Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Salvianolic Acid B Reduces Oxidative Stress to Promote Hair-Growth in Mice, Human Hair Follicles and Dermal Papilla Cells
Authors Thianthanyakij T, Zhou Y , Wu M, Zhang Y, Lin JM, Huang Y, Sha Y, Wang J, Kong SP, Lin J, Liu Q, Wu W
Received 12 January 2024
Accepted for publication 3 April 2024
Published 8 April 2024 Volume 2024:17 Pages 791—804
DOI https://doi.org/10.2147/CCID.S454844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Traidit Thianthanyakij,1,2,* Yinghui Zhou,1,* Mengyi Wu,3,* Yuting Zhang,4 Jui-Ming Lin,1 Yan Huang,4 Yuou Sha,1 Jiayi Wang,1 Sirapath Peter Kong,2 Jinran Lin,1 Qingmei Liu,1 Wenyu Wu1,5,6
1Department of Dermatology, Huashan Hospital, Fudan University, Shanghai Institute of Dermatology, Shanghai, 200040, People’s Republic of China; 2Faculty of Medicine, Thammasat University (Rangsit Campus), Pathumthani, 12120, Thailand; 3Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, People’s Republic of China; 4State Key Laboratory of Genetic Engineering, Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, 200433, People’s Republic of China; 5Department of Dermatology, Jing’an District Central Hospital, Shanghai, 200040, People’s Republic of China; 6Academy for Engineering and Technology, Fudan University, Shanghai, 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qingmei Liu; Wenyu Wu, Email [email protected]; [email protected]
Background: Existing research links oxidative stress and inflammation to hair loss. Salvianolic acid B (SAB) is known for its anti-oxidative, anti-inflammatory, and other beneficial pharmacological properties.
Objective: To assess the efficacy of SAB in modulating hair growth.
Methods: In vivo experiments were conducted using C57BL/6 mice to evaluate the effects of SAB on hair and skin parameters. The study involved ex vivo analysis of human hair follicles (HFs) for hair shaft length and hair growth cycle assessment. In vitro, human dermal papilla cells (hDPCs) were cultured with SAB, and their proliferation, protection against H2O2-induced oxidative damage, and gene/protein expression alterations were examined using various analytical techniques, including Real-Time Cell Analysis (RTCA), DCFH-DA Assay, RNA-seq, and KEGG pathway analysis.
Results: SAB treatment in mice significantly improved hair growth and vascularization by day 21. In human HFs, SAB extended hair shaft length and delayed the transition to the catagen phase. SAB-treated hDPCs showed a notable decrease in the expression of oxidation-antioxidation-related genes and proteins, including reduced phosphorylation levels of ERK and p38.
Conclusion: The study indicates that SAB promotes hDPC proliferation and offers protection against oxidative stress, highlighting its potential as a therapeutic agent for enhancing hair growth and treating hair loss.
Keywords: salvianolic acid B, hair growth, hair cycle, hair follicle, dermal papilla cells
Introduction
Hair loss, a prevalent condition in dermatology, affects not only physical appearance but also has detrimental impacts on patients’ psychological well-being and overall quality of life. Androgenetic alopecia (AGA), commonly known as male pattern alopecia and seborrheic alopecia, affects both genders.1 It is characterized by hair thinning, a receding forehead hairline, and reduced hair volume on the scalp in men, while women typically experience hair thinning with a preserved forehead hairline.1 AGA is marked by a microscopic reduction in hair diameter and a higher proportion of single hair follicles. In China, AGA prevalence is 21.3% in men and 6.0% in women, increasing with age.2
The etiology of AGA remains partially understood, but studies indicate a complex interplay of genetic, hormonal, lifestyle, and environmental factors.2 Genetics and androgens are primary contributors, with 53.3–63.9% of Chinese men with AGA having a family history of the condition.2
The hair growth cycle comprises three phases: anagen (growth phase), catagen (transition phase), and telogen (resting phase). The anagen phase typically lasts 2–6 years, catagen 2–3 weeks, and telogen 1–3 months. In AGA, androgens, particularly dihydrotestosterone (DHT), are implicated in shortening the anagen phase, resulting in smaller hair follicles and thinner hair, leading to hair loss. Perifollicular fibrosis and vascular changes impede blood flow and oxygen delivery to hair follicles, exacerbating hair loss.3 Dermal papilla cells (DPC), highly sensitive to environmental changes like oxidative stress, undergo apoptosis due to the resulting tissue hypoxia and ischemia, leading to hair follicle miniaturization and premature aging.
