Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Scleroderma-Like Lupus Panniculitis: A Case Report and Literature Review
Authors Pinyowiwat P , Rutnin S , Chanprapaph K
Received 20 January 2023
Accepted for publication 4 April 2023
Published 10 April 2023 Volume 2023:16 Pages 995—1001
DOI https://doi.org/10.2147/CCID.S405553
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Prinpat Pinyowiwat, Suthinee Rutnin, Kumutnart Chanprapaph
Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Kumutnart Chanprapaph, Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama IV Road, Ratchatewi, Bangkok, 10400, Thailand, Tel +662-201-1141, Fax +662-201-1211, Email [email protected]
Abstract: Sclerodermic or scleroderma-like lupus erythematosus panniculitis (SLEP) shares both clinical and histopathological features between lupus panniculitis and localized scleroderma. It is exceedingly rare. We herein report a case of SLEP manifested with a solitary, firm-to-hard, erythematous plaque in an Asian woman. This patient responded well to intralesional corticosteroid and antimalarials. We have reviewed the pathogenesis of fibrosis in patients with chronic cutaneous lupus erythematosus as well as documented cases of SLEP in the literature.
Keywords: cutaneous lupus erythematosus, scleroderma-like lupus erythematosus panniculitis, sclerodermic lupus panniculitis, localized scleroderma, lupus profundus, overlap syndrome
Introduction
Lupus erythematosus panniculitis (LEP) is a chronic inflammation of subcutaneous tissue affecting 1–3% of patients with chronic cutaneous lupus erythematosus (CCLE).1 While it usually occurs independently, coexistence with discoid lupus erythematosus (DLE) occurs in approximately one-third of patients.2–4 LEP typically manifests with tender subcutaneous nodules or plaques on the face, proximal extremities, and trunk.1 It displays a female predilection with a female-to-male ratio ranging from 2:1 to 9:1, with onset in third to fifth decades, and a slightly younger onset in the Asian population.1,3 Unique presentations, such as linear configuration or coexistence with localized scleroderma are exceedingly rare. The term “sclerodermic or scleroderma-like lupus erythematosus panniculitis (SLEP)” was used for 5 patients with overlap clinical and histopathologic features between lupus panniculitis and localized scleroderma.5–8 Four of them displayed a linear configuration, and one was described concomitantly with sclerodermic DLE.5,6,8 We hereby present a case of SLEP presented as a solitary, firm to hard erythematous plaque mimicking dermatofibrosarcoma protuberans in a middle-aged Asian woman.
Case Report
A 43-year-old Thai female presented with a solitary indurated erythematous hard plaque at the left lateral neck. The lesion was mildly pruritic, painless, and gradually expanded over the course of 15 months. She had no underlying diseases and denied family history of malignancy or other autoimmune connective tissue diseases. Symptoms of fever, weight loss, joint pain, hair loss, oral ulcer, or photosensitivity were not present. There was no history of trauma preceding the lesion. She also had no history of smoking or alcohol consumption.
Dermatological examination revealed a solitary indurated erythematous plaque with firm to hard consistency on the left lateral side of the neck (Figure 1A), and a faint erythematous plaque with telangiectasia at the left lateral nose. Other examinations including superficial lymph nodes, mucous membranes, scalp, hair, and nails were unremarkable. The initial diagnosis was dermatofibrosarcoma protuberans (DFSP). Other differential diagnoses were cutaneous pseudolymphoma, cutaneous Rosai-Dorfman disease, and lupus tumidus.
Laboratory data showed hemoglobin level of 11.4 g/dL, white blood cell count of 5300/mm3 composed of 59% neutrophils, 34% lymphocytes, and 5% monocytes, and platelet count of 262,000/mm3. Liver function test revealed total and direct bilirubin levels of 0.3 mg/dL and 0.1 mg/dL, respectively, aspartate aminotransferase 20 IU/L, alanine aminotransferase 16 IU/L, alkaline phosphatase 82 IU/L, and gamma-glutamyl transferase 16 IU/L. Renal function test showed blood urea nitrogen of 14 mg/dL and creatinine of 0.84 mg/dL. Urinalysis was within normal limits. Antinuclear antibody titer was 1:80 with coarse speckle pattern.
