Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Sarecycline: A Review of Preclinical and Clinical Evidence
Authors Moore AY, Del Rosso J , Johnson JL , Grada A
Received 22 April 2020
Accepted for publication 15 July 2020
Published 13 August 2020 Volume 2020:13 Pages 553—560
DOI https://doi.org/10.2147/CCID.S190473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Angela Yen Moore,1,2 James Del Rosso,3,4 Jodi L Johnson,5 Ayman Grada6,7
1Arlington Research Center, Inc., Arlington, TX, USA; 2Baylor University Medical Center, Dallas, TX, USA; 3JDR Dermatology Research/Thomas Dermatology, Las Vegas, NV, USA; 4Touro University Nevada, Henderson, NV, USA; 5Departments of Dermatology and Pathology, Feinberg School of Medicine, Northwestern University, Evanston, IL, USA; 6R&D and Medical Affairs, Almirall (US), Exton, PA, USA; 7Department of Dermatology, Boston University School of Medicine, Boston, MA, USA
Correspondence: Angela Yen Moore
Arlington Research Center, Inc., 711 East Lamar, Suite 200, Arlington, TX 76011, USA
Tel +1 817 795-7546 ext. 328
Email [email protected]
Abstract: Sarecycline is a tetracycline-derived oral antibiotic, specifically designed for acne, and is approved by the Food and Drug Administration (FDA) in 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris (AV) in patients 9 years of age and older. It has been decades since a novel systemic antibiotic was approved to treat AV, a disease that affects up to 90% of teenagers and young adults worldwide and lasts well into adulthood. Sarecycline holds promise to yield fewer side effects than other commonly used broad-spectrum tetracyclines, including minocycline and doxycycline. The narrower spectrum of antibacterial activity of sarecycline, which specifically targets C. acnes and some Gram-positive bacteria with little or no activity against Gram-negative bacteria, suggests not only the potential for reduced emergence of antibiotic-resistant bacterial strains but also less disruption of the human gut microflora. Here, we review the key preclinical and clinical evidence on sarecycline.
Keywords: acne vulgaris, antibiotic, narrow spectrum, tetracycline, sarecycline
Introduction
Acne vulgaris (AV) affects nearly everyone, particularly during teenage and young adult years. Over 40% of individuals still suffer from AV well into adulthood. Therefore, much effort continues to be made to identify the most effective and well-tolerated treatments.1,2 Tetracyclines have been prescribed to treat moderate to severe AV since the 1970s. The antibacterial mechanism of action of tetracyclines occurs via inhibition of protein synthesis by preventing the association of aminoacyl-tRNA with the bacterial ribosome of susceptible organisms.3 The therapeutic success of tetracyclines for AV, a disease of the pilosebaceous unit, is believed to correlate directly with both their anti-inflammatory and antimicrobial properties. The anti-inflammatory activity of tetracycline-class drugs is based on multiple reported biologic properties, including inhibition of matrix metalloproteinases (MMPs), suppression of IL-8, TNFα, and IL-6 gene expression from neutrophils and macrophages, suppression of hydrolases such as α-amylases and phospholipase A2, and scavenging of reactive oxygen species.4,5
Tetracyclines, including doxycycline and minocycline, are frequently prescribed for AV when topical treatment alone is deemed unsuccessful or unpractical, in patients that present with moderate-to-severe inflammatory AV.
Because of the increasing problem of antibiotic resistance, strategies to limit antibiotic overuse have emerged. These strategies include limiting the duration of broad-spectrum oral antibiotics and the preferential use of narrow-spectrum or targeted antibiotics when the causative organism is known.6–10 Side effect profiles have also limited the use of oral tetracyclines in some patients, including gastrointestinal (GI) complications, phototoxicity, dizziness, vertigo, benign intracranial hypertension (pseudotumor cerebri), lupus-like syndrome, changes in pigmentation, and urticaria.11–14
Sarecycline is a novel tetracycline-derived drug, specifically designed for acne, with a narrow antibacterial spectrum when compared to previously available broad-spectrum tetracycline-class antibiotics; it was FDA-approved in October 2018 for once-daily treatment of inflammatory lesions of non-nodular moderate to severe AV in patients ≥9 years of age.15 Here, we will review the pharmacology and clinical characteristics of sarecycline that are believed to relate to its therapeutic potential.
