Back to Journals » Journal of Inflammation Research » Volume 13
Salvianolic Acid B Improves Chronic Mild Stress-Induced Depressive Behaviors in Rats: Involvement of AMPK/SIRT1 Signaling Pathway
Authors Liao D, Chen Y, Guo Y, Wang C, Liu N, Gong Q, Fu Y, Fu Y, Cao L, Yao D, Jiang P
Received 11 February 2020
Accepted for publication 18 April 2020
Published 12 May 2020 Volume 2020:13 Pages 195—206
DOI https://doi.org/10.2147/JIR.S249363
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Dehua Liao,1,2,* Yun Chen,1,* Yujin Guo,3 Changshui Wang,4 Ni Liu,1 Qian Gong,1 Yingzhou Fu,1 Yilan Fu,1 Lizhi Cao,1 Dunwu Yao,1 Pei Jiang3
1Department of Pharmacy, Hunan Cancer Hospital, Changsha, Hunan 410013, People’s Republic of China; 2Department of Pharmacy, Second Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China; 3Institute of Clinical Pharmacy & Pharmacology, Jining First People’s Hospital, Jining, Shandong 272000, People’s Republic of China; 4Department of Clinical Translational Medicine, Jining Life Science Center, Jining, Shandong 272000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Pei Jiang Tel/ Fax + 86 537 2106208
Email [email protected]
Introduction: Depression is one of the most common neuropsychiatric illnesses which leads to a huge social and economic burden on modern society. So, it is necessary to develop an effective and safe pharmacological intervention for depression. Accumulating evidence has shown that adenosine monophosphate-activated protein kinase/sirtuin 1 (AMPK/SIRT1) signaling pathway plays a pivotal role in the development of depression. Our present study aimed to investigate the antidepressant effect and possible mechanisms of salvianolic acid B (SalB) in a chronic mild stress (CMS)-induced depression model in rats.
Materials and Methods: The rats were randomly divided into three groups: control group with no stressor, CMS group and CMS+SalB (30 mg/kg/d) group. After administration for 28 consecutive days, the behavior tests were performed. The rats were sacrificed after behavior tests, and the brain tissues were collected for biochemical analysis.
Results: It was observed that the administration of SalB for 28 consecutive days successfully corrected the depressive-like behaviors in CMS-treated rats. SalB could effectively reduce the gene expression of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), as well as nuclear factor-kappa B (NF-κB) p65 protein. In addition, inhibitor of NF-κB (IκB) protein expression was significantly increased after the administration of SalB. Moreover, SalB could effectively decrease protein expression of oxidative stress markers such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) and increase the activity of catalase (CAT). SalB treatment also reversed CMS-induced inhibition of Nrf2 signaling pathway, along with increasing the mRNA expression of NAD(P)H:quinone oxidoreductase (NQO-1) and heme oxygenase 1 (HO-1). Regarding the endoplasmic reticulum (ER) stress markers, the protein expressions of C/EBP-homologous protein (CHOP) and glucose-regulated protein 78 kD (GRP78) were also significantly reduced after SalB administration. Furthermore, the supplementation of SalB could effectively activate the AMPK/SIRT1 signaling pathway, which indicated significant increase in pAMPK/AMPK ratio and SIRT1 protein expression.
Conclusion: Our study demonstrated that SalB relieved CMS-induced depressive-like state through the mitigation of inflammatory status, oxidative stress, and the activation of AMPK/SIRT1 signaling pathway.
Keywords: SalB, depression, CMS, AMPK/SIRT1
Introduction
Major depressive disorder (MDD) is a pervasive neuropsychiatric disorder with high morbidity and mortality,1 which is recognized as a global public issue. MDD is characterized by depressed mood, loss of concentration or interest, decreased food intake, and fatigue.2 A previous study has reported that depression may become the second most prevalent disease worldwide by 2020.3 Several drugs that are based on monoamine neurotransmitters have been used to reduce depressive symptoms. These agents exhibit some therapeutic benefits but are also accompanied by serious side effects.4 Therefore, it is urgent to further understand the pathophysiology of depression and develop new antidepressant drugs.
