Back to Journals » Infection and Drug Resistance » Volume 15
Salmonella Group D1 Subdural Empyema Mimicking Subdural Hematoma: A Case Report
Authors Lu HF , Yue CT, Kung WM
Received 30 August 2022
Accepted for publication 27 October 2022
Published 31 October 2022 Volume 2022:15 Pages 6357—6363
DOI https://doi.org/10.2147/IDR.S388101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Hao-Fang Lu,1,2 Chung-Tai Yue,3 Woon-Man Kung4,5
1Division of General Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Foundation, New Taipei City, 23142, Taiwan; 2School of Medicine, College of Medicine, National Taiwan University, Taipei, 10002, Taiwan; 3Department of Anatomic Pathology, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, 23142, Taiwan; 4Division of Neurosurgery, Department of Surgery, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, 23142, Taiwan; 5Department of Exercise and Health Promotion, College of Kinesiology and Health, Chinese Culture University, Taipei, 11114, Taiwan
Correspondence: Woon-Man Kung, Division of Neurosurgery, Department of Surgery, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, No. 289, Jianguo Road, Xindian District, New Taipei City, 23142, Taiwan, Email [email protected]
Abstract: Subdural empyema is caused by various pathogens. The most typical clinical presentation may include fever, headache, seizures, and altered consciousness. However, Salmonella infections are relatively rare. Representative features of Salmonella infection include fever and gastrointestinal symptoms such as diarrhea, vomiting, and abdominal cramping pain. Extra-gastrointestinal invasion of Salmonella in the central nervous system is unusual. We present the case of an afebrile 58-year-old male who presented with a headache and a progressive dull response for a week. He had a closed head injury approximately 1 week before this visit. A tentative diagnosis led to a subdural hematoma (SDH), and he underwent urgent burr hole surgery. Intraoperative findings showed a large amount of brown-yellow pus in the subdural space instead of the pathognomonic bloody serosanguinous or thick motor oil, which is typical of SDH. The intraoperative culture yielded Salmonella group D1. After initial brain surgery and 52 days of effective intravenous administration of a third-generation cephalosporin (Ceftriaxone 2000 mg per day), the patient recovered fully without neurological deficits. His consciousness and mentality remained normal without focal weakness of the limbs for over 5 years of follow-up. This is a unique case with an atypical initial presentation that leads to a final unexpected diagnosis. Ongoing treatment strategies include a combination of surgical drainage for disease confirmation and appropriate medical antibiotics.
Keywords: Salmonella, subdural, empyema, hematoma
Background
Subdural empyema (SDE) refers to the abscess collection between the dura mater and the arachnoid space. It usually occurs secondary to sinusitis, otitis media, or upper respiratory tract infection. In addition to generalized underlying symptoms, specific symptoms may consist of disturbed consciousness and/or other focal neurological deficits.1 Typical symptoms of SDE include fever, headache, seizures, and altered consciousness.2 Otolaryngologic infections were found in 40–80% of patients with SDE, particularly sinusitis.3 Meanwhile, up to one-fifth of these patients occur due to head injuries or brain surgeries.4 Common pathogens of SDE are Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae.5
The transmission pathway of Salmonella occurs via the fecal-oral route. Ingestion of raw or undercooked foods can cause Salmonella infections. The typical presentations of salmonellosis include fever, diarrhea, vomiting, and abdominal cramping pain. Extra-gastrointestinal invasion of Salmonella into the central nervous system (CNS) is uncommon. According to the latest nomenclature system of the World Health Organization, Salmonella is categorized as Salmonella bongori and Salmonella enterica.6 There are some further subspecies of Salmonella enterica as follows: I-enterica, II-salamae, IIIa-arizonae, IIIb-diarizonae, IV-hountenae, and VI–indica, respectively.6 The most common subspecies is I-enterica. Furthermore, Salmonella group D is one of the “serovars” of I-enterica. After reviewing the current literature, only 11 cases of Salmonella group D SDE have been reported worldwide since 1986 (Table 1). We report an exceptional case of frequent nonhygienic sashimi consumption, initially misinterpreted as subdural hematoma (SDH). Intraoperative culture led to the final diagnosis of Salmonella group D1 SDE.
 |
Table 1 Summary of Reported Cases of Salmonella Group D Subdural Empyema |
Case Presentation
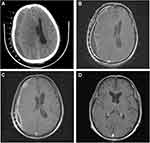
A 58-year-old male suffered from a gradual headache. He experienced progressive confusion (Glasgow Coma Scale [GCS] score: E4V4M6) with a slow response. Subsequently, the patient was brought to the emergency department. Vomiting episodes were also observed. He had a history of closed head injury approximately 1 week ago and his four limbs were moving freely. No known immunocompromising factors exist. The patient had no history of rhinitis, sinusitis, or otitis media before the insult. However, he had a low socioeconomic status and poor housing hygiene. He had consumed unsanitary and uncooked seafood and suffered from trivial recurrent gastrointestinal discomfort in the past few months. All vital signs, including body temperature, were normal. Blood examination showed a slight elevation in white blood cell count (10.33 × 103 /μL). Urgent computed tomography (CT) showed massive hypodense right convexity subdural fluid collection with a midline shift (Figure 1A). Under the working diagnosis of SDH with a significant mass effect, he underwent urgent burr hole drainage. During surgery, a large amount of brown-yellow pus was grossly inspected in the subdural space instead of pathognomonic bloody serosanguinous or thick motor oil, representing SDH (Figure 2). Microscopically, the specimen consisted of numerous neutrophils with abundant background amorphous necrotic material, compatible with a pus/abscess (Figure 3). Surprisingly, neither schistocytes nor red blood cells were present. Due to the frank pus observed by the surgeon, the level of C-reactive protein was elevated (11.35 mg/dL). Empirical broad-spectrum intravenous antibiotics were administered postoperatively for 4 days (Vancomycin 1000 mg per 12 h and Flomoxef 1000 mg per 8 h), routinely applied clinically as intracranial doses aimed at treating CNS infection.