In vitro Antibacterial Activity
Tetracyclines exhibit a broad-spectrum of antibacterial activity, targeting a wide range of both Gram-positive and Gram-negative bacteria, including activity against normal bacterial microflora commonly found in the human GI tract.3,16-19 These normal microbiota include Enterobacteriaceae, Enterococcus, and Escherichia species, of which disruption (dysbiosis) could potentially lead to sequelae associated with gastrointestinal disease,20–22 but also the emergence of antibiotic-resistant strains.23,24 Below, we summarize the main findings of the microbiological profile of sarecycline compared to other tetracyclines.
Gram-Positive Bacteria
Studies have been conducted to determine the antibacterial spectrum of activity of sarecycline compared to other tetracycline-class compounds.19 Similar to other tetracyclines, sarecycline exerts its antibacterial effect mainly as a ribosomal protein inhibitor.19,25 Sarecycline has shown activity against C. acnes that is comparable to doxycycline and minocycline (Table 1). Importantly, the minimum inhibitory concentration (MIC90) for sarecycline was 0.5 μg/mL against both methicillin-susceptible (MSSA) and methicillin-resistant (MRSA) strains of S. aureus and 2 µg/mL against both methicillin-susceptible and -resistant S. epidermidis, only twofold less active than doxycycline and minocycline. Sarecycline was more active than tetracycline and doxycycline against S. haemolyticus. Sarecycline MIC90 was 8–32 μg/mL against S. pyogenes, S. agalactiae, E. faecalis, and E. faecium (both vancomycin susceptible and resistant (Table 2)).
 |
Table 1 Activity of Sarecycline and Comparator Agents Against 55 Clinical Isolates of C. acnes |
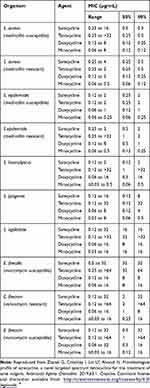 |
Table 2 Activity of Sarecycline and Comparator Agents Against Isolates of Gram-Positive Bacteria Except C. acnes |
Gram-Negative Bacteria
Sarecycline demonstrated very low activity (MIC90 >64 µg/mL) against aerobic Gram-negative bacilli E. cloacae, E. coli, K. pneumoniae, P. mirabilis, and Salmonella spp (Table 3). Sarecycline was least active (2 to 32 fold reduced MIC90 potency compared to minocycline and doxycycline) against isolates of Enterobacteriaceae and other clinically relevant Gram-negative microflora commonly found in the human GI tract.19
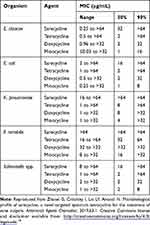 |
Table 3 Activity of Sarecycline and Comparator Agents Against Isolates of Gram-Negative Bacteria |
In vivo Antibacterial Activity
A murine thigh infection model was used to represent a tissue-based in vivo infection. Sarecycline demonstrated in vivo efficacy against systemic infection caused by S. aureus and achieved a 2-log10 reduction in the bacterial burden in the thigh at a dose comparable to doxycycline.19
Available data support that sarecycline is a narrow-spectrum antibiotic, which targets C. acnes, the bacterium involved in AV pathophysiology, while having lower antibacterial activity than conventional tetracyclines against normal microflora, including Enterobacteriaceae, Enterococcus, and Escherichia species.