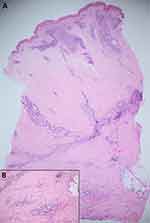
After obtaining informed consent, a punch biopsy from the lesion at the neck was performed. Histopathology revealed normal epidermis, dense superficial and deep perivascular and periadnexal lymphoplasmacytic infiltrates with lobular lymphocytic panniculitis. In addition, thickened homogenized collagen in the lower dermis extending to the broadened fibrous septum and loss of adipocytes around eccrine structures were noted (Figure 2). These findings shared histopathologic findings among lupus panniculitis and scleroderma.
Based on the histopathological features, the diagnosis of SLEP was made. The patient was treated with intralesional corticosteroid along with hydroxychloroquine (200 mg/day). Moderate potency topical steroid was applied twice daily on the lesion at the nose. Strict photoprotection was also advised. At 2-month follow-up, the lesions showed partial improvement with some indentation (Figure 1B). She remained free from signs and symptoms of systemic lupus erythematosus (SLE) after a 2-year follow-up period.
Discussion
Concomitant CCLE and localized scleroderma is a rare overlap syndrome of connective tissue diseases. The first case was described in 1978 by Umbet and Winkelmann with the clinicopathologies of both DLE and scleroderma.9 Later, in 1994, an unusual presentation of lupus panniculitis coexisting with localized morphea was reported.7 Sclerodermic or SLEP is an umbrella term proposed by Marzano for these groups of patients.8 They also share some histopathological findings such as lymphocytic panniculitis, lymphocytic vasculitis, dermal sclerosis, and broadening of fibrous septa.5
Due to the rarity of SLEP, the presence of lymphoid follicles with germinal center or dermal sclerosis can mislead to the diagnosis of cutaneous lymphoma or scleroderma. Previous documented cases are summarized in Table 1. Of six patients, half of them required repeated skin biopsy for the diagnosis of SLEP. Of note, all cases apart from ours were reported from Western countries with age of onset varying from 9 to 63 years. The predominant features are the female predilection and upper body distribution. Four of six lesions displayed a linear configuration. Association with SLE was reported in 2 cases.8 The first developed severe renal involvement 9 years after the onset of lesions, and the second had autoimmune thrombocytopenia 5 years prior to cutaneous presentation and experienced spontaneous abortion a few months before the lesions occurred.8 Marzano proposed that this rare variant could be a warning sign of impending SLE, and long-term follow-up on systemic symptoms are required.8 Most of SLEP lesions responded well to antimalarials and resolved with localized lipodystrophy. There was only one pediatric case that had almost complete healing without lipodystrophy.5 Our case presented with a hard indurated plaque mimicking dermatofibrosarcoma protuberans (DFSP), which is a unique clinical manifestation compared with previous reports on SLEP. The histopathology shared features of both lupus panniculitis and morphea. The superficial and deep perivascular and periadnexal lymphoplasmacytic infiltration and lobular lymphocytic panniculitis were compatible with lupus panniculitis while the sclerotic dermal collagen extending to the broadened fibrous septum were consistent with morphea. The patient had no signs and symptoms of SLE or systemic sclerosis.
 |
Table 1 Demographic Data, Clinical, Laboratory, Histopathological, Immunopathological Characteristics, Treatment and Outcome in Scleroderma-Like Lupus Erythematosus Panniculitis Cases |
The pathomechanism of fibrosis in lupus panniculitis is still unknown. Recent evidence reveals that active SLE neutrophils extracellular traps (NETs) bearing tissue factor and interleukin-17 had a potential role in promoting fibrotic activity in cultured human skin fibroblasts.10 The amount of neutrophil producing NETs was significantly higher in scarring lesions such as lupus panniculitis and DLE than the non-scarring subtypes, such as subacute cutaneous lupus erythematosus, suggesting that NETs might be associated with scarring and tissue damage.11 Current data mostly exhibited possible pathways involving transforming growth factor (TGF)-β to induce fibrosis in CCLE. A microarray study suggested TGF-β-dependent fibrosis formation in DLE.12 An increase of B lymphocytes in scarring DLE was also proposed for a pathogenic role in fibrosis by upregulating collagen production in a TGF-β1-dependent manner.13,14 Julia et al reported a case of CCLE coexisting with morphea and speculated that a lichenoid reaction may be a trigger in stimulating abnormalities in fibroblast-dependent reparative mechanisms leading to tissue sclerosis.15 Further studies on the pathogenesis of SLEP are required.
