214. doi:10.3233/NPM-15814128
5. Hadi AM, Hamdy IS. Correlation between risk factors during the neonatal period and appearance of retinopathy of prematurity in preterm infants in neonatal intensive care units in Alexandria, Egypt. Clin Ophthalmol. 2013;7:831. doi:10.2147/OPTH.S40136
6. Wilkinson AR, Haines L, Head K, Fielder AR. UK retinopathy of prematurity guideline. Eye. 2009;23(11):2137–2139. doi:10.1038/eye.2008.128
7. Fierson WM, Saunders RA, Good W, et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–195. doi:10.1542/peds.2012-2996
8. Honavar SG. Do we need India-specific retinopathy of prematurity screening guidelines? Indian J Ophthalmol. 2019;67(6):711. doi:10.4103/ijo.IJO_973_19
9. Chen Y, Li X. Characteristics of severe retinopathy of prematurity patients in China: a repeat of the first epidemic? Br J Ophthalmol. 2006;90(3):268–271. doi:10.1136/bjo.2005.078063
10. Vinekar A, Dogra MR, Sangtam T, Narang A, Gupta A. Retinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth: ten year data from a tertiary care center in a developing country. Indian J Ophthalmol. 2007;55(5):331. doi:10.4103/0301-4738.33817
11. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999. doi:10.1001/archopht.123.7.991
12. Chiang MF, Quinn GE, Fielder AR, et al. International classification of retinopathy of prematurity. Ophthalmology. 2021;128(10):e51–e68. doi:10.1016/j.ophtha.2021.05.031
13. Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233.
14. Hong EH, Shin YU, Cho H. Retinopathy of prematurity: a review of epidemiology and current treatment strategies. Clin Exp Pediatr. 2022;65(3):115. doi:10.3345/cep.2021.00773
15. Painter SL, Wilkinson AR, Desai P, Goldacre MJ, Patel CK. Incidence and treatment of retinopathy of prematurity in England between 1990 and 2011: database study. Br J Ophthalmol. 2015;99(6):807–811. doi:10.1136/bjophthalmol-2014-305561
16. Rao KA, Purkayastha J, Hazarika M, Chaitra R, Adith KM. Analysis of prenatal and postnatal risk factors of retinopathy of prematurity in a tertiary care hospital in South India. Indian J Ophthalmol. 2013;61(11):640.
17. van Sorge AJ, Termote JU, Kerkhoff FT, et al. Nationwide inventory of risk factors for retinopathy of prematurity in the Netherlands. J Pediatr. 2014;164(3):494–498. doi:10.1016/j.jpeds.2013.11.015
18. Gonçalves E, Násser LS, Martelli DR, et al. Incidence and risk factors for retinopathy of prematurity in a Brazilian reference service. Sao Paulo Med J. 2014;132:85–91. doi:10.1590/1516-3180.2014.1322544
19. Hansen T, Corbet A. Disorders of the transition, persistent pulmonary hypertension of the newborn. In: Taeusch HW, Ballard RA, editors. Avery’s Diseases of the Newborn.
20. Jefferies AL. Retinopathy of prematurity: recommendations for screening. Paediatr Child Health. 2010;15(10):667–670. doi:10.1093/pch/15.10.667
21. Fierson WM, Chiang MF, Good W, et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142(6). doi:10.1542/peds.2018-3061
22. Binenbaum G, Bell EF, Donohue P; G-ROP Study Group. Development of modified screening criteria for retinopathy of prematurity: primary results from the postnatal growth and retinopathy of prematurity study. JAMA Ophthalmol. 2018;136(9):1034–1040. doi:10.1001/jamaophthalmol.2018.2753
23. Valikodath N, Chan RV, Beca F, et al. Retinopathy of prematurity screening criteria based on the ROPE-SOS trial in India. Invest Ophthalmol Vis Sci. 2019;60(9):6522.