Resistance Profile and Mutation Rates of Different Bacteria
The same report that tested activity against various bacteria also looked at spontaneous mutation rates of bacteria (C. acnes, S. aureus, and S. epidermidis) cultured in the presence of sarecycline, vancomycin or minocycline.19 Sarecycline showed a very low propensity to induce bacterial resistance with spontaneous mutation frequencies ranging from 10−9 to 10−11 for C. acnes at 4 and 8-fold the MIC, similar to vancomycin. The spontaneous mutation frequencies were 10−9 for S. aureus and 10–8 for S. epidermidis at 4- and 8-fold the MIC, similar to vancomycin. Sarecycline structural modification at C-7 has been attributed to overcoming tetracycline resistance mechanisms and to changing bacterial ribosome binding.19,26 Possibly because of its unique structural properties and narrow spectrum of antibacterial activity, sarecycline may reduce the emergence of antibiotic-resistant bacterial strains.19
Dosage and Administration in Humans
Phase 2 dosage studies randomized patients to receive 0.75 mg/kg, 1.5 mg/kg, or 3 mg/kg sarecycline or placebo (1:1:1:1).27 At week 12, sarecycline at 1.5 mg/kg and 3.0 mg/kg demonstrated significantly reduced inflammatory acne lesions compared to baseline (52.7% and 51.8%, respectively) vs placebo (38.3%). Since the 3 mg/kg dose was not more effective than the 1.5 mg/kg dose, 1.5 mg/kg was identified as the therapeutic dose. The tablets come in 3 weight-based dosages – 60 mg (33–54 kg), 100 mg (55–84 kg), and 150 mg (>84 kg). Because clinical data did not show any difference in efficacy when sarecycline was taken with or without food, the FDA granted approval for sarecycline to be taken once daily with or without food.15 Tetracyclines are not recommended for use during pregnancy.28 Tetracyclines are not recommended for children below age 9 years due to the risk of tooth discoloration.14
Pharmacokinetic Properties
The maximum plasma concentrations of sarecycline are reached in a median time of 1.5–2.0 hours and reach steady state by day 7 with a mean accumulation ratio of 1.5 to 1.6-fold with repeated dosing. Steady-state exposure increased slightly less than proportionally when the once-daily dose was increased from 60 to 150 mg. While sarecycline can be taken with or without food, taking the drug with a milk-containing meal high in fat and calories reduced drug Cmax (maximum plasma concentration) by ~30% and delayed the Tmax (time to maximum plasma concentration) by approximately half an hour.15 However, these observed changes were not considered clinically relevant since efficacy was not impacted. No recommended adjustment in dosage of sarecycline is required for those with mild or moderate renal or hepatic impairment, as neither hepatic nor renal impairment impacted sarecycline pharmacokinetics.29
Blood–Brain Barrier
Minocycline is a lipophilic tetracycline capable of crossing the blood–brain barrier and is more lipophilic than doxycycline and tetracycline.25,30 Sarecycline has demonstrated low potential for crossing the blood–brain barrier in an animal (rat) model study (Table 4).29 It is believed that crossing of the blood–brain barrier contributes to an increased risk of vestibular side effects such as dizziness and vertigo, which are seen more commonly with minocycline as compared to other tetracyclines.31
Distribution, Metabolism, and Elimination
Sarecycline has a mean volume of distribution of 91.4–97.0 L at steady state and is minimally metabolized by liver microsome enzymes in vitro (<15%).15 The drug is excreted through feces and urine with 42.6% and 44.1% of a single 100 mg oral dose being recovered via these respective routes. Sarecycline has a mean elimination half-life of 21–22 h and a mean oral clearance of around 3 L/hour at steady state.15
Clinical Efficacy
Two identically designed, well-controlled Phase 3, randomized, double-blind clinical trials (SC1401 and SC1402) enrolled n = 483 sarecycline, n = 485 placebo and n = 519 sarecycline, n = 515 placebo patients, respectively (Main findings summarized in Table 5).
 |
Table 4 Reduced Blood–Brain Barrier Penetration of Sarecycline Relative to Minocycline in Rats |
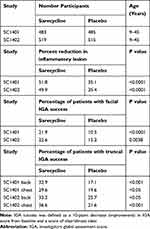 |
Table 5 Clinical Efficacy in Phase 3 Clinical Trials – 12 Weeks |
Efficacy was evaluated primarily in facial AV, but also in truncal AV involving the chest and back.32 Marked improvement was observed in facial inflammatory AV lesions with sarecycline compared to placebo with statistically significant onset of efficacy as early as week 3 (P=0.0003 vs placebo in SC1401 and P<0.0001 vs placebo in SC1402). At the end of treatment (week 12), the clinical efficacy was 51.8% improvement sarecycline vs 35.1% placebo in SC1401, P<0.0001 and 49.9% improvement sarecycline vs 35.4% placebo in SC1402, P<0.0001 (Table 5). Furthermore, statistically significant improvement at week 12 was noticed in truncal acne (P<0.05). The percentage of patients with truncal acne investigator global assessment (IGA) success at week 12 in SC1401 was 32.9% sarecycline versus 17.1% placebo (P<0.001, back) and 29.6% sarecycline versus 19.6% placebo (P<0.05, chest). The percentage of patients with truncal acne IGA success at week 12 in SC1402 was 33.2% sarecycline versus 25.7% placebo (P<0.05, back) and 36.6% sarecycline versus 21.6% placebo (P<0.001, chest). Sarecycline also demonstrated a therapeutic effect on non-inflammatory (comedonal) lesions with greater mean absolute changes from baseline in the sarecycline group than the placebo group beginning at week 6 in study SC1401 and at week 9 in study SC1402 and continuing through week 12 in both studies.32 In study SC1401, sarecycline-treated patients had a mean absolute change from baseline in non-inflammatory lesions at week 12 of −15.1 versus −11.2 in placebo-treated patients (P<0.01), while in study SC1402 the mean absolute change was −16.2 for sarecycline-treated patients’ non-inflammatory lesions versus −13.4 for placebo (P<0.01).
