Back to Journals » International Journal of General Medicine » Volume 15
Screening and Analysis of Potential Critical Gene in Acute Myocardial Infarction Based on a miRNA-mRNA Regulatory Network
Authors Hou R , Guo D , Fan M, Hou Y, Zhao J, Wu X
Received 11 January 2022
Accepted for publication 3 March 2022
Published 10 March 2022 Volume 2022:15 Pages 2847—2860
DOI https://doi.org/10.2147/IJGM.S354641
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ruirui Hou, Dong Guo, Maoxia Fan, Yawei Hou, Jisen Zhao, Xiaoqi Wu
Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China
Correspondence: Dong Guo, Email [email protected]
Background: MicroRNAs (miRNAs) have been shown to be involved in the initiation, progression, and prevention of acute myocardial infarction (AMI), but the underlying mechanism remains unclear.
Objective: Through the GEO database, bioinformatics methods were used to explore the miRNA-mRNA regulatory relationship pairs associated with AMI and to elucidate the underlying mechanism.
Methods: Using the R software Limma package, differential expression analysis was performed using the AMI-related miRNA chip dataset (GSE31568) and mRNA chip dataset (GSE159657) from the GEO database. The miRDB, miRWalk, miRTarBase, and TargetScan databases were used to predict potential downstream target genes regulated by differentially expressed miRNAs, and a miRNA-mRNA regulatory network was built with Cytoscape; GO function and KEGG pathway enrichment analyses of target genes were done with Funrich software, and the protein interaction network of target genes in the regulatory network was built with the STRING database.
Results and Conclusions: A total of 187 differentially expressed miRNAs were experimentally screened, of which 91 were upregulated (such as hsa-miR-302b, hsa-miR-1299), and 96 were downregulated (such as hsa-miR-1201, hsa-miR-1283); 507 differentially expressed mRNAs were identified, of which 430 were upregulated (such as MRM1 and SFXN4), and 77 were downregulated (such as KCTD13 and CCDC134). And 16 miRNAs and 44 mRNAs were used for regulatory network construction. GO and KEGG enrichment analyses mainly focused on Integrins in angiogenesis, angiopoietin receptor Tie2-mediated signaling, and signaling events mediated by stem cell factor receptor (c-Kit). As hub genes in the PPI network, FGF2 and MMP2 may be key targets of AMI. The experimentally constructed miRNA-mRNA regulatory network found that hsa-miR-190b targets to inhibit FGF2, while hsa-miR-330-3p targets to regulate MMP2, which may mediate Integrins in angiogenesis, Angiopoietin receptor Tie2-mediated signaling pathway to induce AMI pathogenesis, providing strong data support and a research direction for the prevention and treatment of AMI.
Keywords: acute myocardial infarction, AMI, GEO database, bioinformatics, differential gene, regulatory networks, miRNA-mRNA, genes, protein interaction
Introduction
Acute myocardial infarction (AMI) is an important factor threatening human life worldwide. According to the existing epidemiological survey data in western countries, in 2017, an estimated 695,000 Americans had a new acute myocardial infarction (AMI), and another 325,000 had a recurrent event.1 Statistical studies suggest reductions in hospital admissions and mortality in developed countries in recent years, but the global burden of CVD and acute myocardial infarction has shifted to low- and middle-income countries, as more than 80% of all CVD deaths currently occur in these countries.2 It is well known that mortality from AMI is higher; although the case fatality rate has been reduced from 30% to approximately 5% in recent years due to the improved sensitivity of the detection of creatine kinase isozymes as well as biomarkers such as troponin,3,4 as well as rapidly developing PCI and medical therapy, there is still a high risk of mortality. Currently, invasive coronary angiography remains the gold standard for the diagnosis of AMI; however, there is a great probability of misdiagnosis in the diagnosis of ECG, and blood-based biomarkers such as CK-MB and cTnT have certain deficiencies in the diagnosis of AMI.5,6 Post-AMI onset progression is very rapid, with many patients dying within 1–2 h of onset, most deaths occurring within a week of onset, and there is a high rate of recurrence1. Some patients with acute myocardial infarction have premonitory symptoms before onset, such as chest pain, but a large part of them are asymptomatic and often can not attract attention. Once the disease occurs, it will cause very serious consequences. Therefore, it is crucial to improve criteria for early and accurate diagnostic workups, which will reduce mortality and improve outcomes. And more methods and biological markers need to be identified to develop diagnostics for AMI. Current research on AMI biomarkers has gradually become a hot spot, and there have been many studies related to gene markers for diagnosis as well as targeted therapy.7,8 This study aimed to enrich the research on AMI biomarkers to perform the next advance work for definite clinical diagnosis and treatment.
