Back to Journals » Research and Reports in Tropical Medicine » Volume 10
Schistosomiasis: Still a Cause of Significant Morbidity and Mortality
Authors Verjee MA
Received 11 February 2019
Accepted for publication 24 October 2019
Published 3 January 2020 Volume 2019:10 Pages 153—163
DOI https://doi.org/10.2147/RRTM.S204345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Video abstract presented by Mohamud A Verjee.
Views: 1240
Mohamud A Verjee
Weill Cornell Medicine – Qatar, Qatar Foundation – Education City, Doha, Qatar
Correspondence: Mohamud A Verjee
University Institution Weill Cornell Medicine – Qatar Qatar Foundation – Education City, Doha P O Box 24144, Qatar
Tel +974 4492 8504
Fax +974 4492 8555
Email [email protected]
Abstract: Tropical diseases remain severe threats to global health with acute or chronic debility. Public health issues are regularly monitored and reported by the WHO. Conditions with high prevalence and virulence such as Schistosomiasis or Malaria still need active treatment. Advances over the decades in the treatment and management of Schistosomiasis have reduced morbidity and mortality in patients. However, poverty, adverse environments, lack of education and awareness, with parasites and vectors that can thrive if uncontrolled, remain issues for the successful global eradication of Schistosomiasis. From the disease’s discovery in 1850, the author relates historical details to its current status. Several countries previously affected, including Japan and Tunisia, have eliminated the disease while others seek the same goal. Africa remains the most severely affected continent with vulnerable women and children, although the infection persists in South America and the Far East of Asia as well. Realistic improvements for continuing health conditions are vogue and emphasized for those at risk or afflicted by the infection, illustrating success models of concerted efforts of extirpation. Constant proximity to infected water, with a parasite host, are hurdles in reducing exposure. Effective medication for acute treatment is available, and prophylaxis by vaccination is promising. Where endemic Schistosomiasis is prevalent, significant morbidity and mortality have far-reaching complications in multiple human organ systems, including irreversible pulmonary hypertension, renal, genitourinary, central nervous system conditions, and neoplasia. Two hundred and thirty million people are estimated to have contracted Schistosomiasis globally, with up to 700 million still at risk of infection, and 200,000 deaths occur annually. The disease may be more prevalent than thought after newer tests have shown increased sensitivity to pathological antigens. The author discusses infectivity risks, investigations, prognosis, treatment, and management, as well as morbidity and mortality.
Keywords: cercariae, egg load, granulomas, miracidia, morbidity, mortality, schistosomulae, cestode, trematode
Introduction - Historical Background
Theodor Bilharz, a German professor of anatomy and chief of surgery at the Kasr El Ani Hospital of Cairo from 1850, first identified an infective organism, Distomum hematobium in 1851, which was renamed Schistosoma haematobium in 1858.1 It arose from a cestode worm, Hymenoleptis nana, lying in the small colon of an Egyptian patient. He also discovered a trematode worm at the same time from an autopsy, thought to be the cause of urinary Schistosomiasis. Bilharz died from typhoid fever in 1862 at the age of 37. The Theodor Bilharz Research Institute in Giza, Egypt, stands as a tribute to him today. F. Milton published the first recorded peer-reviewed article report on Schistosomiasis in 1914.2
Today, over 26,762 peer-reviewed articles exist on the subject, the latest publication published in August 2019.3 Schistosomiasis, also known as bilharzia, primarily a parasitic tropical disease, is as significant for morbidity and mortality as ever in developing countries. It is the third most reported global tropical disease, and the second-most in the sub-Saharan region. A leading authority in the study of Schistosomiasis conservatively estimated at least 230 million people worldwide were infected with Schistosoma species in 2014.4 A WHO estimate cites over 700 million people as being at risk of infection, and approximately 200,000 annual deaths from Schistosomiasis alone.5 The disease now poses diagnostic issues with migrant displacement and relocation of populations to Europe and the West. Schistosomiasis is a zoonosis considered to be a neglected tropical disease (NTD). Five zoonoses have an underestimated burden on global health, leishmaniasis, lymphatic filariasis, intestinal nematodes, cryptosporidiosis, and schistosomiasis.6
Objectives
The objectives of this review were to study the epidemiology and characteristics of the disease, hosts, infectivity, subtypes, clinical presentations, its geographical distribution, prevalence, successes in eradication, persistent endemic areas of infection, modern means of diagnosis, morbidity, treatment, and outcomes.
Methodology
Data was collected scrutinizing PubMed using a MeSH search. Initial word searches included Schistosomiasis; S. mansoni; S. japonicum; S. hematobium; neuroschistosomiasis; best match; morbidity; mortality; prevalence; epidemiology; treatment; elimination; and species. The search period extended from the discovery of the Schistosoma parasite in 1850, as published in 1914, up until August 2019. The “best match“ articles totaled 26,683; plus, “mortality“ 7169; plus, “epidemiology“ 6564; plus, ”prevention” 1867; plus, ”treatment” 1578; plus, ”species” 94. One renowned author had a lifetime production of 186 first name and co-authored papers. Each of the ninety-four papers was read, noting content with context, and selected according to the objectives of the review paper.