Safety
In the pivotal Phase 3 clinical trials (SC1401 and SC1402), treatment-emergent adverse events (TEAEs) occurred in 29.3% and 25% of patients in the sarecycline groups (SC1401 and SC1402, respectively) and in 29.8% and 26.7% of patients in the placebo groups. Adverse events included nausea (4.6% sarecycline, 2.5% placebo), nasopharyngitis (3.1% and 1.7%), headache (2.7% in both groups), and vomiting (2.1% and 1.4%) for SC1401, and nasopharyngitis (2.5% sarecycline and 2.9% placebo) and headache (2.9% and 4.9%) in SC1402, but most were not considered treatment-related.32 There were nearly as many adverse events (AEs) in the placebo groups as in the treatment groups in these studies. In study SC1401, 3 of the 483 patients (0.6%) in the sarecycline group, and 7 of the 481 (1.4%) of patients in the placebo group discontinued the study due to AEs. In study SC1402 11/513 (2.1%) and 6/513 (1.2%) patients in the sarecycline and placebo groups, respectively, discontinued due to AEs. The majority of these discontinuations were judged by the investigator as possibly related or related to the study treatment. Vulvovaginal candidiasis and vulvovaginal mycotic infections were rare (1.1% and 0.7% of female patients, respectively, in SC1401 and 0.3% and 1.0% of female patients, respectively, in SC1402). No cases of these infections occurred in the placebo group in either study (Table 6).32
Long-Term safety
A Phase 3, multicenter, open-label extension study evaluated the long-term safety of sarecycline (1.5 mg/kg/day) in patients with moderate to severe acne vulgaris up to 1 year.33 Patients who had completed 12 weeks of study in SC1401 or SC1402 were followed at 52 centers in the United States for 9 months with study visits occurring at weeks 2, 6, 12, 18, 24, 32, and 40, or at early termination. A total of 483 patients were enrolled in the study, with 354 patients (73.3%) completing the study (Table 6). Overall, 38.9% of patients reported at least 1 TEAE. Only 2.5% withdrew from the study due to an AE. The most common adverse events (>2%) were nasopharyngitis (3.7%), upper respiratory tract infection (3.3%), headache (2.9%), and nausea (2.1%).33
 |
Table 6 Selected Treatment-Emergent Adverse Events Common to Tetracycline-Class Antibiotics |
Rates of TEAEs of special interest due to commonly reported association with other tetracyclines were evaluated in study subjects treated with sarecycline. The outcomes showed dizziness in 0.4%, sunburn in 0.2%, nausea in 2.1%, vomiting in 1.9%, and diarrhea in 0.2%.33 There were no clinically meaningful safety findings new to tetracyclines in the pivotal trials with sarecycline, and no treatment-emergent serious AEs were considered related to study treatment. In the Phase 3 clinical studies of sarecycline, vestibular AEs were low (0.5% in the sarecycline group and 1.1% in the placebo group).