24. Al Hazzani F, Al-Alaiyan S, Kattan A, et al. Short-term outcome of very low-birth-weight infants in a tertiary care hospital in Saudi Arabia over a decade. J Neonatal Perinatal Med. 2021;14(3):427–432. doi:10.3233/NPM-200534
25. Feghhi M, Altayeb SM, Haghi F, et al. Incidence of retinopathy of prematurity and risk factors in the south-western region of Iran. Middle East Afr J Ophthalmol. 2012;19(1):101. doi:10.4103/0974-9233.92124
26. Xu Y, Zhou X, Zhang Q, et al. Screening for retinopathy of prematurity in China: a neonatal units–based prospective study. Invest Ophthalmol Vis Sci. 2013;54(13):8229–8236. doi:10.1167/iovs.13-12297
27. Bassiouny RM, Ellakkany RS, Aboelkhair SA, Mohsen TA, Othman IS. Incidence and risk factors of retinopathy of prematurity in neonatal intensive care units: mansoura, Egypt. J Egypt Ophthalmol Society. 2017;110(3):71. doi:10.4103/ejos.ejos_25_17
28. Gaber R, Sorour OA, Sharaf AF, Saad HA. Incidence and risk factors for Retinopathy of Prematurity (ROP) in biggest neonatal intensive care unit in Itay Elbaroud City, Behera Province, Egypt. Clin Ophthalmol. 2021;15:3467. doi:10.2147/OPTH.S324614
29. Abdel HA, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of incidence and risk factors in NICU of Al-Minya University Hospital in Egypt. J Clin Neonatol. 2012;1(2):76. doi:10.4103/2249-4847.96755
30. Tawfik S, Mansour A, Selim NL, et al. Analysis of a two-year independent screening effort for retinopathy of prematurity in rural Egypt. BMC Ophthalmol. 2021;21(1):1–6. doi:10.1186/s12886-021-02193-x
31. Vedantham V. Retinopathy of prematurity screening in the Indian population: it′ s time to set our own guidelines Indian J Ophthalmol. 2007;55(5):329. doi:10.4103/0301-4738.33816
32. Roohipoor R, Karkhaneh R, Farahani A, et al. Retinopathy of prematurity screening criteria in Iran: new screening guidelines. Arch Dis Childhood-Fetal Neonatal Edition. 2016;101(4):F288–F293. doi:10.1136/archdischild-2015-309137
33. Good WV, Hardy RJ. The multicenter study of Early Treatment for Retinopathy of Prematurity (ETROP). Ophthalmology. 2001;108(6):1013–1014. doi:10.1016/S0161-6420(01)00540-1
34. Austeng D, Källen KB, Hellström A, Tornqvist K, Holmström GE. Natural history of retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden. Arch Ophthalmol. 2010;128(10):1289–1294. doi:10.1001/archophthalmol.2010.234
35. Quinn GE, Ying GS, Bell EF; G-ROP Study Group. Incidence and early course of retinopathy of prematurity: secondary analysis of the postnatal growth and retinopathy of prematurity (G-ROP) study. JAMA Ophthalmol. 2018;136(12):1383–1389. doi:10.1001/jamaophthalmol.2018.4290
36. Cayabyab R, Ramanathan R. Retinopathy of prematurity: therapeutic strategies based on pathophysiology. Neonatology. 2016;109(4):369–376. doi:10.1159/000444901
37. Ashton N. Retinal angiogenesis in the human embryo. Br Med Bull. 1970;26(2):103–106. doi:10.1093/oxfordjournals.bmb.a070758
38. Sun Y, Smith LE. Retinal vasculature in development and diseases. Annual Rev Vision Sci. 2018;4(1):101. doi:10.1146/annurev-vision-091517-034018
39. Meng QY, Cheng Y, Zhao MW, Liang JH. The process of retinal vascularization in retinopathy of prematurity after ranibizumab treatment in China. Int J Ophthalmol. 2019;12(7):1146. doi:10.18240/ijo.2019.07.15
40. Lepore D, Quinn GE, Molle F, et al. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018;125(2):218–226. doi:10.1016/j.ophtha.2017.08.005
41. Palmer EA, Flynn JT, Hardy RJ, et al.; Cryotherapy For Retinopathy of Prematurity Cooperative Group. Incidence and early course of retinopathy of prematurity. Ophthalmology. 2020;127(4):S84–S96. doi:10.1016/j.ophtha.2020.01.034
42. Chiang MF, Arons RR, Flynn JT, Starren JB. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of a comprehensive New York state patient database. Ophthalmology. 2004;111(7):1317–1325. doi:10.1016/j.ophtha.2003.10.030
43. Lundgren P, Kistner A, Andersson EM, et al. Low birth weight is a risk factor for severe retinopathy of prematurity depending on gestational age. PLoS One. 2014;9(10):e109460. doi:10.1371/journal.pone.0109460
44. Slidsborg C, Jensen A, Forman JL, et al. Neonatal risk factors for treatment-demanding retinopathy of prematurity: a Danish national study. Ophthalmology. 2016;123(4):796–803. doi:10.1016/j.ophtha.2015.12.019
45. Yang CY, Lien R, Yang PH, et al. Analysis of incidence and risk factors of retinopathy of prematurity among very-low-birth-weight infants in North Taiwan. Pediatr Neonatol. 2011;52(6):321–326. doi:10.1016/j.pedneo.2011.08.004
46. Chang JW, Hansen RM. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS One. 2019;14(7):e0219934. doi:10.1371/journal.pone.0219934
47. Ying GS, Quinn GE, Wade KC, Repka MX, Baumritter A, Daniel E. Predictors for the development of referral-warranted retinopathy of prematurity in the telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. JAMA Ophthalmol. 2015;133(3):304–311. doi:10.1001/jamaophthalmol.2014.5185
48. Viejo G, Romera Santa Bárbara B. Is patent ductus arteriosus a risk factor for retinopathy of prematurity? InAnales de Pediatria. 2010;74(1):25–30.