Epidemiology
Schistosomiasis is a global cause of morbidity and mortality. Tropical endemic areas are the sources, in rivers, lakes, reservoirs, and irrigation channels, perpetuating the opportunities for infection. In fifty-two countries, S. mansoni causes debility with intestinal Schistosomiasis. They include the Caribbean, Eastern Mediterranean, South American, and most countries in Africa.7,8 It is estimated that more than 207 million people in at least seventy-four countries still have an active schistosomal infection. Approximately 60% are clinically affected, predominantly suffering from chronic anemia and malnutrition. More than 20 million are severely ill. Factors propagating the infection are poverty with poor sanitation, contaminated freshwater irrigation, and manifest schistosomal infestation by human, animal, and snail populations.
Addressing all these factors, particularly improving water quality, medical treatment in endemic areas including China, Brazil, Egypt, and some areas of sub-Saharan Africa, has been successful. However, the level of infection, while reduced, is still significant. Improvement of health in rural Chinese villages demonstrated substantial improvements in the human, snail, and wild mouse schistosomiasis infection rates.9 An earlier review stated that Schistosomiasis in China, dating back for over two millennia, had caused social and economic hardship, which compounded rates of mortality.10 Controlling morbidity from the infection by using one drug improved outcomes with a prevalence reduction from 11.6 million in the mid-1950s to an estimated 694,788 infections by 2000.
The World Health Organization5 stated that the global distribution of Schistosomiasis decreased with its eradication from Japan and the Lesser Antilles Islands. Transmission ceased in Tunisia and remains low in Morocco, Saudi Arabia, Venezuela, and Puerto Rico. Schistosomiasis is more common in males than females from occupational and leisure activities. Age affects the prevalence and severity of schistosomal infections. Children and adolescents are most often more heavily infected. Tourists to endemic areas need to take precautions and remain aware of the risks of contracting the disease during boat trips, rafting, and swimming.11 Even if asymptomatic, follow up within three months of exposure is advisable on returning home to exclude latent Schistosomiasis. Acute symptoms commonly affect non-immune travelers more due to a severe immune response.
A World Health Organization report5 noted that almost ninety million people had treatment for Schistosomiasis in 2016. This figure included 70.9 million school-aged children and approximately 18.3 million adults, mostly in Africa, where nine out of ten infected people live. Where treatment was required, thirty out of forty-one countries reported to the WHO. Almost 75% of treatment coverage was achieved by twelve of the reporting countries, still leaving a shortfall of 25%. Japan and Tunisia are two countries that have successfully eradicated the disease in their populations. The Caribbean Islands and Morocco have made good progress, too, while Brazil, China, and Egypt continue to eliminate Schistosomiasis actively. Neglected tropical diseases in sub-Saharan Africa were deemed to exert “great health, social and financial burden on economies of households and governments.”12 Poverty attributed to Schistosomiasis disrupted child development, morbidly affected pregnancy and reduced workforce production. Factors responsible for continued infection included “global warming, proximity to water, irrigation and dam construction as well as socio-economic factors.” Pre-existing poverty in sub-Saharan Africa is still evident.12 Anecrotic-exudative granuloma surrounds the isolated central egg. Figures 1 and 2 illustrate the parasite’s histological versatility to penetrate human tissue, leading to the formation of granulomas.
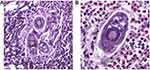 |
Figure 1 (A and B) Eggs of S. haematobium in a urinary bladder biopsy specimen, H & E stain. Notes: Reproduced from Michael E, De Bakey VA. Medical Center, Houston, Texas, United States. Available from: https://www.cdc.gov/dpdx/index.html.13 |
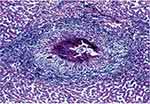 |
Figure 2 Egg of S. mansoni embedded in a liver. Notes: Reproduced from Lambertucci JR. Acute schistosomiasis mansoni: revisited and considered. Mem Inst Oswaldo Cruz. 2010;105(4):422–435. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762010000400012.14 |
More recent concerns appeared with Schistosomal infections arising in Corsica, acquired by French tourists bathing in the Cavu River between 2011 and 2013, necessitating the instigation of a screening program.15 The outbreak was the first reported Schistosomal infection in Europe since a report in Portugal in 1965.
Schistosomiasis infections of any type are uncommon in the United States unless imported. Although an estimated 400,000+ infected persons have emigrated in recent years, neither susceptible snail species nor chronically infected human reservoirs pose risks to contaminate freshwater in North America. Pathogenic schistosomes have survived and replicated in human hosts for years. Migrants may present to Emergency Departments (EDs) with active cases of acute or chronic Schistosomiasis, with associated end-organ complications.