MicroRNAs (miRNAs) constitute a class of small RNAs that are approximately 20–24 nucleotides in length and have various important regulatory roles in the cell. They are widely present in eukaryotic cells in various organisms and can be specifically expressed during cell differentiation and the tissue development stage. They do not encode proteins but can affect the transcriptional, posttranscriptional and epigenetic levels of genes and can inhibit protein translation or promote mRNA degradation. Studies have shown that miRNAs are of great clinical importance in the pathogenesis, prevention, and treatment of cardiovascular diseases and diseases such as diabetes.9,10 However, there are few reports of in-depth exploration into the molecular mechanisms of miRNAs in AMI, and the molecular network involved in the miRNA-mediated regulatory mechanism during AMI disease development remains unclear.
The Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) is an international public repository where researchers may contribute high-throughput microarray and next-generation sequencing functional genomic data sets. The resource allows for the preservation of raw data, processed data, and metadata that is searchable, indexed, and cross-linked.11 All data are freely available for download in a variety of formats. Therefore, an experiment was performed to construct a miRNA-mRNA regulatory network using bioinformatics and AMI-related miRNA and mRNA expression datasets in the GEO database to identify the key miRNA-mRNA regulatory relationship pairs, analyze the functions of targets and related signaling pathways, explore the mechanisms of action, and provide an important theoretical reference and scientific basis for the targeted therapy of AMI. The study flow chart was as follows (Figure 1).
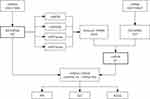 |
Figure 1 Flow chart. |
Materials and Methods
Data Source
Chip data of miRNA expression profiles and mRNA expression profiles associated with AMI were retrieved from the NCBI GEO (Gene Expression Omnibus) database, and the screening criteria were samples of AMI patients and healthy populations. Cell lines or animal models were excluded. The time for this study to search data in geo database is from October 2021 to November 2021.The qualified miRNA expression chip dataset GSE31658 and mRNA expression high-throughput dataset GSE159657 were downloaded. The GSE31658 dataset is based on the GPL9040 platform (Febit Homo Sapiens miRBase 13.0) and contains 454 samples. Twenty whole blood samples from patients with acute myocardial infarction (AMI) and 20 whole blood samples from healthy controls (controls) were selected. The GSE159657 dataset is based on the GPL24676 platform (Illumina NovaSeq 6000 (Homo sapiens)) containing 28 samples selected, including plasma samples from 10 patients with acute myocardial infarction (AMI) and plasma samples from 10 healthy controls (controls). (Database or software for research in Table 1).
 |
Table 1 Database or Software for Research |
Methods
Data Processing and Differential Expression Analysis
The GSE31568 dataset was analyzed using the GEO2R function in the GEO database for differential expression using the Limma package in R software with the filtering criteria set at adj.p.Val<0.01, |log2-fold change (FC)| >1. Differential analysis of the GSE159657 dataset was performed using the NetworkAnalyst database, and the filtering criteria were set at P. Value <0.05, |log2-fold change (FC)| >1. The differential expression data were visualized and displayed with volcano plots and cluster plots.
Target Gene Prediction and miRNA-mRNA Regulatory Network Construction
Using the miRDB, miRWalk, miRTarBase, and TargetScan databases to predict the target genes of differentially expressed miRNAs, the differentially expressed mRNAs predicted by the simultaneous four databases and the GSE159657 analysis were intersected by Jvenn online, and the miRNA-mRNA relationship pairs were defined according to the regulatory relationship between miRNAs and mRNAs. MiRNA-mRNA regulatory network visualization was performed using Cytoscape software.
Functional Enrichment Analysis of Target Genes Regulated by miRNAs
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of target genes in the miRNA-mRNA regulatory network was performed using Funrich software, including biological process (BP), cellular component (CC) and molecular function (MF), setting the p value < 0.05.
miRNA Regulated Target Gene Protein Interaction Network Construction
To further identify the relationship between target genes in the miRNA-mRNA regulatory network, protein–protein interaction analysis (PPI) was performed using the STRING (Search Tool for the Retrieval of Interacting Genes) database, setting the confidence score > 0.4. The PPI network was visualized in Cytoscape software. Each node in the PPI network was evaluated by the “degree” to screen core genes (hub genes). The higher the degree of a node is, the greater its significance in the PPI network.