We report a case of SLEP mimicking DFSP in a middle-aged Thai woman presenting with a slowly growing firm to hard erythematous plaque on the lateral neck. The histopathological findings showed a characteristic pattern of lupus panniculitis as well as thickened homogenized collagen, consistent with morphea. To the best of our knowledge, this is the first report in an Asian patient presenting with a unique feature of SLEP. Due to its rarity, we encourage physicians to keep in mind the coexistence of lupus panniculitis and morphea. The pathogenesis is largely unknown. Whether SLEP is a distinct entity or a coincidental overlapping disease between lupus panniculitis and localized scleroderma remains to be determined. Future studies are warranted.
Conclusion
We report a case of SLEP presented with a solitary firm to hard erythematous plaque, mimicking DFSP and biopsy results were consistent with lupus panniculitis and scleroderma. Due to its rarity, there are limited data on whether SLEP holds a prognostic value for systemic or recurrent diseases. We encourage physicians to keep in mind the coexistence of these two distinct entities.
Ethical Statement
Written informed consent was provided by the patient to have her case details and images published. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Park HS, Choi JW, Kim BK, Cho KH. Lupus erythematosus panniculitis: clinicopathological, immunophenotypic, and molecular studies. Am J Dermatopathol. 2010;32(1):24–30. doi:10.1097/DAD.0b013e3181b4a5ec
2. Rangel LK, Villa-Ruiz C, Lo K, et al. Clinical characteristics of lupus erythematosus panniculitis/profundus: a retrospective review of 61 patients. JAMA Dermatol. 2020;156(11):1264. doi:10.1001/jamadermatol.2020.2797
3. Ng PP, Tan SH, Tan T. Lupus erythematosus panniculitis: a clinicopathologic study. Int J Dermatol. 2002;41(8):488–490. doi:10.1046/j.1365-4362.2002.01510.x
4. Chanprapaph K, Tankunakorn J, Suchonwanit P, Rutnin S. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational Study in Asians. Dermatol Ther. 2021;11(1):131–147. doi:10.1007/s13555-020-00471-y
5. Elbendary A, Griffin J, Li S, Tlougan B, Junkins-Hopkins JM. Linear sclerodermoid lupus erythematosus profundus in a child. Am J Dermatopathol. 2016;38(12):904–909. doi:10.1097/DAD.0000000000000663
6. Schwartz Z, Magro CM. Intralesional overlap syndrome: sclerodermic lupus panniculitis and sclerodermic discoid lupus erythematosus. JAAD Case Rep. 2020;6(3):166–168. doi:10.1016/j.jdcr.2019.10.018
7. Stork J, Vosmik F. Lupus erythematosus panniculitis with morphea-like lesions. Clin Exp Dermatol. 1994;19(1):79–82. doi:10.1111/j.1365-2230.1994.tb01125.x
8. Marzano AV, Tanzi C, Caputo R, Alessi E. Sclerodermic linear lupus panniculitis: report of two cases. Dermatology. 2005;210(4):329–332. doi:10.1159/000084760
9. Umbert P, Winkelmann RK. Concurrent localized scleroderma and discoid lupus erythematosus. Cutaneous ‘mixed’ or ‘overlap’ syndrome. Arch Dermatol. 1978;114(10):1473–1478. doi:10.1001/archderm.1978.01640220022005
10. Frangou E, Chrysanthopoulou A, Mitsios A, et al. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann Rheum Dis. 2019;78(2):238–248. doi:10.1136/annrheumdis-2018-213181
11. Safi R, Al-Hage J, Abbas O, Kibbi AG, Nassar D. Investigating the presence of neutrophil extracellular traps in cutaneous lesions of different subtypes of lupus erythematosus. Exp Dermatol. 2019;28(11):1348–1352. doi:10.1111/exd.14040
12. Sole C, Gimenez-Barcons M, Ferrer B, Ordi-Ros J, Cortes-Hernandez J. Microarray study reveals a transforming growth factor-beta-dependent mechanism of fibrosis in discoid lupus erythematosus. Br J Dermatol. 2016;175(2):302–313. doi:10.1111/bjd.14539
13. O’Brien JC, Hosler GA, Chong BF. Changes in T cell and B cell composition in discoid lupus erythematosus skin at different stages. J Dermatol Sci. 2017;85(3):247–249. doi:10.1016/j.jdermsci.2016.12.004
14. Francois A, Chatelus E, Wachsmann D, et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther. 2013;15(5):R168. doi:10.1186/ar4352
15. Julia M, Mascaro JM
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.