Current AGA treatments include drug therapy, laser therapy, and hair transplantation. However, these approaches cannot cure AGA, only managing to delay its progression. The US FDA-approved drugs for AGA treatment are finasteride and minoxidil, both associated with varying degrees of side effects. Finasteride, a selective type II 5α-reductase inhibitor, impedes the conversion of testosterone to DHT. Its common side effects include sexual dysfunction, depression, and gynecomastia.4 Minoxidil, used topically, stimulates ATP-sensitive potassium channels in smooth muscle cells, enhancing blood flow around hair follicles and promoting epithelial cell proliferation. Its primary adverse reaction is scalp irritation. The mechanism of laser therapy remains unclear, and it can cause mild pain, swelling, itching, dryness, and erythema. Hair transplantation, while effective, is costly and invasive, limiting its widespread adoption.5
In recent years, extracts and monomers from traditional Chinese medicine have garnered significant interest for their potential in enhancing hair follicle cell function, reducing hair loss, and promoting hair regeneration. Salvia miltiorrhiza, a commonly used herb in traditional Chinese medicine for promoting blood circulation and alleviating blood stasis, is notable in this regard. Its primary medicinal components include lipophilic diterpene quinones, hydrophilic phenolic acids, flavonoids, triterpenes, and sterols. Salvia miltiorrhiza plays a crucial role as a pharmaceutical ingredient in various traditional Chinese medicine formulas aimed at hair growth, such as Bailing Shengfa Decoction and Yixue Shengfa Decoction. This suggests that the active extracts of Salvia miltiorrhiza may possess pharmacological properties conducive to promoting hair growth.
Among the various components extracted from Danshen, salvianolic acid B (SAB) is particularly notable for its high concentration and potent activity. SAB, also referred to as shikonin steroidal acid B, has a molecular formula of C36H30O16 and a molecular weight of 718.6 Modern pharmacological research has revealed that SAB exhibits anti-oxidative, anti-inflammatory, autophagy-promoting, anti-fibrotic, and angiogenesis-promoting functions. Its capacity to protect against myocardial ischemia-reperfusion, enhance coronary blood flow, safeguard vascular endothelial cells, and mitigate atherosclerosis has been documented.7 Considering the pathological mechanisms of AGA, including hair follicle fibrosis and impaired follicular blood flow, it is hypothesized that SAB could effectively counteract the oxidative stress in dermal papilla cells and prevent apoptosis, thereby offering a potential treatment for AGA. Consequently, this study concentrates on exploring the effects of SAB on hair growth and the underlying mechanisms, potentially introducing novel therapeutics for AGA treatment.
Methods
Evaluating the Effect of Salvianolic Acid B on Depilated Mice
A group of ten C57BL/6 mice, aged 7–8 weeks, were selected as subjects to assess hair growth quality. All animal care and experimental procedures adhered to the Animals (Scientific Procedures) Act 1986. Additionally, the handling, feeding, and experimental procedures involving these animals were conducted in compliance with the Regulations on the Administration of Experimental Animals. This study received approval from the Biomedical Ethics Committee of Fudan University and conformed to the ARRIVE guidelines. The mice were evenly distributed into two groups: a control group and an SAB group, with five mice in each. After being anesthetized with pentobarbital, the mice had their dorsal fur removed using beeswax. This day of depilation was designated as day 0. The control group received daily subcutaneous injections of 100µL normal saline, while the experimental group was administered 100µL of 1mg/mL SAB (Salvia polyphenols; Shanghai Green Valley Pharmaceutical Co., Ltd.) daily. Both groups were treated continuously for 21 days. Fur length measurements and photographic documentation were taken every two days. Following the 21-day period, all mice were euthanized, and skin biopsies from their backs were collected for comparative analysis of fur size and capillary growth using a stereomicroscope (Macro zoom stereo microscope, ZEISS).
Assessing the Effect of Salvianolic Acid B on Hair Follicles
Isolation and Culture of Anagen Hair Follicles
Healthy follicular units were obtained from the occipital region of hair transplant patients. These units were placed in a 12-well plate, each well containing 1mL of follicle medium (enriched with 2 mM glutamine, 10 ng/mL hydrocortisone, 100 U/mL penicillin, and 100 mg/mL streptomycin in Williams E medium). Initial length measurements were taken, followed by a subsequent measurement after 24 hours. Hair follicles exhibiting a length increase of 0.3–0.5 mm and in the anagen phase were selected for subsequent experiments.8
Hair Follicle Grouping and Treatment
Anagen phase hair follicles were divided into two groups: a control group and a treatment group. The treatment group’s hair follicles were exposed to 1 mg/mL SAB, while the control group received normal saline. Hair follicle lengths in the 12-well plate were measured on days 2, 4, and 6.
Hematoxylin and Eosin (HE) Staining
Anagen phase hair follicles were fixed in 10% paraformaldehyde overnight. The fixed tissues were dehydrated through a graded ethanol series (70%, 80%, 90%, and 95%) for one hour at each concentration, followed by two rounds of dehydration in 100% ethanol, each lasting one hour. The tissues were then cleared in a n-butanol xylene mixture for 40 minutes, embedded using a paraffin embedding machine, and sectioned into 3mm slices using a paraffin microtome. These sections were dewaxed twice in xylene for 15 minutes each, rehydrated sequentially in 100%, 80%, and 75% ethanol (3 minutes each), and then rinsed in distilled water for 5 minutes. Staining was performed using hematoxylin and eosin Y solutions, with the sections subsequently observed under a light microscope.