Regarding phototoxicity, in a Phase I study, one patient (0.2%) in the sarecycline group had a mild non-treatment-related sunburn. Another patient (0.2%) reported hyperpigmentation on his upper forehead, but this was thought to be due to excessive sun exposure and not related to study treatment. The patient went on to complete the study.33
Side Effects Among Broad-Spectrum Tetracyclines
Sarecycline has not been compared head to head with other tetracyclines, including either doxycycline or minocycline, in any clinical studies. However, historical reports of commonly recognized AEs often associated with tetracycline-class antibiotics show rates that are generally higher for both doxycycline and minocycline than those reported in the recent clinical trials for sarecycline (Table 6). The use of doxycycline has been associated with an increased risk of developing irritable bowel diseases (IBS) and inflammatory bowel diseases (IBD).34–36 In a large retrospective study, patients who were prescribed doxycycline for their acne had a 2.25-fold greater risk of developing Crohn’s disease.35 Rare but significant systemic AEs have occurred primarily with the use of minocycline.31,37 English literature was analyzed on doxycycline and minocycline adverse drug reactions (ADRs) between 1966 and 2003 by Smith and Leyden who found that minocycline was associated with 333 ADRs compared to 130 doxycycline-associated ADRs.14 The ranges in incidence of AEs were 0% to 61% for doxycycline (24 clinical trials, n = 3833) and 11.7% to 83.3% for minocycline (11 clinical trials, n = 788). Extended-release formulations of minocycline that incorporate weight-based dosing have been shown to exhibit lower rates of acute vestibular side effects compared to immediate-release formulations of minocycline.38,39
Conclusion
Sarecycline is an oral antibiotic, specifically designed for acne, and approved by the FDA in 2018 for the treatment of AV in patients 9 years old and above. It has demonstrated clinical efficacy in moderate to severe AV, as early as 3 weeks. It has been studied in both inflammatory (face and trunk) and non-inflammatory (comedonal) acne lesions with few adverse events and a safety profile established for up to 1 year.32,33 Its narrow-spectrum antibacterial activity, demonstrated by reduced in-vitro activity against Gram-negative bacteria commonly found in the human gut microbiota, along with lower penetration of the blood-brain barrier may have contributed to not only the fewer adverse events observed in clinical trials but also the low potential for inducing bacterial resistance. Further studies are needed to continue monitoring adverse events. Altogether, sarecycline holds great promise as a new treatment for acne and the first novel tetracycline-class drug to be approved for acne treatment in several decades.
Acknowledgments
We acknowledge DerMEDit for technical author support and preparation of this manuscript. Writing assistance by DerMEDit was funded by Almirall LLC.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
JLJ is an employee of DerMEDit and reports personal fees from Almirall, during the conduct of the study. AYM receives funds as consultant (C), clinical study investigator (I), and speaker (SP) from Almirall (C,I,SP), Foamix (I,SP), Galderma (I), Mayne Pharma (C,I), and Naked Biome (I). JDR has served as a consultant, researcher and speaker for Almirall and Bausch Health and as a consultant and speaker for Mayne Pharma and EPI Health. AG is serving as the Director of R&D and Medical Affairs at Almirall (US). The authors report no other conflicts of interest in this work.
References
1. Marson JW, Baldwin HE. An overview of acne therapy, Part 1: topical therapy, oral antibiotics, laser and light therapy, and dietary interventions. Dermatol Clin. 2019;37(2):183–193. doi:10.1016/j.det.2018.12.001
2. Moore AY, Charles JEM, Moore S. Sarecycline: a narrow spectrum tetracycline for the treatment of moderate-to-severe acne vulgaris. Future Microbiol. 2019;14:1235–1242. doi:10.2217/fmb-2019-0199
3. Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260. doi:10.1128/MMBR.65.2.232-260.2001
4. Di Caprio R, Lembo S, Di Costanzo L, Balato A, Monfrecola G. Anti-inflammatory properties of low and high doxycycline doses: an in vitro study. Mediators Inflamm. 2015;2015:329418. doi:10.1155/2015/329418
5. Perret LJ, Tait CP. Non-antibiotic properties of tetracyclines and their clinical application in dermatology. Australas J Dermatol. 2014;55(2):111–118. doi:10.1111/ajd.12075
6. Farrah G, Tan E. The use of oral antibiotics in treating acne vulgaris: a new approach. Dermatol Ther. 2016;29(5):377–384. doi:10.1111/dth.12370
7. Centers for Disease Control and Prevention (CDC). Antibiotic Stewardship Statement for Antibiotic Guidelines – recommendations of the HICPAC. 2017. Available from: https://www.cdc.gov/hicpac/recommendations/antibiotic-stewardship-statement.html.
8. Melander RJ, Zurawski DV, Melander C. Narrow-spectrum antibacterial agents. Medchemcomm. 2018;9(1):12–21. doi:10.1039/C7MD00528H
9. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973. e933. doi:10.1016/j.jaad.2015.12.037
10. Gerber JS, Ross RK, Bryan M, et al. Association of broad-vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325–2336. doi:10.1001/jama.2017.18715
11. Haidari W, Bruinsma R, Cardenas-de la Garza JA, Feldman SR. Sarecycline review. Ann Pharmacother. 2019;1060028019873111.