Results
Differentially Expressed miRNAs
Compared with AMI and healthy control whole blood samples in the GSE31568 dataset, 243 differentially expressed miRNAs were obtained after removing duplicates, and 187 differentially expressed miRNAs, marked with *were obtained, including 91 genes with upregulated expression (eg, hsa-miR-302b, hsa-miR-1299, hsa-miR-613, hsa-miR-609, hsa-miR-190b, hsa-miR-1468, hsa-miR-1258, hsa-miR-508-3p, hsa-miR-1262, and hsa-miR-373) and 96 genes with downregulated expression (eg, hsa-miR-491-3p, hsa-miR-1201, hsa-miR-1283, hsa-miR-1245, hsa-miR-217, hsa-miR-518a-3p, hsa-miR-1291, hsa-miR-1271, hsa- miR-621, and hsa-miR-515-5p). The differential expression miRNA volcano plot is presented in Figure 2A, and the cluster plots of the top 50 differential miRNAs with larger fold differences are presented in Figure 2B.
Differentially Expressed mRNAs
Compared with AMI and healthy control plasma samples in the GSE159657 dataset, 507 differentially expressed mRNAs were acquired, including 430 upregulated and 77 downregulated mRNAs. Volcano plots of all differential mRNAs are shown in Figure 3A, and cluster plots of the top 50 differential mRNAs with large absolute values of fold difference are shown in Figure 3B.
Target Gene Prediction and Regulatory Network Construction
Downstream target prediction of differentially expressed miRNAs was performed using the miRDB, miRWalk, miRTarBase and TargetScan databases. There were 2634 predicted targets in the four databases, and the predicted targets were intersected with the aforementioned 507 differentially expressed mRNAs in the dataset, resulting in a total of 81 candidate target genes (Figure 4, Table 2).
 |
Table 2 Intersection of Differentially miRNA Predicted Target Genes and Differentially Expressed mRNAs Candidate Target Genes in AMI |
According to the negative regulatory relationships of miRNAs and mRNAs, 44 miRNA-mRNA relationship pairs consisting of 16 differentially expressed miRNAs and 44 differentially expressed mRNAs were ultimately screened out. The miRNA-mRNA regulatory network was constructed and visualized using Cytoscape software (Figure 5, Table 3).
 |
Table 3 AMI Related miRNA mRNA Regulatory Relationship Pairs |
Functional Analysis of Network Target Gene
GO function and KEGG pathway enrichment analysis of target genes in the miRNA-mRNA regulatory network were performed in Funrich software. After screening according to p value <0.05, 25 GO enrichment entries, including 2 BP entries, 17 CC entries and 6 MF entries, were obtained. According to the magnitude of enrichment values, BP was mainly enriched in Protein metabolism and Signal transduction; CC was mainly enriched in Cell-substrate junction, Cell body fiber, Interleukin-28 receptor complex, Vehicle, Costamere, and Microtubule basal body; MF was mainly enriched in Fucosyltransferase activity, Peroxidase activity, Carboxypeptidase activity, Transmembrane receptor activity, Metallopeptidase activity and Receptor signaling complex scaffold activity. The most significantly enriched BP, CC, and MF terms are shown in Figure 6A. KEGG pathway enrichment analysis resulted in 29 signaling pathways, mainly Integrins in angiogenesis, Angiopoietin receptor Tie2-mediated signaling, Signaling events mediated by Stem cell factor receptor (c-Kit), Stabilization and expansion of the E-cadherin adherens junction, E-cadherin signaling in the nascent adherens junction, and E-cadherin signaling events. The most significantly enriched pathways are shown in Figure 6B, and the relationships between the major pathways and the enriched genes are shown in Table 4.
 |
Table 4 Main KEGG Pathways and Enriched Genes of the 44 Target Genes in the miRNA-mRNA Regulatory Network |
Target Gene PPI Network Construction
The 44-candidate target gene PPI network in the miRNA mRNA regulatory network was predicted and constructed using the STRING database (see Figure 7), and the network consisted of 10 nodes and 17 edges. Of these, FGF2 and MMP2 had higher degree values, and it was coincident that they all had the highest absolute values of fold difference, while all had more than three direct connections to other nodes and thus were of significance in the PPI network. Among these nodes, FGF2 and SULF1 were downregulated genes, while the others were upregulated genes.