Investigating the Effects of Salvianolic Acid B on Dermal Papilla Cells
Isolation, Culture, and Validation of Dermal Papilla Cells
Dermal papilla cells were isolated from a single hair follicle obtained from a patient undergoing hair transplantation. Under a stereomicroscope, the hair follicle’s dermal papilla was separated using a needle and then transferred to a new culture dish. This dish was prepared with DMEM medium supplemented with 100 U/L streptomycin, penicillin, 15% fetal bovine serum, 0.3 g/L L-glutamic acid, and 10 mmol/L hydroxyethylpiperazineethanesulfonate. Dermal papilla cells from passages 2 and 3 were selected for the experiments.
For validation, the cells were tested using an alkaline phosphatase chromogenic kit (SIGMAFAST BCIP®/NBT). The chromogenic substrate 5-Bromo-4-chloro-3-indole phosphate (BCIP) is hydrolyzed by alkaline phosphatase (ALP) to form a product that reacts with nitro blue tetrazolium (NBT), resulting in a dark blue to blue-violet precipitate. The culture medium was aspirated, and the cells were rinsed thrice with PBS before being fixed with 4% paraformaldehyde for 1–2 minutes. After aspirating the fixative, the cells were washed three times with TBST and then incubated with a color-developing working solution at room temperature in the dark for 5–30 minutes until the desired color depth was achieved. The cells were then rinsed once with PBS and stained with DAPI staining solution for 5 minutes at room temperature. Following the DAPI staining, the cells were washed 2–3 times with PBS for 3–5 minutes each. Observations were made using a fluorescence microscope (Leica, Germany).
RTCA Cell Proliferation Assay
To determine the optimal concentration of salvianolic acid B for treating cells and its effect on cell proliferation, an RTCA cell proliferation assay was conducted.
Determining the Optimal Cell Number for Hair Papilla Cells
Dermal papilla cells were digested with 0.25% trypsin and counted. A 50 µL volume of complete medium was added to a 16-well E-plate, and background impedance was measured using RTCA for 15–30 minutes. Following a 10–15-minute background equilibration, dermal papilla cells were seeded into E-plate proliferation plates at different densities (8000 cells per well) in a 50 µL volume. The plates were left at room temperature for 30 minutes to facilitate passive adherence of the cells to the gold electrode-coated bottom of the electronic board. Afterward, the electronic board was placed in the RTCA unit (xCELLigence RTCA Analyzer; ACEA Biosciences) within a 5% CO2 humidified incubator. Cell impedance was monitored every 5 minutes for a duration of over 100 hours.
Administration of Drug
SAB was prepared in serial dilutions, resulting in concentrations of 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL. Once the dermal papilla cells entered the linear growth phase, the prepared concentrations of SAB, or medium (as a control), were added to the E-plate in a 50 μL volume. Subsequently, cell impedance was continuously monitored.9
Assessment of Cellular ROS Levels
Dermal papilla cells were dissociated using 0.25% trypsin, counted, and adjusted to the required cell density for the experiment. The cells were then seeded into 6-well plates. Upon reaching 80% confluency, drug treatment was initiated. Cells in the blank control group and the hydrogen peroxide group received solvent stimulation, while the cells in the salvianolic acid group were treated with 50 μg/mL of salvianolic.
Analysis of Cell Gene Expression Levels
RNA Extraction and Quantification
When the dermal papilla cells reached 80% confluency, drug treatments were administered. The blank control group (Group C) received solvent stimulation, while the salvianolic acid group (SAB group) was treated with 50 μg/mL salvianolic acid B. Following 24 hours of treatment, RNA was extracted for quantitative analysis. To initiate cell lysis directly in the Petri dish, 1 mL of TRIZOL reagent (Sigma) was added to a 3.5 cm diameter dish. The cells were lysed through repeated aspiration. The dish was then left at room temperature for 5 minutes. Subsequently, 0.2 mL of chloroform was added, and the mixture was transferred to an EP tube and vigorously shaken for 15 seconds. After a 2–3-minute incubation at room temperature, the sample was centrifuged at 10,000 g for 15 minutes at a low temperature. This process resulted in three distinct layers: the red phenol-chloroform phase, the middle phase, and the upper colorless aqueous phase. RNA, present in the upper aqueous phase, was aspirated and transferred to a new EP tube. To precipitate the RNA, 0.5 mL of isopropanol was added, followed by a 10-minute room temperature incubation and subsequent centrifugation at 10,000 g for 10 minutes at 2–8°C. The supernatant was discarded, and the RNA pellet was washed with 1 mL of 75% ethanol, followed by vortex mixing. The sample was then centrifuged at 7500 g for 5 minutes at 4°C. After air-drying the RNA pellet at room temperature for 5–10 minutes, the RNA was redissolved in DEPC water (diethylpyrocarbonate, Sigma), with an addition of 10 μL DEPC water per 1 mL TRIZOL used. RNA concentration was determined using a Nanodrop (Nanodrop ND-1000; Thermo Fisher).