Numerous studies have shown that oxidative stress and neuroinflammatory are responsible for the development of anxiety and depression.5,6 It was reported that oxidative damage was significantly enhanced in depressed patients, and the levels of oxidative stress markers were decreased after chronic treatments with antidepressants.7 Our previous study demonstrated that the administration of doxorubicin induced an overexpression of hydroxyl radicals and superoxide radicals, accompanied with the alteration of oxidative stress.8 It is widely accepted that there is a strong link between oxidative stress and inflammation. A previous study showed that inflammation triggers the generation of elevated levels of cellular reactive oxygen species that cause cellular oxidative damage.9 In addition, various nuclear factor-kappa B (NF-κB) mediated pro-inflammatory mediators are released when inflammatory cells respond to oxidative stress.10 Generally, oxidative stress always leads to a strong induction of neuroinflammation, which results in neuronal damage in neurodegenerative disorders. As the resident macrophage-like cells of the central nervous system, microglia will always be activated during most neuropathological conditions, including ischemia, depression, and chronic neurodegenerative diseases. Previous studies have reported that activated microglia contribute to neuronal damage through the release of toxic mediators such as cytokines and free radicals in the central nervous system.11,12
Adenosine monophosphate-activated protein kinase (AMPK) is a highly conserved serine/threonine protein kinase, which alters energy metabolism via regulating the expressions or activities of downstream molecules.13 Sirtuin 1 (SIRT1), a major downstream molecule in AMPK signaling pathway, correlates closely to the cellular physiological and biochemical processes, including neuroinflammation.14 SIRT1 is required for the maintenance of normal cognitive function and synaptic plasticity regulation.15 Numerous studies have shown that AMPK/SIRT1 pathway plays a pivotal role in the development of many diseases, such as diabetes,16 fatty liver,17 tumors,18 neurodegenerative disorders,19 and cardiovascular disease.20 AMPK/SIRT1 pathway has also been reported to have neuroprotective effects on neurons in nervous system diseases, such as Parkinson’s disease and Alzheimer’s disease. Wang et al21 reported that AMPK signaling pathway was involved in the regulation of irisin on depressive-like behaviors in a chronic unpredictable mild stress rat model. Luo et al22 reported that SIRT1 expression was significantly downregulated in the peripheral blood of patients with major depressive disorder compared with comparison subjects. The activation of AMPK participates in the suppression of inflammatory responses by inhibiting inflammatory signaling, such as the NF-κB pathway.23 Chen et al24 have also reported that SIRT1 overexpression exhibited strong neuroprotective effects by suppressing NF-κB signaling. Moreover, the inflammatory effect of tumor necrosis factor tumor necrosis factor α (TNF-α) is triggered by the activation of the inflammatory signaling networks, including AMPK and SIRT1 pathways. The biological process of oxidative stress is also regulated by SIRT1. Some important transcription factors including nuclear erythroid factor 2-related factor 2 (Nrf2) are regulated by SIRT1, which induces the transcription of antioxidant enzymes and subsequently affects the cellular redox state.25 Hence, the neuroprotective effects of AMPK/SIRT1 pathway are closely involved in the regulation of biological processes of inflammation and oxidative stress.
Salvianolic acid B (SalB), a polyphenolic compound isolated from traditional Chinese herb Salvia miltiorrhiza, has a broad range of pharmacological potentials including anti-inflammatory, anti-oxidant and anti-apoptotic in vitro and in vivo.26,27 After injection with a dose of 20 mg/kg SalB, the pharmacokinetics of SalB in Wistar rat were as follows: area under the curve (AUC): 1130±329 g/mL•min, total body clearance (CLT): 23.5±6.0 mL/min/kg, steady-state volume of distribution (Vss): 3.61±1.16 L/kg, absorption half-life, t1/2α: 22.7±4.29 min, elimination half-life, t1/2β: 176±30.4 min.28 Numerous evidence has shown that SalB alleviates neuroinflammation and exerts neuroprotective activities in several neurological disorders.29,30 Zhang et al31 reported that the antidepressant-like effects of SalB are associated with anti-inflammatory activity in chronic mild stress (CMS)-treated C57BL/6 mice. Several studies have suggested that SalB mediates anti-inflammation via inhibiting NF-κB signaling.32,33 In addition, the anti-oxidative effects of SalB also play an important role in neuroprotective activities. Zeng et al34 demonstrated that SalB is a potent activator of SIRT1. Lv et al35 have also reported that SalB attenuated brain injury by reducing inflammation and apoptosis through the activation of SIRT1 signaling. Moreover, SalB provides protection against subarachnoid hemorrhage (SAH)-triggered oxidative damage by upregulating the Nrf2 antioxidant signaling pathway, which may be modulated by SIRT1 activation.36 In addition, SalB can traverse the blood-brain barrier37 and act directly on the central nervous system. However, the detailed mechanism underlying the antidepressant effects of SalB as related to AMPK/SIRT1 pathway in the brain still remain unknown.
Therefore, in our present study, we aimed to investigate the antidepressant-like effect of SalB in CMS-induced rats. The neuroinflammation, oxidative stress and endoplasmic reticulum stress in the brain of the animal model were also determined in our study. In addition, the activation of AMPK/SIRT1 pathway in the brain was analyzed to gain further insight into the mechanism underlying the antidepressant effect of SalB.
Materials and Methods
Animals
Male Sprague-Dawley rats (200 ± 20g) were obtained from Hunan Cancer Hospital Animal Centre. Rats were housed in polycarbonate cages under controlled temperature (23 ± 2°C) with a 12h light/dark cycle. Food and water were freely available except prior to sucrose preference test (SPT). The study was approved by the animal ethics committee members of Hunan Cancer Hospital (protocol number 021/2019). All experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals (Chinese Council).
CMS Procedure
After one week of adaptation, the CMS procedure was performed according to the method described previously,38 with slight modification. Briefly, the rats were subjected to several different stressors according to a semi-random schedule for 4 consecutive weeks, and each cage housed 4 rats during CMS procedure. The stressors consisted of 24h food deprivation followed by 24h water deprivation, 45° cage tilting for 24 h, restraint for 4 h in an empty water bottle, 20 min of noise, 1 min tail clamping and damp bedding. In order to make the stress procedure unpredictable, all stressors were applied individually, continuously, and randomly.