49. Akkawi MT, Shehadeh MM, Shams AN, et al. Incidence and risk factors of retinopathy of prematurity in three neonatal intensive care units in Palestine. BMC Ophthalmol. 2019;19(1):1–7. doi:10.1186/s12886-019-1180-4
50. Araz-Ersan B, Kir N, Akarcay K, et al. Epidemiological analysis of retinopathy of prematurity in a referral centre in Turkey. Br j Ophthalmol. 2013;97(1):15–17. doi:10.1136/bjophthalmol-2011-301411
51. Sabzehei MK, Afjeh SA, Farahani AD, Shamshiri AR. Retinopathy of prematurity: incidence, risk factors, and outcome. Arch Iran Med. 2013;16(9):507.
52. Tremblay S, Miloudi K, Chaychi S, et al. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Invest Ophthalmol Vis Sci. 2013;54(13):8125–8139. doi:10.1167/iovs.13-12496
53. Şahin A, Şahin M, Türkcü FM, et al. Incidence of retinopathy of prematurity in extremely premature infants. Int Scholar Res Notices. 2014;2014:45.
54. Procianoy RS, Garcia‐Prats JA, Hittner HM, Adams JM, Rudolph AJ. An association between retinopathy of prematurity and intraventricular hemorrhage in very low birth weight infants. Acta Pædiatrica. 1981;70(4):473–477. doi:10.1111/j.1651-2227.1981.tb05725.x
55. O’Keefe M. Ocular significance of intraventricular haemorrhage in premature infants. Br j Ophthalmol. 2001;85(3):357–359. doi:10.1136/bjo.85.3.357
56. Hand I, Shrier E. Lack of association of intraventricular hemorrhage with retinopathy of prematurity. J Pediatr Neurol. 2019;17(06):219–222. doi:10.1055/s-0038-1661344
57. Pascal AJ, Shaikh A, Hajee ME, Lazzaro DR, Shrier EM. Association of intraventricular hemorrhage with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2011;52(14):3141.
58. Del Vecchio A, Henry E, D’Amato G, et al. Instituting a program to reduce the erythrocyte transfusion rate was accompanied by reductions in the incidence of bronchopulmonary dysplasia, retinopathy of prematurity and necrotizing enterocolitis. J Maternal-Fetal Neonatal Med. 2013;26(sup2):77–79. doi:10.3109/14767058.2013.830836
59. Lust C, Vesoulis Z, Jackups R, Liao S, Rao R, Mathur AM. Early red cell transfusion is associated with development of severe retinopathy of prematurity. J Perinatol. 2019;39(3):393–400. doi:10.1038/s41372-018-0274-9
60. Yau GS, Lee JW, Tam VT, et al. Incidence and risk factors of retinopathy of prematurity from 2 neonatal intensive care units in a Hong Kong Chinese population. Asia-Pacific J Ophthalmol. 2016;5(3):185–191. doi:10.1097/APO.0000000000000167
61. Christensen RD, Ilstrup SJ, Hartnett ME. Retinopathy of prematurity and transfusion practice. Transfusion. 2014;54(4):960–961. doi:10.1111/trf.12510
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.