Gene Expression Analysis Using qPCR
cDNA was synthesized via reverse transcription using the MonScriptTM RTIIII All-in-One Mix with dsDNase Kit, following the manufacturer’s protocol. Quantitative PCR (qPCR) was performed using the QuantStudioTM 7 Flex Real-Time PCR System (Applied Biosystems) and Antibody Dye Quantitative PCR Master Mix (QIAGEN). Primers (detailed in Supplemental Table 1) were used to assess the expression levels of genes including iNOS, RAC-1, and EC-sod. The expression levels of these genes, related to oxidation-antioxidation processes, were normalized to those of GAPDH. Relative gene expression was calculated using the 2–ΔΔCt method and presented as mean ± standard error values from a minimum of three independent experiments. The primers required for qPCR were synthesized by GENEWIZ Company, with the sequences provided in Supplemental Table 1.
Detection of Protein Expression Levels in Cells
When the confluency of dermal papilla cells reached 80%, treatments were administered. The blank control group (control group) received solvent stimulation, while the salvianolic acid group (SAB group) was treated with 50 μg/mL salvianolic acid B. Following 48 hours of treatment, proteins were extracted using RIPA Lysis Solution (Beyotime) for target protein expression analysis. The protein concentration was quantified using the BCA detection kit (Thermo Scientific). Proteins were separated on a polyacrylamide gel of appropriate concentration, then loaded into an electrophoresis device (Electrophoresis System; BioScience) along with protein markers (Protein Ladder; Thermo Scientific). Electrophoresis was conducted at 80V for the stacking gel and 120V for the separating gel. Post-electrophoresis, the gel was transferred to a membrane transfer device for further processing. Total cell lysates (10 µg/lane) were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore). These membranes were blocked at room temperature for 2 hours using TBST containing 5% BSA and incubated overnight at 4°C with primary antibodies including mouse anti-GAPDH (Yisheng, Shanghai), caspase-3 (Yisheng, Shanghai), p-38 (Abcam), and p-ERK (Abcam). Following primary antibody incubation, membranes were incubated with secondary antibodies for 1 hour at room temperature. Protein bands were visualized using ECL luminescent substrate (Suner Biotechnology, Shanghai), developed, captured using ImageQuantTLLAS 4000 mini (General Electric), and analyzed for grayscale intensity.
RNA-Seq Detection and Data Analysis
RNAs extracted from the control and SAB-treated dermal papilla cells were qualitatively assessed using 1% agarose gel electrophoresis at 150V for 15 minutes and visualized under a UV gel imaging system. For valid results, RNA bands needed to be clear and without smearing. RNA quantification was performed using NanoDrop. Transcriptomic data sequencing was completed by Novo Company using Illumina RNA-Seq technology. Tophat software was utilized for aligning RNA-seq data reads to the genome, and cufflinks software for splicing reads into transcription units. SeqArray software performed quantitative RNA analysis and differential gene expression analysis at the exon level. GO functional annotation analysis (http://circe.med.uniroma1.it/fidea/index.php) categorized differentially expressed genes into three levels: cellular component, molecular function, and biological process. KEGG analysis (http://circe.med.uniroma1.it/fidea/index.php) conducted significant enrichment analysis of metabolic pathways on multiple genes, defining pathways with P ≤ 0.05 as significantly enriched among differentially expressed genes. Pathway significance enrichment analysis was used to identify relevant signal transduction pathways and biochemical pathways involved in differentially expressed genes.
Protein Interaction Analysis
For this analysis, the STRING database (http://string-db.org/) was utilized. The procedure involved entering the names of differentially expressed genes, identified before and after treatment with SAB, into the database’s search interface. The database’s search algorithm drew from experimental data and text mining results, including PubMed abstracts and other database records. Bioinformatics tools were employed to predict interactions between both known and predicted proteins. These interactions were then weighted using a scoring mechanism, integrating results obtained from various methods to assign a comprehensive score to each interaction.
Identification of Target Gene Expression Level
The expression levels of targeted genes were identified using the quantitative PCR (qPCR) method.
Statistical Analysis
Data analysis was conducted using SPSS20.0 software. Results are presented as mean ± standard deviation. For measurement data exhibiting normal distribution and homogeneity of variance, the independent sample t-test was applied. In cases where these conditions were not met, an independent sample nonparametric test was used. A p-value of less than 0.05 was considered to indicate statistical significance.
Results
SAB Promoted Hair Growth in Mice
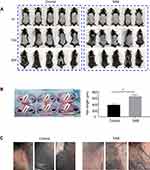
Figure 1A illustrates the initial skin condition of the mice following hair removal. On the first day, the skin in the depilated area was pink for both groups. By the tenth day of SAB administration, the skin color transitioned from pink to gray-black, more noticeably in the SAB group compared to the control. On the twentieth day, the SAB group exhibited a larger area of hair regrowth, with the hair appearing thick and almost fully covering the back. In contrast, the control group showed significantly less hair regrowth. This experiment demonstrated SAB’s effectiveness in promoting hair growth in depilated mice.
On day 21, skin samples from the backs of the mice were collected, and fur size was measured under a stereo microscope. Hair lengths on the backs of both groups were quantified, as shown in Figure 1B. The hair in the SAB group was significantly longer than in the control group (P<0.05).