Characteristics Of Clinical Schistosomiasis
The parasitic trematode flatworm Schistosoma causes Schistosomiasis.16 Freshwater snails are intermediate hosts, contaminating water by releasing larval forms of parasitic worms, which penetrate the skin. Mature infective larvae reproduce in the blood vessels, liver, kidneys, and intestines. Released egg antigens, trapped within organ tissues, stimulate granulomatous reactions involving T cells, macrophages, and eosinophils resulting in clinical disease. Symptoms and signs depend upon the load and location of trapped eggs. While initial inflammatory reactions are reversible, later stages of the disease are more debilitating. Pathological collagen deposition increases organ fibrosis that may not be fully reversible.
Ectopic eggs can settle in the skin, brain, muscle, adrenal glands, eyes, and genitourinary system, leading to new clinical syndromes. Granulomas may develop in the uterus, fallopian tube, and ovaries. The central nervous system (CNS) is prone to egg embolization from the portal-mesenteric system traveling to the brain and spinal cord tissue via the paravertebral venous plexus.17 S. haematobium egg retention and granuloma formation in the urinary tract can cause hematuria, dysuria, bladder polyps, ulcers, and obstructive uropathies. Central nervous system involvement rarely causes transverse myelitis.
Infectivity Risks
Poor hygiene, playing in riverbank mud and contaminated water, exposes children to a higher risk of infection. Some forty million women of childbearing age were affected in 2010.18 Almost ten million women in Africa sustained Schistosomiasis during pregnancy.19 The intensity and prevalence of infection rise with age and peaks between 15 to 20 years of age. In older adults, no significant change occurs in the incidence of disease, as the parasite burden, and intensity decrease.17 Women washing clothes in contaminated water, or living near open water, lakes or rivers, were also at higher risk of infection.20 Uganda has a unique feature with virtually no occurrence of infection at altitudes higher than 1400m or where land is drier and the annual rainfall is less than 900 mm.21
Most human Schistosomiasis commonly arises from three species, Schistosoma haematobium, Schistosoma japonicum, and Schistosoma mansoni. Less common species, such as Schistosoma mekongi and Schistosoma intercalatum, may also cause systemic human disease.22
Snail Hosts
Schistosomiasis is endemic in seventy-four developing countries, 80% of those infected living in sub-Saharan Africa with humans acting as the parasite’s reservoir. A seven-year study (2005–2012) in Nanchang City, China, indicated the use of health education for susceptible populations.23 Morbidity fell from 46% in 2005 to 0% by 2012. The different species of Schistosoma infecting specific types of snails as intermediate hosts are listed in Table 1. Biomphalaria glabrata, an example of a snail host (Figure 3), is illustrated.24
 |
Figure 3 Biomphalaria glabrata, an example of a snail host, is illustrated. Notes: Reproduced from Blouin laboratory, Oregon State University. Available from: https://www.flickr.com/photos/oregonstateuniversity/16730248430/in/photolist-ruoTZ9. |
 |
Table 1 Snail Carriers And Their Respective Infective Schistosoma Species And Snail Carriers |
Acute And Chronic Schistosomiasis
Acute Schistosomiasis, (Katayama fever), is a “serum sickness-like illness that develops after several weeks elapse from infection by new schistosomal infections.”21 The first cycle of egg deposition promotes marked peripheral eosinophilia and circulating immune complexes. While symptoms usually resolve over several weeks, infections can overwhelm the immune response. Paradoxically, early treatment with bactericidal drugs may exacerbate this syndrome. Steroid therapy usually settles the syndrome’s inflammatory reaction. Water contact, i.e., bathing and swimming, is the most common finding in a history of infection. Mild, maculopapular skin lesions can develop in an acute infection within hours after exposure to cercariae. Tourists visiting endemic areas are at high risk of infection and may present diagnostic problems to home physicians unfamiliar with the schistosome lifecycle.
Chronic Schistosomiasis may occur when adult worms absorb host proteins and can live for years in the bloodstream coated with host antigens if unaffected by the immune system. Bowel granulomas with egg retention can cause bloody diarrhea, bowel cramps, and inflammation associated with colonic polyposis. The bowel wall becomes less resistant to infections such as Salmonella, allowing a higher opportunity for bacteremia. Chronic Schistosomiasis can complicate acute appendicitis.25 Eggs penetrate the bowel adjacent to mesenteric vessels where adult worms reside. Unshed eggs lodged in the portal circulation, induce granulomatous reactions in the portal tracts.