Experimental Design
The 24 rats were randomly divided into three groups (n=8): control group with no stressor, CMS group and CMS+SalB (30 mg/kg/d) group. All the rats were exposed to CMS for 4 weeks. Treatment group (CMS+SalB) rats were intraperitoneally injected with SalB at a dose of 30 mg/kg/d for 4 weeks, control rats were intraperitoneally injected with equal volume of saline without CMS exposure for 4 weeks, and the rats in CMS group were administered an equal volume of saline for 4 weeks. All the injection operations were conducted once daily 60 min before each stressor was applied for 4 weeks. All the experimental protocols are shown in Figure 1.
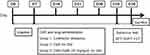 |
Figure 1 Schematic representation of experimental protocol. |
SalB was provided by the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). SalB was dissolved in normal saline for injection.
After behavior tests, all the rats were sacrificed and the brains of all the 24 rats were used for the following molecular analysis. The left hemispheres of the brains were prepared for histopathological examination and immunohistochemical staining. While the hippocampi in the right hemispheres were used for Western blot determination and polymerase chain reaction analysis, including the biomarkers of neuroinflammation, oxidative stress, endoplasmic reticulum stress, and AMPK/SIRT1 signaling pathway.
SPT
The SPT was performed using the same method as in our previous study.8 In brief, 2 days before the experiment, all the rats were each cage separated and habituated to drink 1% sucrose solution in two bottles on each side. After deprivation of water for 14 h, all the rats had full access to two pre-weighed bottles (one with only tap water and the other with 1% sucrose). After 1 h, the bottles were weighed again, and the consumed weights of 1% sucrose solution and tap water were recorded. The percentage preference for sucrose was calculated as follows: sucrose preference (%) = sucrose consumption/(sucrose consumption + water consumption).
Forced Swimming Test (FST)
The FST was performed as described previously39 with minor modifications. In short, rats were separated and forced to swim in the glass chamber (40 cm height × 25 cm diameter) filled with water (up to 25 cm) at room temperature for a 15 min pretest. After 24 h, the rats were again placed in this container for 5 min. Immobility time was defined as floating with only small movement necessary to keep the head above water, and this parameter was scored under double blind conditions.
Novelty-Suppressed Feeding Test (NSFT)
Before NSFT, the rats were food-deprived for 24 h in their home cages. A small amount of food was placed on a sheet of white paper (10×10 cm) which was placed in an open field (75×75×40 cm). The rats were allowed to explore the open field for 8 min. The time it took for the rat to approach and take the first bite of the food was defined as latency time and recorded in our study. After that, the animals were transferred to their home cage, and the total food intake for the next 5 min was also weighed to avoid the influence of the animals’ appetite.
Real-Time PCR Analysis
According to the manufacturer’s instructions, trizol reagent (Invitrogen Corp., Carlsbad, CA, USA) was used to extract total RNA from the hippocampus. The mRNA level of interleukin-6 (IL-6), interleukin-1β (IL-1β), TNF-α, NAD(P)H: quinone oxidoreductase (NQO-1) and heme oxygenase 1 (HO-1) was determined in our present study. Quantitative polymerase chain reaction (PCR) was performed on Bio-Rad Cx96 Detection System (Bio-Rad, Hercules, CA, USA) using SYBR green PCR kit (Applied Biosystems Inc., Woburn, MA, USA) and gene-specific primers. Table 1 shows the sequences of gene-specific primers. A 5 ng cDNA sample was used with 40 cycles of amplification. Each cDNA was determined in triplicate. β-actin was used as an internal standard to normalize the signals.
 |
Table 1 Primers Used in Real-Time PCR Analyses of mRNA Expression |
Western Blot Analysis
For Western blot analysis, total protein was isolated from the hippocampus and its concentration was determined by Bradford method. The concentration of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was 12%. The gel was transferred to a polyvinylidene fluoride (PVDF) membrane and the filters were blocked for 1 h in 5% non-fat dry milk in TBS-T (25 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween-20). Blots were incubated at 4°C overnight with antibodies as follows: anti-C/EBP-homologous protein (anti-CHOP) (Cell Signaling; 1:1000), anti glucose-regulated protein 78 kD (anti-GRP78) (Proteintech; 1:1000), anti-inhibitor of NF-κB (anti-IκB) (Cell Signaling; 1:1000), anti-p65 (Proteintech; 1:800), anti-AMPK (Cell Signaling; 1:2000), anti-pAMPK (Cell Signaling; 1:2000), anti-SIRT1 (abcam; 1:1000), anti-β-actin (Proteintech; 1:4000). After washing, the membranes were then probed with horseradish peroxidase-conjugated secondary antibody. The bands were then detected by a chemiluminescence detection system. β-actin was used as an internal standard for all of the gels.
Measurement of Oxidative Stress
The contents of malondialdehyde (MDA) and catalase (CAT) activity of the hippocampus were measured by lipid peroxidation MDA Assay Kit and CAT Assay Kit (Nanjing Jiancheng Bioengineering Institute, China), respectively, following the manufacturer’s instructions.
Nissl Staining
Nissl staining was used to detect necrotic cell death according to morphological changes in the brain. Paraffin-embedded hippocampi were cut into 5 µm thick sections, and the sections were stained with 0.1% cresyl violet (Sigma Aldrich), dehydrated with ethanol, and cover-slipped with Entellan. The staining was observed under a light microscope (400× magnification). Five sections from each animal were selected for counting viable neuron numbers, using Image J software.