SAB Enhanced Capillary Growth in Mice
Given that poor blood flow and reduced oxygen supply to hair follicles can impede hair growth, the impact of SAB on capillary proliferation in the mice’s skin was examined. Post 21 days of SAB administration, stereomicroscopic analysis (Figure 1C) revealed a higher number of capillaries in the SAB group compared to the control group. Thus, SAB effectively promoted vascularization, potentially enhancing blood flow around the hair follicles and stimulating hair growth on the mice’s backs.
Acquisition of Anagen Hair Follicles
The anagen phase hair follicles displayed an expanded hair bulb encompassing the dermal papilla, resembling an inverted funnel. HE-stained sections revealed distinct layers within the hair follicle structure, as depicted in Figure 2A.
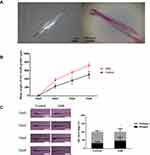
SAB Promoted in vitro Hair Follicle Growth
Hair follicles cultured in vitro were measured every two days. In the control group, the hair follicle growth length was 227.24±55.54 μm on day two, 365.11±83.96 μm on day four, and 504.72±91.87 μm on day six. With SAB treatment (1mg/mL), the growth length was approximately 337.24±51.21 μm on day two, 561.24±53.01 μm on day four, and 725.34±74.33 μm on day six, as shown in Figure 2B. Visually, the growth length of hair follicles treated with SAB was significantly greater than in the control group (P<0.05).
Effect of SAB on Hair Follicle Growth Cycle
The morphology of hair follicles cultured in vitro was examined using a macroscopic zoom microscope to determine their growth cycle phase, as illustrated in Figure 2C. After six days of in vitro culture, the distribution of hair follicles in the anagen and catagen phases was assessed. In the control group, 33% of the hair follicles were in the anagen phase and 67% in the catagen phase. In contrast, in the SAB group, 47% of hair follicles were in the anagen phase and 53% in the catagen phase. These findings indicate that SAB significantly increased the proportion of hair follicles in the anagen phase compared to the control group (P<0.05). This suggests that SAB effectively prolongs the anagen phase of hair follicles in vitro and delays their transition to the catagen phase.
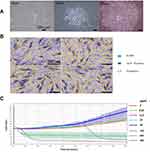
Acquisition, Culture, and Verification of Hair Papilla Cells
Figure 3A presents microscopic images of dermal papilla cells isolated and cultured over 14 days. On day 11 post-isolation, cells predominantly displayed irregular, triangular, polygonal, and spindle-shaped morphologies, radiating from the periphery of the follicles. The growth rate accelerated over time, with cells extending outwards in sheets. By day 14, the cells had developed a spindle-shaped, fibroblast-like morphology with multiple protrusions and elongated bodies. ALP staining of the isolated dermal papilla cells was positive, confirming their identity (Figure 3B).
SAB Promotes Proliferation of Dermal Papilla Cells
To evaluate SAB’s impact on dermal papilla cell proliferation and to establish an appropriate concentration for subsequent qPCR and Western blot assays, the RTCA system was employed for real-time monitoring. In Figure 3C, the x-axis represents the treatment duration with salvianolic acid B, and the y-axis shows the cell index (CI) value. SAB, at concentrations of 50μg/mL or lower, enhanced the proliferation of hair follicle cells in a dose-dependent manner. However, at 100μg/mL or higher, SAB exhibited cytotoxicity, leading to inhibited proliferation and cell death.
Thus, SAB within a specific dose range (below 50μg/mL) can stimulate the proliferation of hair follicle cells, while higher concentrations are cytotoxic. Based on these results, a concentration of 50μg/mL was selected for cell treatment in subsequent qPCR and Western blot experiments.
Reduction of Cellular Reactive Oxygen Species by SAB
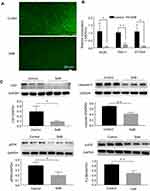
SAB is known to act predominantly through its antioxidant properties. In this study, the effect of SAB on protecting hair follicle cells against hydrogen peroxide-induced oxidative damage was examined. Compared to the control group, SAB treatment significantly lowered reactive oxygen species levels. A model was established using a 100 μmol/L hydrogen peroxide treatment for 24 hours to simulate oxidative damage. In this model, SAB-treated dermal papilla cells showed a marked reduction in DCFH-DA staining fluorescence intensity, indicating effective protection against hydrogen peroxide-induced intracellular ROS increase (Figure 4A).
Regulation of Oxidation-Antioxidation Related Gene Expression by SAB
Oxidative stress is crucial in AGA pathogenesis, with SAB demonstrating potent antioxidant effects. To delve deeper into SAB’s impact on dermal papilla cells’ oxidative levels, its influence on oxidation-antioxidation gene expression was analyzed. Specifically, SAB inhibited the assembly and activation of the NADPH oxidase system by downregulating Rac1 expression, a key player in ROS production. Concurrently, SAB treatment for 24 hours at 50μg/mL led to downregulation of superoxide dismutase (SOD) gene expression, suggesting reduced ROS generation and improved cellular oxidative stress levels (Figure 4B). Additionally, SAB’s effect on 3-NT, an oxidative damage-related protein, was confirmed through Western blot analysis, showing a downregulation in its expression (Figure 4C).