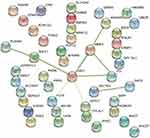 |
Figure 7 PPI network of target genes in the miRNA-mRNA regulatory network. |
Discussion
In this study, both data sets were compared between patients with AMI and healthy people. The key step of this study is to find out the differential mRNA and miRNA between patients with AMI and healthy people, and then construct the corresponding regulatory relationship. Then, PPI network construction, GO and KEGG enrichment analysis were carried out for miRNAs in the regulatory relationship. Further analyze the miRNAs closely related to the disease. These miRNAs may affect the occurrence and development of AMI through multiple targets, multiple pathways and multiple pathways, and provide new ideas and strategies for the early diagnosis and treatment of AMI.
Since the first microRNA (miRNA) was identified in Caenorhabditis elegans by Lee et al12 in 1993, miRNAs have been widely studied in physiology and pathophysiology. A large body of evidence indicates that miRNAs play key roles in cardiovascular diseases, such as cardiac remodeling, nonischemic heart disease, atherosclerosis, myocardial repair, apoptosis and angiogenesis after AMI, and these findings may change our traditional understanding of the cardiovascular field, regulate the levels of some miRNAs after AMI, and help limit tissue damage, promote neovascularization and inhibit ventricular remodeling, thereby improving long-term prognosis. Numerous studies have provided a biological basis for miRNAs as early AMI biomarkers, such as miRNA-1, miRNA-133a7, and miR-181a, with significantly increased concentrations in the short term of onset. Most of the above studies focus on the upstream and downstream interaction between single or several miRNAs, genes or pathways, but the occurrence and development of the disease is the result of the synergy of multiple targets, multiple pathways, multiple pathways and multiple links. If we only study the relationship between a single miRNA or gene and AMI, the study of the mechanism of AMI will be limited to a certain extent.
With the development of transcriptomics, the molecular network patterns of diseases during occurrence and development can be studied using bioinformatics. The GEO database is one of the central resources important for bioinformatics research, where large amounts of high-throughput data are deposited, but most of the data are underutilized. Domestic and foreign scholars apply high-throughput data because of the differences in research purposes and the limitation of data independence, and the gene information of miRNAs and mRNAs in AMI research has not been integrated and compared. For other diseases, some people have done relevant research with this method and draw effective conclusions. Such as using miRNA-mRNA Regulatory Networks to study the pathogenesis of Osteonecrosis of the Femoral Head.13 However, this research method has not been applied to study the pathogenesis of AMI. In this study, the differentially expressed genes were analyzed by mining the chip data of miRNAs and mRNAs associated with AMI from the GEO database, and then a miRNA-mRNA regulatory relationship network was constructed in the hope of providing data support and a theoretical basis for the occurrence, development and prevention of AMI.
A total of 187 differentially expressed miRNAs were screened in this experiment, of which 91 were upregulated and 96 were downregulated; and 507 differentially expressed mRNAs were screened out, of which 430 were upregulated and 77 were downregulated. Using the miRDB, miRWalk, miRTarBase and TargetScan databases, 2634 downstream target genes were predicted to be present in 4 databases simultaneously, 16 miRNAs and 44 mRNAs were collated for regulatory network construction, and 44 regulatory network relationship pairs were constructed. These differential miRNAs and mRNAs may be the key nodes in the pathophysiology of AMI, and the PPI network of the 44 candidate target genes in the miRNA-mRNA regulatory network showed that the degree values of FGF2 and MMP2 were high, indicating that they were the core nodes in the regulatory network.
FGF2 is an important protein of the fibroblast growth factor family. FGF2, activated by binding to FGFR1, regulates the differentiation and proliferation of cells, playing an important role in the inflammatory response.14 FGFR1 has a high affinity for FGF2 and is mainly responsible for endothelial cell signaling, which can directly affect vascular endothelial cell growth, migration, and angiogenesis, among others. Hsa-miR-190b upregulated and thereby regulated FGF2 downregulation, thereby alleviating the inflammatory response and vascular endothelial injury and activating FGF2 and FGFR1 protein expression. MMP-2 is a zinc ion-dependent protease secreted and activated by macrophages/foam cells and T lymphocytes in a prozymogen form. Macrophage invasion into atherosclerotic lesions promotes the secretion of a large number of factors, such as MMP-2, which injures endothelial cells when acting to cleave extracellular matrix components, collagen and elastase, and accelerates the formation of intravascular thrombi, thus accelerating atherosclerosis and plaque formation.15,16
GO functional annotation and KEGG pathway enrichment analyses of the 44 target genes in the miRNA-mRNA regulatory network were performed and highlighted Integrins in angiogenesis, Angiopoietin receptor Tie2-mediated signaling, Signaling events mediated by Stem cell factor receptor (c-Kit), Stabilization and expansion of the E-cadherin adherens junction, E-cadherin signaling in the nascent adherens junction, and E-cadherin signaling events.