12. Ochsendorf F. Minocycline in acne vulgaris: benefits and risks. Am J Clin Dermatol. 2010;11(5):327–341. doi:10.2165/11319280-000000000-00000
13. Sanchez AR, Rogers RS
14. Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 2005;27(9):1329–1342. doi:10.1016/j.clinthera.2005.09.005
15. Deeks ED. Sarecycline: first global approval. Drugs. 2019;79(3):325–329. doi:10.1007/s40265-019-1053-4
16. Roca-Saavedra P, Rodriguez JA, Lamas A, et al. Low-dosage antibiotic intake can disturb gut microbiota in mice. Cyta J Food. 2018;16(1):672–678. doi:10.1080/19476337.2018.1474264
17. Boynton FDD, Ericsson AC, Uchihashi M, Dunbar ML, Wilkinson JE. Doxycycline induces dysbiosis in female C57BL/6NCrl mice. BMC Res Notes. 2017;10(1):644. doi:10.1186/s13104-017-2960-7
18. Haas K, Notay M, Rodriguez W, et al. 383 Doxycycline effects on the gut and skin microbiomes and lipidome in acne. J Invest Dermatol. 2018;138(5):S65. doi:10.1016/j.jid.2018.03.389
19. Zhanel G, Critchley I, Lin LY, Alvandi N. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1. doi:10.1128/AAC.01297-18
20. Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2015;6:1543.
21. Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156(Pt 11):3216–3223. doi:10.1099/mic.0.040618-0
22. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi:10.1186/s13073-016-0294-z
23. Rashid MU, Panagiotidis G, Backstrom T, Weintraub A, Nord CE. Ecological impact of doxycycline at low dose on normal oropharyngeal and intestinal microflora. Int J Antimicrob Agents. 2013;41(4):352–357. doi:10.1016/j.ijantimicag.2012.11.014
24. Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79(6):471–489. doi:10.1016/j.jinf.2019.10.008
25. Cunha BA, Baron J, Cunha CB. Similarities and differences between doxycycline and minocycline: clinical and antimicrobial stewardship considerations. Eur J Clin Microbiol Infect Dis. 2018;37(1):15–20. doi:10.1007/s10096-017-3081-x
26. Robertsen HL, Musiol-Kroll EM. Actinomycete-derived polyketides as a source of antibiotics and lead structures for the development of new antimicrobial drugs. Antibiotics (Basel). 2019;8:4.
27. Leyden JJ, Sniukiene V, Berk DR, Kaoukhov A. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a Phase 2, dose-ranging study. J Drugs Dermatol. 2018;17(3):333–338.
28. Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73(8):779–787. doi:10.1007/s40265-013-0060-0
29. Data on file with Almirall.
30. Barza M, Brown RB, Shanks C, Gamble C, Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother. 1975;8(6):713–720. doi:10.1128/AAC.8.6.713
31. Kim S, Michaels BD, Kim GK, Del Rosso JQ. Systemic antibacterial agents. In: Wolverton SE, editor. Comprehensive Dermatologic Drug Therapy.
32. Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, Phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17(9):987–996.
33. Pariser DM, Green LJ, Lain EL, et al. Safety and tolerability of sarecycline for the treatment of acne vulgaris: results from a Phase III, multicenter, open-label study and a Phase I phototoxicity study. J Clin Aesthetic Dermatol. 2019;12(11):E53–E62.
34. Lee TW, Russell L, Deng M, Gibson PR. Association of doxycycline use with the development of gastroenteritis, irritable bowel syndrome and inflammatory bowel disease in Australians deployed abroad. Intern Med J. 2013;43(8):919–926. doi:10.1111/imj.12179
35. Margolis DJ, Fanelli M, Hoffstad O, Lewis JD. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2610–2616. doi:10.1038/ajg.2010.303
36. Theochari NA, Stefanopoulos A, Mylonas KS, Economopoulos KP. Antibiotics exposure and risk of inflammatory bowel disease: a systematic review. Scand J Gastroenterol. 2018;53(1):1–7. doi:10.1080/00365521.2017.1386711
37. Lebrun-Vignes B, Kreft-Jais C, Castot A, Chosidow O. French Network of Regional Centers of P. Comparative analysis of adverse drug reactions to tetracyclines: results of a French national survey and review of the literature. Br J Dermatol. 2012;166(6):1333–1341. doi:10.1111/j.1365-2133.2012.10845.x
38. Garner SE, Eady A, Bennett C, Newton JN, Thomas K, Popescu CM. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;8:CD002086.
39. Fleischer AB
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