Inhibition of Dermal Papilla Cell Apoptosis by SAB
Given the role of oxidative stress in inducing apoptosis, this study investigated SAB’s impact on dermal papilla cell apoptosis. In Figure 4C, Western blot analysis revealed that SAB effectively downregulated caspase-3, an apoptosis-related protein, suggesting a protective role against oxidative stress-induced apoptosis in these cells.
SAB’s Inhibition of ERK and p38 Phosphorylation
Excessive ROS under pathological conditions can activate the MAPK signaling pathway, influencing various cellular processes including survival, growth, and apoptosis. This study also assessed SAB’s effect on the MAPK pathway, particularly its impact on ERK and p38 expression in skin cells. The findings indicated that SAB significantly reduced the phosphorylation levels of ERK and p38 in dermal papilla cells by approximately 50%, implying a protective mechanism against oxidative damage through the inhibition of these signaling pathways (Figure 4C).
Discussion
Hair loss is a prevalent dermatological condition that, while not life-threatening, significantly impacts the psychological well-being and quality of life of patients. The etiology of AGA remains elusive, with current research pointing to genetic, hormonal, sleep-related, and environmental factors as key contributors.10 Moreover, oxidative stress and inflammatory responses have been implicated in hair loss.
Danshen, a traditional Chinese medicine, contains SAB, which pharmacological studies have identified as having antioxidative, anti-inflammatory, autophagy-promoting, anti-fibrotic, and angiogenic properties.11 These attributes suggest that SAB could potentially enhance hair growth and mitigate hair loss. However, its role in protecting hair follicles from oxidative stress remains under-explored. To address this gap, our study employed in vivo C57BL/6 mice models, ex vivo human hair follicle cultures, and genetic expression analyses to examine SAB’s effects on hair growth.
C57BL/6 mice, commonly used in hair growth research, were administered 1 mg/mL of SAB in our study to assess its pharmacological impact on hair growth. Results indicated enhanced hair growth and vascularization in the treated group. This aligns with findings from Lin et al, who reported SAB’s angiogenic stimulation in rat models of skin flap transplantation.12 These consistent results suggest that SAB facilitates hair growth by augmenting blood supply, thereby optimizing nutrient absorption and waste removal for hair follicles. The mechanism appears to involve the upregulation of vascular endothelial growth factor (VEGF), a critical angiogenesis mediator.13,14
The hair follicle, a mini-organ with complex structures, can be used ex vivo to simulate in vivo hair growth conditions. Our study demonstrated that SAB promotes hair follicle proliferation in a dose-dependent manner, with 50 μg/mL being the most efficacious concentration. At 100 μg/mL, SAB exhibited prooxidant properties, leading to cytotoxic effects at concentrations above 100 μg/mL. The prooxidant effect, intensifying at higher concentrations, was associated with decreased cell viability, as determined by the Real-time Cell Analysis (RTCA) system. This system, which uses a microelectrode array to monitor cell behavior, provided insights into cell quantity, morphological changes, and viability.15 Intriguingly, SAB’s dual antioxidant/prooxidant behavior underscores its multifaceted potential in pharmaceutical applications, with lower concentrations aiding cell proliferation and higher concentrations inducing apoptosis, a mechanism relevant to its antitumor effects. Supporting our observations, several studies have documented SAB’s antitumor efficacy, highlighting its role in growth inhibition and apoptosis across various tumor cell types.8,11,16
Studies indicate that environmental stressors like hydrogen peroxide (H2O2), ultraviolet radiation, and DHT contribute to damage in dermal papilla cells (DPCs), ultimately leading to hair loss. SAB demonstrates a protective effect against oxidative stress-induced damage in hair follicle cells, effectively maintaining hair growth promoters amidst these stress factors. To measure reactive oxygen species (ROS) in DPCs, the DCFH-DA Assay Kit was employed. DCFH-DA, non-fluorescent by itself, permeates cell membranes and is oxidized to DCF in the presence of ROS like hydrogen peroxide, superoxide radicals, hydroxylates, and peroxides. This oxidation process yields a green fluorescence under a 502 nm excitation wavelength, with fluorescence intensity proportionate to ROS levels within the cells. Our findings revealed that pre-treatment of hair follicle cells with 50 μg/mL SAB before exposure to 100 μmol/l hydrogen peroxide significantly diminished ROS levels. Similar results were observed by Liu et al in their study on H2O2-induced apoptosis in rat cerebral microvascular endothelial cells.15
Furthermore, the activation of NADPH oxidase, SOD, and inducible nitric oxide synthase (iNOS) are closely linked to the generation of ROS, impacting various cellular components and potentially triggering apoptosis.9 This study demonstrated that SAB influenced the expression of these oxidation-antioxidation-related genes. Notably, the NADPH oxidase system, a primary ROS source in cells, and Rac1, a small GTPase critical for NADPH oxidase assembly and activation, were key targets of SAB. SAB was found to inhibit NADPH oxidase by down-regulating Rac-1 expression, thereby curtailing ROS production in cells. This aligns with findings from studies on SAB-treated rat hepatic stellate cells, which showed a reduction in NADPH oxidase activity.17
Secondly, the expression of iNOS is directly associated with inflammatory processes and oxidative stress.18 In this study, a notable decrease in iNOS expression in DPCs was observed. These results align with those of Zhu et al, where SAB was reported to suppress iNOS expression, thereby mitigating inflammation and cell apoptosis in cartilage cells.19 These findings suggest that SAB serves as an effective anti-inflammatory and antioxidant agent, preventing oxidative stress and cellular apoptosis.