Systems Most Severely Affected From Schistosoma Infection
- Cardiopulmonary (associated with hepatic disease)
- Gastrointestinal tract
- Genitourinary tract
- Central nervous system
Cardiopulmonary Arterial Hypertension Linked To Hepatic Disease
Hepatic disease is more prevalent with heavy infestations, promoting collateral veins, and enabling eggs to reach the pulmonary circulation. Subsequent pulmonary granulomatosis and fibrosis can lead to irreversible pulmonary hypertension and frank cor pulmonale with a high mortality rate.26 This critical complication develops in about 7.7% of patients with a hepatosplenic disease from S. mansoni, S. japonicum, and possibly S. mekongi infections. The prevalence of the disease’s pulmonary artery complication worldwide was estimated to exceed 270,000 individuals in 2009.26
Gastrointestinal
Gallbladder cancer has an association with schistosomal infections. S. haematobium infection can increase the rate of bladder cancer, usually squamous cell, rather than the transitional cell type.27 Local tissue invasion by eggs stimulates toxin and enzyme release, provoking a Th2–mediated immune response.28 When glomerulonephritis occurs, the renal changes are often irreversible, and pathological symptoms can persist for years. Periportal fibrosis, also termed Symmers pipestem fibrosis, is the most common complication of gastrointestinal Schistosomiasis (GS). The fibrosis leads to portal hypertension and gastrointestinal bleeding. Liver failure is uncommon, except in the presence of chronic hepatitis B or cirrhosis.26 S. mansoni tends to be the species associated with the rapid progression of liver disease. GS patients with low worm loads are difficult to diagnose accurately and more challenging to control.29,30
Genitourinary Tract
Long after the initial infection, a parasitic infection may cause renal failure from obstructive uropathy, pyelonephritis, or bladder carcinoma. The five main schistosome parasitic species have a widespread distribution Table 2. illustrates. Schistosomiasis infections during pregnancy can cause debility with severe anemia. Low birth weights are common.5 Infant and maternal mortality increases. Placental infection, with an infant confirmed with the disease, establishes congenital infection.4 Vulval genital Schistosomiasis, spread by S. haematobium causes significant social and medical problems. It increases the risk factors of spreading certain sexually transmitted diseases, such as HIV and human papillomavirus (HPV) by up to 30%.31 S. haematobium increases male genital organ pathology in the epididymis, spermatic cord, testes, and prostate gland, which can lead to sterility.
 |
Table 2 Parasitic Species And Geographical Distribution Of Schistosomiasis |
CNS Schistosomiasis
S. japonicum is mainly responsible for 60% of all Schistosoma brain infections because of its small egg size.32
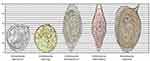 |
Figure 4 Eggs of Schistosoma, S. hematobium, S. intercalatum, and S. mansoni with their typical spines and comparative sizes. Notes: Reproduced from CDC. Atlanta, United States. Figure 6. Available from: https://www.cdc.gov/dpdx/diagnosticprocedures/stool/morphcomp.html.33 |
CNS involvement occurs in 2 – 4% of all S. japonicum infections. S. mansoni has a larger egg size and can affect spinal tissue. S. haematobium can also affect the brain. S. mekongi, limited to the Mekong River basin in Laos and Cambodia, affects an estimated 140,000 people at high risk of being infected. Temporal brain masses, causing paresthesia of the arm and leg with dysphasia, are associated with this species. Neurologic symptoms such as cauda equina syndrome, anterior spinal artery syndrome, and quadriparesis can develop months after the initial infection.34
Lifecycle
Schistosomes in endemic areas infect specific freshwater snails, with intrinsic parasitic species. The infected snails release cercariae four to six weeks after infection. The fork-tailed cercariae are free-swimming larvae approximately 1mm in length, capable of freshwater survival for up to 72 hrs. To live, they must attach to human skin or another suitable animal host. Once connected using oral and ventral suckers, the larvae progressively burrow through intact skin, entering dermal veins. Following access, the larvae migrate to the pulmonary vasculature. During this phase, the cercariae metamorphose and develop an outer coating, which resists a sustained host immune attack. As schistosomulae worms, they reach pulmonary capillaries through the systemic circulation, maturing in the portal veins of the liver.
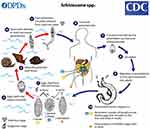 |
Figure 5 The life cycle of the schistosoma parasite. Notes: Reproduced from CDC. Atlanta, United States. Image courtesy of DPDx, Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/parasites/schistosomiasis/biology.html. 35 |
The highly antigenic eggs migrate through the bowel or bladder wall by fecal or urinary shedding. Over approximately ten days, the organisms begin to mature into miracidia. Unshed eggs may remain in the tissues or flow back to the portal circulation from the mesenteric vessels, or the pulmonary circulation from the vesicular vessels via the inferior vena cava. The free-swimming miracidia shed into fresh water survive one to three weeks. They must complete their life cycle by infecting a snail. Two generations of sporocysts multiply, mature into free-swimming cercariae, and exit the snail to seek a human host and begin a new cycle. S. japonicum, most likely the species with the highest risk of complications, infect cattle and other domesticated animals. Its life-cycle pattern provides a source of the disease that often thwarts control efforts based on treating human infection and reducing snail populations. Comparative Schistosoma species are illustrated for egg size in Figure 4 and the life cycle of the schistosoma parasite in Figure 5.