Immunohistochemical Staining
For immunohistochemical analysis, 5 µm thick sections of paraffin-embedded hippocampi were processed by standard procedure. The tissue was then incubated with Iba-1 antibody (rabbit, monoclonal, 1:4000) with gentle shaking for 4 h at room temperature and then overnight at 4°C, followed by washing with PBS 3× and 30 min incubation with biotinylated secondary antibody at room temperature. Staining was developed by 3, 3′-diaminobenzidine solution and counterstained with hematoxylin. Five sections from each animal were selected for counting Iba-1 positive cells by using Image J software (400× magnification).
Immunofluorescence Staining
The procedures of immunofluorescence were performed as in a previous study.40 5 µm thick sections of paraffin-embedded hippocampi were incubated at room temperature with primary antibodies against 4-hydroxynonenal (4-HNE) (1:100 Abcam, Cambridge, MA). After washing three times with PBS, the sections were incubated with a secondary antibody: Cy3-conjugated goat anti-rat (1:300; Wuhan goodbio technology CO, LTD). The nucleus was counter-stained with 4,6-diamidino-2-phenylindole (DAPI). The sections were then mounted with a fluorescent mounting medium and imaged. Colocalization of brains with 4-HNE with DAPI was observed under a fluorescence microscope. The cells with DAPI labeling (blue) overlapping with 4-HNE (green) in the brains were counted by an investigator blinded to the experimental design.
Statistical Analysis
Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). All data were analyzed by one-way analysis of variance (ANOVA) with least significant difference (LSD) post hoc multiple comparisons. The homogeneity of variance and normality of all the data were tested before ANOVA. All variables were expressed as mean ± standard deviation (SD), and values of p<0.05 were considered statistically significant.
Results
Effects of SalB on Behavioral Tests
As shown in Figure 2, in comparison with the rats in control group, the sucrose preference in SPT was significantly reduced in CMS group (Figure 2A, F2, 21=11.6, p<0.01). The immobility time in FST (Figure 2B, F2, 21=13.7, p<0.01) and latency time in NSFT (Figure 2C, F2, 21=15.2, p<0.01) were all significantly prolonged when compared with control group. In comparison with the CMS group, the administration of SalB markedly increased sucrose preference (Figure 2A, F2, 21=11.6, p<0.05), decreased immobility time (Figure 2B, F2, 21=13.7, p<0.01), and latency time (Figure 2C, F2, 21=15.2, p<0.01). In addition, no significant difference of food intake was observed in NSFT (Figure 2D, F2, 21=1.73, p>0.05).
Effects of SalB on CMS-Induced Neuronal Apoptosis and Microglia Activation
Nissl staining was used to determine the neurons’ viability in the hippocampus in our study. As shown in Figure 3A, a large amount of neurons showed physalides, karyopyknosis, cellular structure loss in CMS-treated rats when compared with the rats in control group. Meanwhile, the administration of SalB significantly decreased the degree of degeneration and necrosis of neurons in CMS-treated rats. The quantitative results are shown in Figure 3B, in comparison with the rats in control group, viable neuronal cells were significantly decreased in CMS group (Figure 3B, F2, 21=22.6, p<0.01). In contrast, SalB treatment significantly rescued neuronal cells from CMS-induced injury in the hippocampus (Figure 3B, F2, 21=22.6, p<0.01). Immunohistochemical staining for Iba-1 was used to investigate whether the activation of microglia was regulated by SalB. The expression of Iba-1 was found to be upregulated in CMS group, and the expression of Iba1 proteins was attenuated after administration of SalB (Figure 3A). The quantitative results are shown in Figure 3C, in which a significant increase in the microglia density of Iba-1 positive cells was found in the CMS group compared with the control group (Figure 3C, F2, 21=57.1, p<0.01), and SalB co-treatment significantly decreased the microglia density of Iba-1 positive cells in the hippocampus (Figure 3C, F2, 21=57.1, p<0.01).
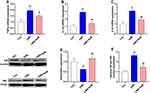
Effects of CMS and SalB on Neuroinflammation
To investigate inflammatory alterations triggered by CMS stimulation, the mRNA expression of TNF-α, IL-1β, and IL-6 were determined in our present study. After exposure to CMS, the mRNA levels of TNF-α (Figure 4A, F2, 21=24.3, p<0.01), IL-1β (Figure 4B, F2, 21=43.6, p<0.01), and IL-6 (Figure 4C, F2, 21=34.7, p<0.01) in hippocampus were all significantly elevated when compared to those in control group. Nevertheless, the administration of SalB remarkably decreased the mRNA level of TNF-α (Figure 4A, F2, 21=24.3, p<0.05), IL-1β (Figure 4B, F2, 21=43.6, p<0.01), and IL-6 (Figure 4C, F2, 21=34.7, p<0.01) compared to those of rats in CMS group. The protein expression of IκB (Figure 4D, F2, 21=19.8, p<0.01) was significantly suppressed in CMS group as compared to the vehicle treated control group, and the treatment of SalB significantly mitigated the reduction of IκB protein level (Figure 4D, F2, 21=19.8, p<0.01) when compared to the rats in CMS group. The nuclear protein expression of NF-κB p65 (Figure 4E, F2, 21=25.7, p<0.01) was significantly increased in CMS group, while SalB effectively decreased the nuclear protein expression of NF-κB p65, which is consistent with the modulating effects of SalB on the inflammatory cytokines. (Figure 4E, F2, 21=25.7, p<0.01).