Integrins are heterodimeric transmembrane cell adhesion molecules consisting of alpha (α) and beta (β) subunits arranged in numerous dimeric pairings. These complexes have varying affinities for extracellular ligands. Integrins regulate cellular growth, proliferation, migration, signaling, and cytokine activation and release and thereby play important roles in cell proliferation and migration, apoptosis, tissue repair, and all processes critical to inflammation, infection, and angiogenesis. Several studies have shown that soluble fragments of ECM proteins suppress angiogenesis. Many of these proteins appear to bind to and suppress the function of endothelial cell integrins. These include but are not limited to Stupack and Cheresh proteolytic cleavage fragments of plasminogen (angiostatin), MMP-2 (PEX), collagen 18 (endostatin), and the NC domains from the alpha 2, alpha 3 (tumstatin), and alpha 6 chains of type IV collagen. These proteins may act to block interactions with immobilized ECM components and may therefore elicit apoptosis through a variety of mechanisms.17 It was suggested that this inhibition is due to blocked MMP2 binding to avb3. The organic molecule TSRI265 can disrupt avb3/MMP2 formation, and although it does not block vitronectin binding, it does block tumor angiogenesis in vivo.18 Thus, the importance of MMPs in angiogenesis has been established, but a direct interaction of MMP2 with avb3 is still somewhat debated.19
Regarding angiopoietin receptor Tie2-mediated signaling, the current study suggests that the development of a functional cardiovascular system is dependent on the regulated proliferation, migration and differentiation of endothelial cells in two discrete processes known as vasculogenesis and angiogenesis. Angiogenesis is the formation of new capillaries from pre-existing vessels, whereas vasculogenesis is de novo capillary formation from EPCs (endothelial precursor cells). New capillaries arise from pre-existing larger vessels to give rise to a more complex vascular network with a hierarchy of both large and small vessels.20 These sequential vascular developments are tightly regulated by a range of pro- and antiangiogenic factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), thrombospondin, angiopoietins and, more recently, angiopoietin-like proteins (eg, Angptl).21 Angiopoietins are a new family of growth factor ligands that bind specifically to TIE2/Tek RTK (Receptor Tyrosine Kinase). To date, four angiopoietins (Ang1 to 4) bind Tek and behave as either agonists (Ang1 and Ang4) or context-dependent antagonists (Ang2 and Ang3) of Tek kinase activity.22 Angiopoietin mainly regulates two pathways that mediate cell motility, the first being through activation of the Phosphatidylinositol-3 Kinase pathway and the second involving the Ras pathway.23 However, there are currently no studies to clarify the mechanism of FGF2 and MMP2 in angiopoietin receptor Tie2-mediated signaling, which can be investigated as a new direction for future research.
Despite the key role of miRNAs in cardiovascular diseases, the early release and ultrasensitivity of miRNAs in myocardial infarction give them the advantage of competing with the traditional myocardial biomarker CTN, but their specificity still needs validation in a large number of clinical trials. The diverse regulatory functions of miRNAs in the field of CVD revolutionize the current understanding of CVD, which may provide a novel approach in predicting, diagnosing, and treating CVD. Although the experiments constructed a potential miRNA-mRNA regulatory network based on bioinformatics, there were certain limitations. The experiments did not distinguish between different types of AMI, and the high-throughput datasets of AMI included in the GEO database and the number of samples were small, especially datasets that lacked documents from the same population and the same platform. More studies, such as dual-luciferase reporter assays, will be designed in the future to verify the biological functions of miRNA-mRNA regulatory network patterns in vitro and in vivo.
Conclusions
In summary, based on GEO high-throughput datasets and with the aid of bioinformatics, 44 miRNA-mRNA regulatory relationship pairs related to AMI were experimentally constructed, and the complex net regulation of AMI multiple targets and multiple pathways was elucidated. The experimentally constructed miRNA-mRNA regulatory network found that hsa-miR-190b targets to inhibit FGF2, while hsa-miR-330-3p targets to regulate MMP2, which may mediate Integrins in angiogenesis, Angiopoietin receptor Tie2-mediated signaling pathway to induce AMI pathogenesis, providing strong data support and a research direction for the prevention and treatment of AMI.