Prognosis
Early treatment of disease produces better outcomes, even those affected by a hepatic, urinary, or neuro-cerebral disease. Hepatosplenic Schistosomiasis has a relatively good prognosis with preserved hepatic function until the end of the disease, in the absence of variceal bleeding. Late-stage treatment provides less benefit with multiple system involvement, especially with a past heavy worm load in tissues.
Morbidity And Mortality
Acute Schistosomiasis, or Katayama fever,36 can increase the mortality rate by up to 25%. Even with asymptomatic disease, morbidity affects health. Hepatic, cardiac, pulmonary, renal, neurological diseases, along with malignancy, are the main problems. In women, genital infections can complicate pregnancy. Repeated courses of therapy are often required as reinfection is a constant risk in endemic areas. Schistosomiasis often develops over a while, leading to the delay of diagnosis (Table 3 & 4).
Some signs and symptoms are common to a large number of disorders.
The result can lead to serious systemic complications and multidimensional organ involvement (Table 4).
Acute Manifestations
Patients with acute Schistosomiasis, usually present about four to eight weeks after exposure to infected water. S. japonicum or S. mansoni can be symptomatic after two weeks. Katayama syndrome should be suspected when fever, lethargy, malaise, and myalgia occur. Milder symptoms include a cough, headache, anorexia, and rashes.36 Care should be taken not to confuse Schistosomiasis with malaria or viral infections, the rash at the entry site of the worm being definitive.
Chronic Manifestations
As patients with symptomatic chronic Schistosomiasis may present many years after primary exposure, a carefully elicited travel history is imperative for a firm diagnosis. Five species of Schistosoma can cause intestinal or liver disease: S. mansoni, S. mekongi, S. intercalatum, and S. japonicum. However, S. hematobium only rarely causes intestinal or liver disease, more commonly causing urinary tract disease.37
Physical Findings In Schistosomiasis
A comparison of physical findings differentiates acute from chronic pathology with specific entities in Table 5. Hepatosplenomegaly is a standard feature for both.
 |
Table 3 Schistosomiasis Signs and Symptoms (WHO, 2016) |
 |
Table 4 System Complications |
 |
Table 5 Physical Findings of Schistosomiasis (WHO, 2016) |
 |
Table 6 Definitions of Words |
Laboratory Investigations
Primary tests include serology and polymerase chain reaction (PCR) assays. A complete blood count (CBC) is necessary, looking for peripheral eosinophilia, particularly in acute infection and anemia. Alkaline phosphatase and gamma-glutamyltransferase (GGT) levels increase, seen with hepatic granulomatosis. Transaminase levels are generally unaffected, and elevated levels are due to coexisting hepatitis. Checking renal function is vital as it may deteriorate in severe obstructive nephropathy. Patients with persistent or recurrent fever and those with recurrent Salmonella infection with severe enteric Schistosomiasis should have blood cultures. Urinary microbiology is imperative when diagnosing vesicular infections from S. haematobium. Macroscopic and microscopic hematuria is common. Egg load measurement itself is an unreliable measure in relating the severity of the disease. Fecal microbiology is essential for a diagnosis with any bowel infection.
Imaging Studies
Several imaging investigations enable a more accurate assessment of a patient’s condition.38 Ultrasonography (US) is helpful for hepatic and renal assessments. An intravenous pyelogram (IVP) may also be required. A chest X-ray and echocardiogram establish cardiopulmonary status. Plain abdominal X-rays, computerized tomography (CT), magnetic resonance imaging (MRI) also have a place according to the clinical condition. Rarely, is a barium swallow or endoscopy, with contrast studies of the small and large bowel, required.
Biopsy And Other Procedures
Tissue biopsies for egg detection are necessary only when stool sample findings are negative, or if the infection is light. Occasionally, a mucosal or liver biopsy can reveal further pathology. Additional procedures may include a sigmoidoscopy after proctoscopy, cystoscopy, bronchoscopy, lumbar puncture, or upper endoscopy.
Treatment & Management
Two anthelmintic medications are effective for Schistosomiasis. Praziquantel (PZQ) is the drug of choice for mass community treatment. It is the cheapest, effective medication with a cure rate of 65–90% after a single oral dose. Its pharmacological action affects the membrane permeability of the parasite by vacuolation of the tegument. While mature worms are paralyzed and attacked by the host immune system, developing schistosomula are more resistant, allowing the infection to continue. Praziquantel is safe to use in pregnant and lactating women.39 Praziquantel resistance is also well defined.40 Mild adverse effects from the reactions of dying worms lasting about twenty-four hours comprise dizziness, headache, nausea, vomiting, diarrhea, abdominal discomfort, bloody stool, urticaria, and fever following initiation of treatment.19
Oxamniquine, used since 1972, is only useful for S. mansoni infections and is not advised during pregnancy. The drug predominantly damages male worms with the loss of their female conjugal partners. Egg production profoundly reduces or stops.