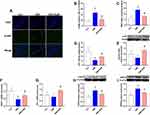
Effects of CMS and SalB on Oxidative Stress and Endoplasmic Reticulum Stress
As shown in Figure 5A, a large number of 4-HNE positive cells in hippocampus was observed in CMS group, and few of cells were labeled with 4-HNE in control group and CMS+SalB group. As expected, 4-HNE positive cells increased markedly in CMS group compared with control group (Figure 5B, F2, 21=22.9, p<0.01). However, SalB treatment significantly decreased the number of 4-HNE positive cells in hippocampus (Figure 5B, F2, 21=22.9, p<0.01). Exposure to CMS resulted in a significant increase in MDA concentration (Figure 5C, F2, 21=32.6, p<0.01) as well as a significant decrease in CAT activity (Figure 5D, F2, 21=37.2, p<0.01) in hippocampus compared to control group. The administration of SalB induced a significant elevation in the CAT activity (Figure 5D, F2, 21=37.3, p<0.01) as well as a significant reduction in MDA concentration (Figure 5C, F2, 21=32.6, p<0.01) in hippocampus compared to CMS group. The results of Western blot showed that in comparison with control group, the protein expression of Nrf2 in nuclear was significantly suppressed after exposure to CMS (Figure 5E, F2, 21=14.7, p<0.05). The administration of SalB significantly reversed CMS-induced decrease of nuclear Nrf2 protein level (Figure 5E, F2, 21=14.7, p<0.01), which indicated that SalB promoted Nrf2 nuclear translocation. The mRNA expressions of anti-oxidative protein were also assessed in our study. The gene level of NQO-1 (Figure 5F, F2, 21=10.6, p<0.01) was significantly decreased after exposure to CMS when compared to vehicle treated group. In addition, compared with the rats in CMS group, both NQO-1 (Figure 5F, F2, 21=10.6, p<0.01) and HO-1 (Figure 5G, F2, 21=11.2, p<0.01) mRNA levels were significantly upregulated after administration of SalB. The protein expressions of endoplasmic reticulum (ER) stress marker (GRP78 and CHOP) were also determined in our study. The protein levels of both CHOP (Figure 5H, F2, 21=21.4, p< 0.01) and GRP78 (Figure 5I, F2, 21=17.9, p<0.01) were all significantly increased in CMS group. Meanwhile, the up-regulation of CHOP (Figure 5H, F2, 21=21.4, p<0.01) and GRP78 protein level (Figure 5I, F2, 21=17.9, p<0.05) were effectively inhibited by SalB treatment.
Effects of SalB on the Activation of AMPK/SIRT1 in CMS-Treated Rats
As shown in Figure 5, pAMPK/AMPK ratio (Figure 6A, F2, 21=9.8, p<0.01) and protein expression of SIRT1 (Figure 6B, F2, 21=19.5, p<0.01) in CMS group were all significantly decreased when compared with the rats in normal treated group. However, the administration of SalB obviously reversed CMS-induced decrease of pAMPK/AMPK ratio (Figure 6A, F2, 21=9.8, p<0.01) and SIRT1 protein (Figure 6B, F2, 21=19.5, p<0.01) expression.
Discussion
Our present study demonstrated the anti-depressant role of SalB in a CMS-induced depression model. Depressive-like behaviors (SPT, FST, and NSFT) were observed in rats exposed to CMS, and chronic administration of SalB normalized behavioral changes in rats under CMS. Besides, we also found that chronic administration of SalB could attenuate neuroinflammation, oxidative stress, and ER stress in the hippocampi of rats under CMS. Furthermore, our study suggested that the potential antidepressant mechanism of SalB might be related to the activation of AMPK/SIRT1 signaling pathways.
The CMS model is a widely used animal model of depression, and most effects of CMS could be effectively reversed by antidepressant agents.41 The behavioral changes were determined by SPT, FST, and NSFT in our present study. Significantly reduced sucrose preference with longer immobility time in FST and longer latency time were observed in the rats exposed to CMS. Moreover, the administration of SalB displayed significant improvement in sucrose preference and shorter immobility time in FST and shorter latency time in NSFT, which indicated the potential anti-depressant activity of SalB. The results were consistent with previous studies. Huang et al and Zhang et al31,42 have reported that 3 weeks of treatment with SalB could increase sucrose consumption in SPT and result in shorter immobility time in FST of rats under CMS, thus reported as potent anti-depressant activity.