Additionally, SOD, a critical antioxidant system in cells, catalyzes the conversion of oxygen free radicals to H2O2. Prior studies have indicated that excessive H2O2 impedes hair growth via the down-regulation of β-catenin,20 a key player in the Wnt/β-catenin pathway that is vital for the morphogenesis, development, and growth of hair follicles. In this study, SAB’s role in reducing ROS generation and oxidative stress in DPCs led to a down-regulation of SOD-related genes. This observation is in line with findings from Govindarasu et al, where reduced SOD activity was noted in SAB-treated colon tissues.21 Consequently, SAB-induced down-regulation of SOD may spare β-catenin levels, thereby preserving hair growth. However, further research is required to elucidate SAB’s direct impact on β-catenin expression.
Furthermore, DPCs interact with other cells within hair follicles (HFs) to regulate hair growth and development, partly through cytokine release that activates various pathways. Previous studies have shown that increased ROS levels elevate SOD activity, leading to H2O2 flux and the activation of redox-sensitive pathways.22 One such pathway is the mitogen-activated protein kinases (MAPK) signaling pathway, crucial for regulating cellular functions such as survival, growth, metabolism, differentiation, proliferation, and apoptosis. Our findings demonstrate that SAB effectively down-regulated the phosphorylation levels of MAPK subfamilies, specifically ERK and p38, in DPCs. This is consistent with the work of Liu et al, showing SAB’s inhibitory effect on the MAPK/ERK pathway in skin fibroblast proliferation.23 This suggests that SAB may protect cells from oxidative damage by targeting the ERK and p38 signaling pathways.
Additionally, SAB was observed to inhibit cell apoptosis. Caspase-3, a crucial protein in the apoptosis pathway, is instrumental in initiating and executing cell apoptosis. In this study, SAB was found to decrease caspase-3 activity, a finding corroborated by Dai et al, who reported a reduction in caspase-3 activity in nucleus pulposus cells following SAB treatment.24 These results suggest that SAB may play a protective role by suppressing DPC apoptosis induced by oxidative stress.
Our study indicates that SAB holds potential as a treatment for AGA by preventing apoptosis in hair follicle cells under conditions of inflammation and oxidative stress. The proposed mechanism involves SAB’s inhibition of NADPH oxidase, SOD, and iNOS expression, which consequently down-regulates the MAPK/ERK signaling pathway. Furthermore, the observation that SAB down-regulates the Wnt/β-catenin pathway and promotes hair growth in C57BL/6 mice, primarily through enhancing vascularization via up-regulation of VEGF, suggests that SAB may influence other signaling pathways that support and sustain hair growth.
Despite the promising potential of SAB in treating AGA, this study is pioneering in assessing its effects on DPCs. Consequently, the mechanisms underlying SAB’s action have not been thoroughly investigated. It however, adds to a valuable list of conditions to treat, those being heartache, stomachache, joint pain, and irregular menstruation.25 Future research is planned to explore the interactions between SAB and various signaling pathways. This comprehensive understanding is crucial before considering the use of SAB in clinical settings for AGA treatment.
Funding
This study was supported by the grants from Shanghai Engineering Research Center of Hair Medicine (19DZ2250500), National Science Foundation of China (82373507, 82173442 and 82103759), Leading Talent Project of Shanghai Health Commission (2022LJ017), Jing’an District Clinical Advantage Special Disease Construction Project (2021ZB01), Clinical Research Plan of SHDC (SHDC22022302), Shanghai Municipal Commission of Health and Family Planning (2023ZZ02018), Shanghai Municipal Key Clinical Specialty (shslczdzk01002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen S, Xie X, Zhang G, Zhang Y. Comorbidities in androgenetic alopecia: a comprehensive review. Dermatol Ther. 2022;12(10):2233–2247. doi:10.1007/s13555-022-00799-7
2. Mu Z, Gao Y, Li K, Liu H, Zhang J. Androgenetic alopecia among hospital staff: a study of prevalence, types and a comparison with general population in a secondary hospital in China. Clin Cosmet Invest Dermatol. 2021;14:1387–1392. doi:10.2147/CCID.S333789
3. English RS. A hypothetical pathogenesis model for androgenic alopecia: clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Med Hypotheses. 2018;111:73–81. doi:10.1016/j.mehy.2017.12.027
4. Traish AM. Post-finasteride syndrome: a surmountable challenge for clinicians. Fertil Sterility. 2020;113(1):21–50. doi:10.1016/j.fertnstert.2019.11.030
5. Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021;20(12):3759–3781. doi:10.1111/jocd.14537
6. Xiao Z, Liu W, Mu YP, et al. Pharmacological effects of salvianolic acid B against oxidative damage. Front Pharmacol. 2020;11:572373. doi:10.3389/fphar.2020.572373
7. Wang J, Xiong X, Feng B. Cardiovascular effects of salvianolic Acid B. Evid Based Complement Alternat Med. 2013;2013:247948. doi:10.1155/2013/247948
8. Yan F. Effects of salvianolic acid B on growth inhibition and apoptosis induction of ovarian cancer SKOV3. Eur J Gynaecol Oncol. 2016;37(5):653–656.