Hospital admissions provide fuller assessments and enable definitive diagnosis after considering differential diagnoses. For acute schistosomiasis illness (Katayama fever), anti-schistosomal drugs and high-dose corticosteroids are the best treatments. A second course of therapy, several weeks after the first, is often considered. When there is a central nervous system (CNS) infiltration, oral prednisone therapy of 40mg daily for five days is supportive and is strongly recommended together with PZQ. Together, they reduce inflammation, edema, and hypersensitivity around eggs, which damages neural tissue. Anticonvulsant therapy may be necessary if seizures are induced by developing cysticerci larvae. The goals of chemotherapy are to cure the disease and decrease morbidity, then to eliminate transmission of the parasite in the endemic areas. Artemether, an antimalarial drug, and PZQ combined can kill schistosomula during the first three weeks of infection. Individuals co-infected with Plasmodium and Schistosoma treated with a mefloquine-artesunate combination may experience a dual benefit with a clearance of malaria parasitemia and a reduction of schistosomiasis-related morbidity.41 Occasional excision of tumors, specifically pulmonary and bladder granulomas, ligation of esophageal varices, and fashioning porta-caval shunts, are surgical options.
Outpatient Care
Egg count evaluation assesses any treatment response. For two weeks afterward, egg counts may not decrease as eggs laid before the treatment are not shed for up to two weeks and may still be excreted for up to two months post-treatment.
Measuring worm antigen levels, five to ten days after treatment, may help determine the therapeutic response. When egg antigens circulate persistently, residual infection is present, necessitating further PZQ treatment. Clear stool and urine samples tested for up to six months after treatment ensure absent egg excretion. The presence of hematuria and eosinophilia are more important than checking antigen serology, which can remain positive for years.
Prevention
Current research for a human vaccine against Schistosomiasis is promising for both humans and bovine animals.42 While PZQ is the most effective drug, it has no prophylactic effect. Clinical trials are in progress to develop an effective vaccine. Some promising results have been found in DNA vaccinated naive mice.43 The use of recombinant S. mansoni fatty acid binding protein (smFABP) shows significant acquired protection of 74.2% (p<0.01) against S. mansoni infection. Also, vaccination revealed an enhanced curative effect in subsequent PZG treated mice. Clinical studies show that Artemether may have potential as a prophylactic agent when given once every two to four weeks. Trials also show that while used effectively as an antimalarial treatment, the medication is active against all three major schistosomal parasites.
Traveler Advice
Travelers to endemically infected areas should be aware of the exposure risk to freshwater larvae. Early treatment reduces morbidity and is advisable on accurate diagnostic test results, or if there is a high clinical suspicion of Schistosomiasis. Topical lotions of DEET (N, N-diethyl-m-toluamide) suffice for effectively killing schistosome cercariae, with minimal risk to any person.44
Control Of Infection In Endemic Areas
Several factors are essential in the control of Schistosomiasis in endemic areas:
- Providing population-based preventive chemotherapy
- Ensuring a safe water supply
- Health education for the improvement of water sanitation
- Avoiding schistosome-contaminated urine or stool is a prerequisite
- Removing snails decreasing the transmission of parasites adds further control.
Elimination Of Schistosoma
The task ahead to eliminate global human Schistosoma infection is extensive and will require investment. Public Health measures undertaken with determination need infrastructure, and government or commercial support. Focused clearing of the parasites and snail hosts from community accessed water, needs proper sanitation, and attention to hygiene (WASH) as well as mass drug administration (MDA).45 However, “hotspots” may remain and give rise to new infections, reversing the attempts of control.46 The prevalence of the three common Schistosoma species impacting human morbidity may be higher than detected. Evidence that older analytic tools were not picking up the correct degree was confirmed when worm antigens were tested in the urine of previously infected people, particularly using the circulating anodic antigen (CAA).47
Limitations
The plethora of over 27,000 publications available for review posed challenges in assembling this article. While the World Health Organization (WHO) was an invaluable resource, the data used for Schistosomiasis is subject to being updated continuously. The author endeavored to use the latest qualitative and quantitative data available. A list of definitions is outlined in Table 6.
Conclusion
Schistosomiasis continues to be a scourge on health. Effective public health policies with improved hygiene and population education, have improved the quality of life in areas now cleared of the infection. The advent of new, economic vaccines may aid future eradication of the parasite. All the previous determinants of health need active support. Pregnant women and young children are more severely affected by the debilitating disease. Significant complications can arise in multiple organ systems, contributing to morbidity and mortality. Countries have shown that by developing focused policies, Schistosomiasis can be reduced and eliminated. Increasing public health awareness, reducing the incidence both in endemic regions, and lifting people out of poverty are both keys to the continued eradication of the infection. Progress to date has been encouraging, but global warming may negatively impact outcomes, and monitoring the parasite’s prevalence will continue to be a significant public health issue.
Acknowledgments
The author acknowledges CDC, Atlanta, Georgia, US; Michael De Bakey V A Veteran Center, Texas, US; Oregon State University, US; Genome Research Limited, US; JR Lambertucci; and CDC Atlanta, Georgia, US.
As per Creative Commons license. All selected images are in the public domain and have been included without any changes and are fully referenced.