The histopathology result in our present study showed that the number of viable neurons was significantly increased while Iba-1 positive microglia density was significantly decreased under CMS, and the administration of SalB could effectively reverse this phenomenon, which indicated that the administration of SalB could effectively alleviate CMS-induced damage to neurons in the hippocampus. The neuroprotective effects of SalB have also been widely reported in previous studies.30,43
It is well accepted that activated microglia perform a critical role among neurodegenerative diseases, and the activation of microglia is accompanied with the release of pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α in the central nervous system.44,45 A previous study showed that microglia could be activated under CMS, accompanied with expression of pro-inflammatory cytokines.46 Dannehl et al47 reported that the protein expressions of IL-6, IL-1β, and TNF-α in blood were higher in a depressed patient. Lv et al35 reported that SalB alleviates inflammation as evidenced by the decrease of TNF-α and IL-1β levels in brain tissue of SD rat. Huang et al42 also showed that the protein levels of inflammatory cytokines like IL-6, IL-1β, and TNF-α were significantly increased in the brain of rats under CMS, and the administration of SalB could effectively reduce the concentration of these inflammatory markers in the brain of CMS-induced rats. The results in our present study are in agreement with the previously mentioned studies. SalB successfully reversed CMS-induced upregulation of the gene expression of IL-6, IL-1β, and TNF-α in the hippocampus. NF-κB is necessary for the transcription of inflammatory cytokines and a key regulator known to exacerbate inflammatory diseases.48 Under normal circumstances, NF-κB proteins combined with IκB generally exist in the cytoplasm almost inactively.49,50 NF-κB is activated by phosphorylation when stimulated by inflammatory mediators, and then translocated into the nucleus,51 which promotes the expression of pro-inflammatory cytokines. Numerous studies have revealed that the anti-inflammatory effect of SalB was accompanied by inhibiting of transcription factor NF-κB activation.33,52 Therefore, the nuclear NF-κB p65 protein expression and IκB protein expression in the hippocampus were determined in our present study to access the effect of SalB on the NF-κB signaling pathway in rats under CMS. Our results showed that SalB could effectively decrease nuclear NF-κB p65 protein expression, and increase the protein level of IκB in the meantime, which means SalB could inhibit CMS-induced NF-κB activation. These results indicated that SalB may exhibit an anti-inflammatory effect by modulating the NF-κB signal pathway.
Previous studies have shown that oxidative stress is activated in depression.53 The hippocampus lipid peroxidation and antioxidant parameters were determined in our present study to unveil the effect of SalB on oxidative stress in CMS-induced rats. Our study found that oxidative stress marker MDA and 4-HNE positive cells observed in immunofluorescence staining were significantly increased in rats under CMS, while CAT activity was significantly decreased. Furthermore, our study found that SalB effectively reversed the increase of both MDA and 4-HNE positive cells and the reduction of CAT. The same results were also observed in a previous study.42 The most possible mechanism of this phenomenon may be the potent free radical scavenging and anti-lipid peroxidation activities of SalB.54 The activation of the nucleus transcription factor Nrf2 is pivotal in antioxidant stress systems of cells. Nrf2 translocates into the nucleus once it encounters oxidative stress stimulation, and subsequently induces antioxidant enzyme expression, including NQO-1 and HO-1.55 Our results showed SalB could successfully reverse CMS-induced decrease of Nrf2 protein in nucleus, which indicated that SalB could activate the Nrf2 pathway. The increase of gene expression of NQO-1and HO-1 after administration of SalB further demonstrated that SalB may exert an antioxidant effect by activating Nrf2. The imbalance of ER stress is another important inducer of depression. The results in our study showed that protein levels of the typical marker of ER stress (CHOP and GRP78) were all significantly elevated under CMS and the chronic administration of SalB successfully reversed these elevations. To the best of our knowledge, although previous studies have demonstrated that the inhibition of ER stress is a pivotal mechanism of SalB in cardiac protection, our study is the first to demonstrate that ER stress was involved in neuroprotection of SalB.
The activation of AMPK and SIRT1 has been reported to have protective effects on neurons in nervous system diseases. Previous studies have shown that the expression of antioxidant defense related gene could be promoted with the activation of AMPK.56 Furthermore, massive studies have also suggested that AMPK and SIRT1 were involved in the production of pro-inflammatory cytokines.57,58 The expressions of inflammatory factors were significantly decreased after the activation of AMPK/SIRT1 signaling pathway, which in turn further inhibited the inflammatory response. Studies also showed that the inhibitory effects of AMPK activation on pro-inflammatory cytokine production is realized by altering NF-κB activation.45 Our present study found that pAMPK/AMPK ratio was significantly decreased under CMS, and the administration of SalB significantly reversed this phenomenon. The same result was also observed for the expression of SIRT1. Thus, it was suggested that SalB improves CMS-induced depression through anti-inflammatory and anti-oxidative activities in a AMPK/SIRT1 dependent way.
However, a limitation should be noted in our study. We found that the anti-depressant mechanism of SalB was the activation of AMPK/SIRT1 signaling pathway. But we did not provide direct evidence to demonstrate that the reduction in proinflammatory cytokines and oxidative stress by SalB were induced by the activation of the AMPK/SIRT1 pathway. This limitation will be further investigated in our future study.
Conclusion
Our present study has demonstrated that SalB exerted great potential to reverse the depressive-like behavior in CMS-induced rats. The underlying mechanism is largely mediated by suppressing the expressions of pro-inflammatory cytokines and oxidative stress via the activation of AMPK/SIRT1 signaling pathway.