9. Koppula S, Kumar H, Kim IS, Choi DK. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of parkinson’s disease. Mediators Inflamm. 2012;2012:823902. doi:10.1155/2012/823902
10. Yeo IK, Jang WS, Min PK, et al. An epidemiological study of androgenic alopecia in 3114 Korean patients. Clin Exp Dermatol. 2014;39(1):25–29. doi:10.1111/ced.12229
11. Shahzadi I, Ali Z, Bukhari S, Narula AS, Mirza B, Mohammadinejad R. Possible applications of salvianolic acid B against different cancers. Explor Target Antitumor Ther. 2020;1(4):218–238. doi:10.37349/etat.2020.00014
12. Lin J, Lin R, Li S, et al. Salvianolic acid B promotes the survival of random-pattern skin flaps in rats by inducing autophagy. Front Pharmacol. 2018;9:1178. doi:10.3389/fphar.2018.01178
13. Lay IS, Chiu JH, Shiao MS, Lui WY, Wu CW. Crude extract of Salvia miltiorrhiza and salvianolic acid B enhance in vitro angiogenesis in murine SVR endothelial cell line. Planta Med. 2003;69(1):26–32. doi:10.1055/s-2003-37034
14. Chen J, Wang Y, Wang S, Zhao X, Zhao L, Wang Y. Salvianolic acid B and ferulic acid synergistically promote angiogenesis in HUVECs and zebrafish via regulating VEGF signaling. J Ethnopharmacol. 2022;283:114667. doi:10.1016/j.jep.2021.114667
15. Liu CL, Xie LX, Li M, Durairajan SS, Goto S, Huang JD. Salvianolic acid B inhibits hydrogen peroxide-induced endothelial cell apoptosis through regulating PI3K/Akt signaling. PLoS One. 2007;2(12):e1321. doi:10.1371/journal.pone.0001321
16. Katary MA, Abdelsayed R, Alhashim A, Abdelhasib M, Elmarakby AA. Salvianolic acid B slows the progression of breast cancer cell growth via enhancement of apoptosis and reduction of oxidative stress, inflammation, and angiogenesis. Int J Mol Sci. 2019;20(22):5653. doi:10.3390/ijms20225653
17. Tsai M-K, Lin Y-L, Huang Y-T. Effects of salvianolic acids on oxidative stress and hepatic fibrosis in rats. Toxicol Appl Pharmacol. 2010;242(2):155–164. doi:10.1016/j.taap.2009.10.002
18. Sun J, Druhan LJ, Zweier JL. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch Biochem Biophys. 2010;494(2):130–137. doi:10.1016/j.abb.2009.11.019
19. Zhu B, Wang X, Teng J. Retracted article: salvianolic acid B inhibits inflammatory response and cell apoptosis via the PI3K/Akt signaling pathway in IL-1β-induced osteoarthritis chondrocytes. RSC Adv. 2018;8(64):36422–36429. doi:10.1039/C8RA02418A
20. Ohn J, Kim SJ, Choi SJ, Choe YS, Kwon O, Kim KH. Hydrogen peroxide (H(2)O(2)) suppresses hair growth through downregulation of β-catenin. J Dermatol Sci. 2018;89(1):91–94. doi:10.1016/j.jdermsci.2017.09.003
21. Govindarasu M, Ansari MA, Alomary MN, et al. Protective effect of salvianolic acid B in acetic acid-induced experimental colitis in a mouse model. Processes. 2021;9(9):1589. doi:10.3390/pr9091589
22. Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915–1928. doi:10.1083/jcb.201708007
23. Liu Q, Lu J, Lin J, et al. Salvianolic acid B attenuates experimental skin fibrosis of systemic sclerosis. Biomed Pharmacother. 2019;110:546–553. doi:10.1016/j.biopha.2018.12.016
24. Dai S, Liang T, Shi X, Luo Z, Yang H. Salvianolic acid B protects intervertebral discs from oxidative stress-induced degeneration via activation of the JAK2/STAT3 signaling pathway. Oxid Med Cell Longev. 2021;2021:6672978. doi:10.1155/2021/6672978
25. He G, Chen G, Liu W, et al. Salvianolic acid B: a review of pharmacological effects, safety, combination therapy, new dosage forms, and novel drug delivery routes. Pharmaceutics. 2023;15(9):2235. doi:10.3390/pharmaceutics15092235
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.