The author also expresses grateful thanks to Ms. Cathleen De Groot, DeLib Library Manager, Reference and Instruction, Weill Cornell Medicine - Qatar, Education City, Qatar Foundation, Doha, Qatar, for her support and assistance with reference citations.
The publication of this article was funded by the Qatar National Library, Doha, Qatar.
Disclosure
The author reports no conflicts of interest in his work.
References
1. Beaver PC, Jung RC, Cupp EW. Clin Parasitol.
2. Milton F. Does Bilharzia (Schistosomiasis) exist in India? Ind Med Gaz. 1914;49(10):10–14.
3. Andrianjafitrimo HT, Ranaivomanana VF, Ravelomampitoniainarivony TM, Ramiandrasoa LA, Randrianjafisamindrakotroka NS. Schistosomiasis of the female genital tract: a two-center study. Med Sante Trop. 2019;29(3):306–309. doi:10.1684/mst.2019.0910
4. Colley DG, Bustinduy AL, Secor WE, King CH. Human Schistosomiasis. Lancet. 2014;383(9936):2253–2264. doi:10.1016/S0140-6736(13)61949-2
5. Schistosomiasis: WHO reports substantial treatment progress for school age children. 2016. Available from: http://www.who.int/neglected_diseases/news/WHO_schistosomiasis_reports_substantial_treatment_progress_sac/en/.
6. Pisarski K. The global burden of disease of zoonotic parasitic diseases: top 5 contenders for priority consideration. Trop Med Infect Dis. 2019;4(1):44. doi:10.3390/tropicalmed4010044
7. John R, Ezekiel M, Philbert C, Andrew A. Schistosomiasis transmission at high altitude crater lakes in western Uganda. BMC Infect Dis. 2008;8:110. doi:10.1186/1471-2334-8-110
8. CH K. Toward the elimination of Schistosomiasis. N Engl J Med. 2009;360(2):106–109. doi:10.1056/NEJMp0808041
9. Wang LD, Chen HG, Guo JG, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360(2):121–128. doi:10.1056/NEJMoa0800135
10. Zhou XN, Wang LY, Chen MG, et al. The public health significance and control of Schistosomiasis in China – then and now. Acta Trop. 2005;96(2–3):97–105. doi:10.1016/j.actatropica.2005.07.005
11. Röser D, Bjerrum S, Helleberg M, et al. Adventure tourism and Schistosomiasis: serology and clinical findings in a group of Danish students after white-water rafting in Uganda. JMM Case Rep. 2018;5(4):e005141. doi:10.1099/jmmcr.0.005141
12. Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human Schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19(2):196–205. doi:10.1016/j.bjid.2014.11.004
13. Michael E, De Bakey VA. Medical Center, Houston, Texas, United States. Centers for Disease Control. Available from: https://www.cdc.gov/dpdx/index.html. Figures E & F.
14. Lambertucci JR. Acute schistosomiasis mansoni: revisited and considered. Mem Inst Oswaldo Cruz. 2010;105(4):422–435. Figure 6 Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762010000400012. Accessed June 14, 2019. doi:10.1590/S0074-02762010000400012.
15. Beltrame A, Zammarchi L, Zuglian G, et al. Schistosomiasis screening of travelers from Italy with possible exposure in Corsica, France. Emerg Infect Dis. 2015;21(10):1887–1889. doi:10.3201/eid2110.150869
16. CIA World Factbook. Puerto Rico Major infective diseases; 2018. Available from: https://www.indexmundi.com/puerto_rico/major_infectious_diseases.html.
17. Corachan M. Schistosomiasis and international travel. Clin Infect Dis. 2002;35(4):446–450. doi:10.1086/cid.2002.35.issue-4
18. Nour N. Schistosomiasis: health effects on women. Rev Obstet Gynecol. 2010;3(1):28–32.
19. Friedman JF, Mital P, Kanzaria HK, Olds GR, Kurtis JD. Schistosomiasis and pregnancy. Trends Parasitol. 2007;23(4):159–164. doi:10.1016/j.pt.2007.02.006
20. Kabatereine N, Brooker S, Tukahebwa E, Kazibwe F, Onapa AW. Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Trop Med Int Health. 2004;9(3):372–380. doi:10.1046/j.1365-3156.2003.01176.x
21. Ahmed SH Schistosomiasis. Medscape; 2018. Available from: https://emedicine.medscape.com/article/228392-overview.
22. Houston S, Kowalewska-Grochowska K, Naik S, McKean J, Johnson ES, Warren K. First report of Schistosoma mekongi infection with brain involvement. Clin Inf Dis. 2004;38:e1–e6. doi:10.1086/379826
23. Peng GH, Hu ZH, Zhou YS, et al. Epidemic situation of acute Schistosomiasis in Nanchang City of Jiangxi Province from 2005 to 2012. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013;25(5):552–554.