Acknowledgments
This study was supported by Health and Family Planning Commission Foundation of Hunan Province (grant number B20180252, C2019064) and the Project of Hunan Provincial Science & Technology Department (grant number 2018JJ6032, 2019JJ80093).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007;3:137–158. doi:10.1146/annurev.clinpsy.3.022806.091444
2. Matthew C, Keller MCN, Kenneth S. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164:8. doi:10.1176/ajp.2007.164.5.712
3. O’Neil A, Fisher AJ, Kibbey KJ, et al. Depression is a risk factor for incident coronary heart disease in women: an 18-year longitudinal study. J Affect Disord. 2016;196:117–124. doi:10.1016/j.jad.2016.02.029
4. Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi:10.1523/JNEUROSCI.20-24-09104.2000
5. Sulakhiya K, Kumar P, Jangra A, et al. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur J Pharmacol. 2014;744:124–131. doi:10.1016/j.ejphar.2014.09.049
6. Thakare VN, Dhakane VD, Patel BM. Potential antidepressant-like activity of silymarin in the acute restraint stress in mice: modulation of corticosterone and oxidative stress response in cerebral cortex and hippocampus. Pharmacol Rep. 2016;68(5):1020–1027. doi:10.1016/j.pharep.2016.06.002
7. Ng F, Berk M, Dean O, et al. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi:10.1017/S1461145707008401
8. Liao D, Xiang D, Dang R, et al. Neuroprotective effects of dl-3-n-butylphthalide against doxorubicin-induced neuroinflammation, oxidative stress, endoplasmic reticulum stress, and behavioral changes. Oxid Med Cell Longev. 2018;2018:1–13. doi:10.1155/2018/9125601
9. Yang YC, Chou HY, Shen TL, et al. Topoisomerase II-mediated DNA cleavage and mutagenesis activated by nitric oxide underlie the inflammation-associated tumorigenesis. Antioxid Redox Signal. 2013;18(10):1129–1140. doi:10.1089/ars.2012.4620
10. Chaudhari N, Talwar P, Parimisetty A, et al. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014;8:213. doi:10.3389/fncel.2014.00213
11. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi:10.1038/nn1997
12. Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85(3):352–370.
13. Salt IP, Hardie DG. AMP-activated protein kinase: an ubiquitous signaling pathway with key roles in the cardiovascular system. Circ Res. 2017;120(11):1825–1841. doi:10.1161/CIRCRESAHA.117.309633
14. Libert S, Guarente L. Metabolic and neuropsychiatric effects of calorie restriction and sirtuins. Annu Rev Physiol. 2013;75:669–684. doi:10.1146/annurev-physiol-030212-183800
15. Wang F, Shang Y, Zhang R, et al. A SIRT1 agonist reduces cognitive decline in type 2 diabetic rats through antioxidative and antiinflammatory mechanisms. Mol Med Rep. 2019;19(2):1040–1048. doi:10.3892/mmr.2018.9699
16. Kume S, Koya D. Autophagy: a novel therapeutic target for diabetic nephropathy. Diabetes Metab J. 2015;39(6):451–460. doi:10.4093/dmj.2015.39.6.451
17. Song YM, Lee Y-H, Kim J-W, et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11(1):13. doi:10.4161/15548627.2014.984271
18. Talero E, Alcaide A, Avila-Roman J, et al. Expression patterns of sirtuin 1-AMPK-autophagy pathway in chronic colitis and inflammation-associated colon neoplasia in IL-10-deficient mice. Int Immunopharmacol. 2016;35:248–256. doi:10.1016/j.intimp.2016.03.046
19. Domise M, Vingtdeux V. AMPK in neurodegenerative diseases. Exp Suppl. 2016;107:153–177. doi:10.1007/978-3-319-43589-3_7
20. Sciarretta S, Hariharan N, Monden Y, et al. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart?. Pediatr Cardiol. 2011;32(3):275–281. doi:10.1007/s00246-010-9855-x
21. Wang S, Pan J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem Biophys Res Commun. 2016;474(1):22–28. doi:10.1016/j.bbrc.2016.04.047
22. Luo XJ, Chen Z. Down-regulation of SIRT1 gene expression in major depressive disorder. Am J Psychiatry. 2016;173(10):1046. doi:10.1176/appi.ajp.2016.16040394
23. O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–355. doi:10.1038/nature11862
24. Chen J, Zhou Y, Mueller-S S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280(48):40364–40374. doi:10.1074/jbc.M509329200
25. Do MT, Kim HG, Choi JH, et al. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1alpha/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med. 2014;74:21–34. doi:10.1016/j.freeradbiomed.2014.06.010
26. Kim DH, Park SJ, Kim JM, et al. Cognitive dysfunctions induced by a cholinergic blockade and Abeta 25–35 peptide are attenuated by salvianolic acid B. Neuropharmacology. 2011;61(8):1432–1440. doi:10.1016/j.neuropharm.2011.08.038
27. Wang SX, Hu LM, Gao XM, et al. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem Res. 2010;35(7):1029–1037. doi:10.1007/s11064-010-0151-1
28. Zhang Y, Akao T, Nakamura N, et al. Extremely low bioavailability of magnesium lithospermate B, an active component from Salvia miltiorrhiza, in rat. Planta Med. 2004;70(2):138–142.