Ethical Review
The ethics committee of the Affiliated Hospital of Shandong University of Chinese Medicine certifies that the study belongs to the data mining class of papers, and all data are derived from publicly available databases, Permission to use the database does not need to be obtained. The study did not involve animal or human experimentation and did not involve ethical issues. Therefore, it is hereby declared that the ethics committee of the Affiliated Hospital of Shandong University of Chinese medicine reviewed the study and certified that the study does not require ethical review.
Funding
National Academic Schools of Traditional Chinese Medicine (XSLP-2013-35).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Castro-Dominguez Y, Dharmarajan K, McNamara RL. Predicting death after acute myocardial infarction. Trends Cardiovasc Med. 2018;28(2):102–109. doi:10.1016/j.tcm.2017.07.011
2. Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376(21):2053–2064. doi:10.1056/NEJMra1606915
3. Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. doi:10.1093/eurheartj/ehv32
4. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868–877. doi:10.1056/NEJMoa0903515
5. Abbas NA, John RI, Webb MC, et al. Cardiac troponins and renal function in nondialysis patients with chronic kidney disease. Clin Chem. 2005;51(11):2059–2066. doi:10.1373/clinchem.2005.055665
6. Røsjø H, Varpula M, Hagve TA, et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37(1):77–85. doi:10.1007/s00134-010-2051-x
7. Liebetrau C, Möllmann H, Dörr O, et al. Release kinetics of circulating muscle-enriched microRNAs in patients undergoing transcoronary ablation of septal hypertrophy. J Am Coll Cardiol. 2013;62(11):992–998. doi:10.1016/j.jacc.2013.05.025
8. Dawson K, Wakili R, Ordög B, et al. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127(14):1466–1475. doi:10.1161/circulationaha.112.001207
9. Longgang H, An Y. Expressions and clinical significance of plasma microRNA-1 and urothelial carcinoma associated 1 gene in patients with acute myocardial infarction. Chin. J Evid Based Cardiovasc Med. 2017;12:1468–1470.
10. Latronico MV, Catalucci D, Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circ Res. 2007;101(12):1225–1236. doi:10.1161/circresaha.107.163147
11. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991–D995. doi:10.1093/nar/gks1193
12. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi:10.1016/0092-8674(93)90529-y
13. Yu L, Yao T, Jiang Z, et al. Integrated analysis of miRNA-mRNA regulatory networks associated with osteonecrosis of the femoral head. Evid Based Complement Alternat Med. 2021;2021:8076598. doi:10.1155/2021/8076598
14. Suh J, Kim D-H, Lee Y-H, et al. Fibroblast growth factor-2, derived from cancer-associated fibroblasts, stimulates growth and progression of human breast cancer cells via FGFR1 signaling. Mol Carcinog. 2020;59(9):1028–1040. doi:10.1002/mc.23233
15. Liu H, Sun J. Relationship between serum levels of visfatin, matrix metalloproteinase in acute myocardial infarction patients. Chin J Geriatr Heart Brain Vessel Dis. 2018;21:389–391.
16. Nigro N, Winzeler B, Suter-Widmer I, et al. Mid-regional pro-atrial natriuretic peptide and the assessment of volaemic status and differential diagnosis of profound hyponatraemia. J Intern Med. 2015;278(1):29–37. doi:10.1111/joim.12332
17. Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;207–238. doi:10.1016/s0070-2153(04)64009-9
18. Bafetti LM, Young TN, Itoh Y, et al. Intact vitronectin induces matrix metalloproteinase-2 and tissue inhibitor of metalloproteinases-2 expression and enhanced cellular invasion by melanoma cells. J Biol Chem. 1998;273(1):143–149. doi:10.1074/jbc.273.1.143
19. Hodivala-Dilke KM, Reynolds AR, Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 2003;314(1):131–144. doi:10.1007/s00441-003-0774-5
20. Szekanecz Z, Besenyei T, Szentpétery A, et al. Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 2010;22(3):299–306. doi:10.1097/BOR.0b013e328337c95a
21. Yang YH, Wang Y, Lam KS, et al. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28(5):835–840. doi:10.1161/atvbaha.107.157776
22. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi:10.3389/fnmol.2011.00051
23. Audero E, Cascone I, Maniero F, et al. Adaptor ShcA protein binds tyrosine kinase Tie2 receptor and regulates migration and sprouting but not survival of endothelial cells. J Biol Chem. 2004;279(13):13224–13233. doi:10.1074/jbc.M307456200
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.