24. Biomphalaria glabrata image. Blouin laboratory, Oregon State University. Available from: https://www.flickr.com/photos/oregonstateuniversity/albums/72157618063567647/page21.
25. Badmos KB, Komolafe AO, Rotimi O. Schistosomiasis presenting as acute appendicitis. East Afr Med J. 2006;83(10):528–532. doi:10.4314/eamj.v83i10.9464
26. Lapa M, Dias B, Jardim C, Fernandes CJ, Dourado PM, Figueiredo M. Cardiopulmonary manifestations of hepatosplenic Schistosomiasis. Circulation. 2009;119(11):1518–1523. doi:10.1161/CIRCULATIONAHA.108.803221
27. Nmorsi O, Ukwandu N, Egwungenya O, Obhiemi N. Evaluation of CD4(+)/CD8(+) status and urinary tract infections associated with urinary Schistosomiasis among some rural Nigerians. Afr Health Sci. 2005;5(2):126–130.
28. Coutinho HM, Acosta LP, Wu HW, et al. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection. J Infect Dis. 2007;195(2):288–295. doi:10.1086/521712
29. Giboda M, Smith JM. Schistosoma resistance to praziquantel: fact or fiction? Parasitol Today Abstract. 1997;13(4):158. doi:10.1016/S0169-4758(97)89816-X
30. Tsang VC, Wilkins PP. Immunodiagnosis of Schistosomiasis. Immunol Invest. 1997;56(1):107–112.
31. Mosunjac MB, Tadros T, Beach R, Majmudar B. Cervical schistosomiasis, human papilloma virus (HPV), and human immunodeficiency virus (HIV): a dangerous coexistence or coincidence? Gynecol Oncol. 2003;90(1):211–214. doi:10.1016/S0090-8258(03)00191-4
32. Walker M, Zunt JR. Parasitic central nervous system infections in immunocompromised hosts. Clin Infect Dis. 2005;40(7):1005–1015. doi:10.1086/cid.2005.40.issue-7
33. Centers for Disease Control (CDC). Atlanta, United States. Figure 6. Available from: https://www.cdc.gov/dpdx/diagnosticprocedures/stool/morphcomp.html.
34. Vennervald BJ, Dunne DW. Morbidity in Schistosomiasis: an update. Curr Opin Infect Dis. 2004;17(5):439–447. doi:10.1097/00001432-200410000-00009
35. Centers for Disease Control (CDC). Atlanta, United States. Available from: https://www.cdc.gov/dpdx/schistosomiasis/modules/Schistomes_LifeCycle_lg.jpg . Accessed October 17, 2019.
36. Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama syndrome. Lancet Infect Dis. 2007;7(3):218–224. doi:10.1016/S1473-3099(07)70053-1
37. Kjetland EF, Mduluza T, Ndhlovu PD, Gomo E, Gwanzura L, Midzi N. Genital schistosomiasis in women: a clinical 12-month in vivo study following treatment with praziquantel. Trans R Soc Trop Med Hyg. 2006;100(8):740–752. doi:10.1016/j.trstmh.2005.09.010
38. Lambertucci JR, Serufo JC, Gerspacher-Lara R, et al. Schistosoma mansoni: assessment of morbidity before and after control. Acta Trop. 2000;77(1):101–109. doi:10.1016/S0001-706X(00)00124-8
39. Olds GR. Administration of praziquantel to pregnant and lactating women. Acta Trop. 2003;86(2–3):185–195. doi:10.1016/S0001-706X(03)00033-0
40. Ismael M, Botros S, Metwally A, et al. Resistance to Praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60(6):932–935. doi:10.4269/ajtmh.1999.60.932
41. Keiser J, N’Guessan NA, Adoubryn KD, et al. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2010;50(9):1205–1213. doi:10.1086/649516
42. Mo AX, Colley DG. Workshop report: schistosomiasis vaccine development and product characteristics. Vaccine. 2016;34(8):995–1001. doi:10.1016/j.vaccine.2015.12.032
43. Aly I, ELnain G, Hamad RS, et al. DNA vaccination using recombinant Schistosoma mansoni fatty acid binding protein (smFABP) gene. Exp Parasitol. 2018;194:53–59. doi:10.1016/j.exppara.2018.09.018
44. Secor WE, Colley DG. When Should the Emphasis on Schistosomiasis Control Move to Elimination? Trop Med Infect Dis. 2018;3(3):85. doi:10.3390/tropicalmed3030085
45. Ramaswamy K, He YX, Salafsky B, Shibuya T. Topical application of DEET for Schistosomiasis. Trends Parasitol. 2003;19(12):551–555. doi:10.1016/j.pt.2003.10.001
46. Kittur N, Binder S, CH C, et al. Defining hotspots: areas that fail to decrease meaningfully in prevalence after multiple years of mass drug administration with praziquantel for control of Schistosomiasis. Am J Trop Med Hyg. 2017;97(6):1810–1817. doi:10.4269/ajtmh.17-0368
47. Colley DG, Andros TS, Campbell CH
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