29. Lee YW, Kim DH, Jeon SJ, et al. Neuroprotective effects of salvianolic acid B on an Abeta25–35 peptide-induced mouse model of Alzheimer’s disease. Eur J Pharmacol. 2013;704(1–3):70–77. doi:10.1016/j.ejphar.2013.02.015
30. Zhou J, Qu XD, Li ZY, et al. Salvianolic acid B attenuates toxin-induced neuronal damage via Nrf2-dependent glial cells-mediated protective activity in Parkinson’s disease models. PLoS One. 2014;9(7):e101668. doi:10.1371/journal.pone.0101668
31. Zhang JQ, Wu XH, Feng Y, et al. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol Sin. 2016;37(9):1141–1153. doi:10.1038/aps.2016.63
32. Xu S, Zhong A, Bu X, et al. Salvianolic acid B inhibits platelets-mediated inflammatory response in vascular endothelial cells. Thromb Res. 2015;135(1):137–145. doi:10.1016/j.thromres.2014.10.034
33. Wang R, Yu XY, Guo ZY, et al. Inhibitory effects of salvianolic acid B on CCl(4)-induced hepatic fibrosis through regulating NF-kappaB/IkappaBalpha signaling. J Ethnopharmacol. 2012;144(3):592–598. doi:10.1016/j.jep.2012.09.048
34. Zeng W, Shan W, Gao L, et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci Rep. 2015;5:16013. doi:10.1038/srep16013
35. Lv H, Wang L, Shen J, et al. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res Bull. 2015;115:30–36. doi:10.1016/j.brainresbull.2015.05.002
36. Zhang X, Wu Q, Lu Y, et al. Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2- and SIRT1-dependent pathways. Free Radic Biol Med. 2018;124:504–516. doi:10.1016/j.freeradbiomed.2018.06.035
37. Zhang YJ, Wu L, Zhang QL, et al. Pharmacokinetics of phenolic compounds of Danshen extract in rat blood and brain by microdialysis sampling. J Ethnopharmacol. 2011;136(1):129–136. doi:10.1016/j.jep.2011.04.023
38. Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):625–631. doi:10.1016/S0278-5846(03)00051-4
39. Kumar S, Mondal AC. Neuroprotective, neurotrophic and anti-oxidative role of Bacopa monnieri on CUS induced model of depression in rat. Neurochem Res. 2016;41(11):3083–3094. doi:10.1007/s11064-016-2029-3
40. Wang R, Tu J, Zhang Q, et al. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling. Hippocampus. 2013;23(7):634–647. doi:10.1002/hipo.22126
41. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:10.
42. Huang Q, Ye X, Wang L, et al. Salvianolic acid B abolished chronic mild stress-induced depression through suppressing oxidative stress and neuro-inflammation via regulating NLRP3 inflammasome activation. J Food Biochem. 2019;43(3):e12742.
43. Xu S, Zhong A, Ma H, et al. Neuroprotective effect of salvianolic acid B against cerebral ischemic injury in rats via the CD40/NF-kappaB pathway associated with suppression of platelets activation and neuroinflammation. Brain Res. 2017;1661:37–48. doi:10.1016/j.brainres.2017.02.011
44. Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front Aging Neurosci. 2017;9:176. doi:10.3389/fnagi.2017.00176
45. Peixoto C, Oliveira W, Araujo S, et al. AMPK activation: role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol. 2017;298(Pt A):31–41. doi:10.1016/j.expneurol.2017.08.013
46. Kong H, Yang L, He C, et al. Chronic unpredictable mild stress accelerates lipopolysaccharide- induced microglia activation and damage of dopaminergic neurons in rats. Pharmacol Biochem Behav. 2019;179:142–149. doi:10.1016/j.pbb.2019.01.004
47. Dannehl K, Rief W, Schwarz MJ, et al. The predictive value of somatic and cognitive depressive symptoms for cytokine changes in patients with major depression. Neuropsychiatr Dis Treat. 2014;10:1191–1197. doi:10.2147/NDT.S61640
48. Yang R, Yang L, Shen X, et al. Suppression of NF-kappaB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2012;674(2–3):391–396. doi:10.1016/j.ejphar.2011.08.029
49. Li S, Wang R, Wu B, et al. Salvianolic acid B protects against ANIT-induced cholestatic liver injury through regulating bile acid transporters and enzymes, and NF-kappaB/IkappaB and MAPK pathways. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(9):1169–1180. doi:10.1007/s00210-019-01657-8
50. Lou Y, Wang C, Zheng W, et al. Salvianolic acid B inhibits IL-1beta-induced inflammatory cytokine production in human osteoarthritis chondrocytes and has a protective effect in a mouse osteoarthritis model. Int Immunopharmacol. 2017;46:31–37. doi:10.1016/j.intimp.2017.02.021
51. Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–468. doi:10.1146/annurev-biophys-083012-130338
52. Zhou Z, Liu Y, Miao AD, et al. Salvianolic acid B attenuates plasminogen activator inhibitor type 1 production in TNF-alpha treated human umbilical vein endothelial cells. J Cell Biochem. 2005;96(1):109–116. doi:10.1002/jcb.20567
53. Black CN, Bot M, Scheffer PG, et al. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi:10.1016/j.psyneuen.2014.09.025
54. Jiang YF, Liu ZQ, Cui W, et al. Antioxidant effect of salvianolic acid B on hippocampal CA1 neurons in mice with cerebral ischemia and reperfusion injury. Chin J Integr Med. 2015;21(7):516–522. doi:10.1007/s11655-014-1791-1
55. Lee JM, Calkins MJ, Chan K, et al. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278(14):12029–12038. doi:10.1074/jbc.M211558200
56. Essick EE, Sam F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev. 2010;3(3):9. doi:10.4161/oxim.3.3.12106
57. Feng K, Chen Z, Pengcheng L, et al. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J Cell Physiol. 2019;234(10):18192–18205. doi:10.1002/jcp.28452
58. Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011;585(7):986–994. doi:10.1016/j.febslet.2010.11.047
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.