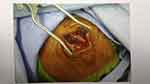 |
Figure 2 Intraoperative burr hole drainage gross photograph showing large amount of brown-yellow pus in subdural space. |
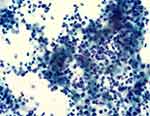 |
Figure 3 Histopathological photomicrograph: Papanicolaou stain, magnification ×200 showing numerous neutrophils with abundant background amorphous necrotic material, compatible with pus/abscess. |
Following the surgical procedure, the GCS score decreased to E2V2M5-6 over the next 4 days. Simultaneously, he experienced dense left hemiparesis with a decrease in muscle power down to 0–1 point, which should be the natural course of Salmonella SDE. In 2011, Chen et al reported 17 cases of Salmonella SDE, in which increased intracranial pressure, seizures, and limb paralysis were present in 47% of the patients.7 A gadolinium-enhanced magnetic resonance (MR) imaging revealed thick residual subdural fluid collection with midline shift. It showed iso-to-low T1-weighted images with rim enhancement under contrast and high T2-weighted images that were consistent with SDE (Figure 1B). Subsequent culture of the specimen revealed Salmonella group D1, which was sensitive to ceftriaxone. The antibiotic was then switched to the therapeutic drug.
Echocardiography did not reveal vegetation on the cardiac valves. Moreover, no bacterial growth was observed in the blood culture; therefore, infection was excluded as the source of SDE. Another possible cause of SDE is periodontitis. Although this patient had chronic periodontitis, common gingival pathogens (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythia)8 are inconsistent with the findings of our final culture report. After 52 consecutive days of effective antibiotic treatment with ceftriaxone at a dose of 2000 mg administered intravenously per day, the patient not only fully recovered to clear consciousness (GCS: E4V5M6) but also regained strength in his left limb. On discharge, MR showed a significant reduction in SDE with only a trivial midline shift (Figure 1C). Clinical findings revealed clear consciousness with full muscle power for over 5 years. Another MR performed after a 5-year follow-up showed a near-normal appearance with nonspecific findings (Figure 1D). The patient’s final outcomes showed that timely and effective antibiotic therapy not only saved the patient from life-threatening SDE but also cured the CNS infection in long-term follow-up.
Discussion
Epidemiology
CNS infections include meningitis, ventriculitis, encephalitis, brain abscess, and subdural/epidural empyema.9 Among all, SDE is one of the life-threatening etiologies, accounting for 20% of total CNS infections.10 According to a recent large-scale systematic review and meta-analysis, Salmonella infects approximately 535,000 people worldwide annually.11 It is unique to have Salmonella infected in the subdural space. Clinical considerations and management of Salmonella group D1 SDE can be challenging.
Diagnosis
For infectious diseases of the CNS, early identification of intracranial infections and identification of pathogens and pathways of infection are the main concerns. Therefore, in addition to pathogen culture and drug resistance testing to clarify the type and nature of the pathogen, it is also important to identify possible routes of infection. SDE develops most frequently from sinusitis, otitis media, or upper respiratory tract infections. Less frequent etiologies leading to SDE include head injury and brain surgery.4 A gut-vascular barrier prevents microbiomes passage from the gut to the blood, while the distinct Salmonella is able to penetrate this barrier.12 To our knowledge, we postulate that our patient experienced disseminated subdural infection through possible occult Salmonella group D1 enteric infection translocated into the systemic bloodstream. Initial neuroimaging analysis is often contrast-enhanced CT due to its universal convenience in general healthcare systems. However, contrast-enhanced MR is the optimal choice to evaluate SDE due to its higher sensitivity compared to its companion CT.13
The Rarity of Salmonella Infection in This Case
Of the uncommon Salmonella SDE reported in the past, infants and children were more prevalent. Twelve adults have been reported in the literature, and some of them were immunocompromised, such as those with malignancies and acquired immune deficiency syndrome. Our reported 58-year-old patient, although without any immunocompromising factors, had a habit of consuming raw seafood in an unhygienic Japanese restaurant. In a study from Saudi Arabia, the percentage of raw seafood Salmonella contamination is 39.9%.14 However, the incidence of Salmonella SDE is not high.15 We recommend that SDE should always be considered as a differential diagnosis in patients with atypical signs and symptoms upon the diagnosis of SDH, not only in developing countries but also in developed ones.
Among the clinical manifestations of Salmonella SDE fever accounts for approximately 70% of cases. Other common clinical symptoms include headache, seizures, and altered consciousness levels. Our case with a final diagnosis of Salmonella SDE presented no fever during the course of the disease, which is a very distinct manifestation.
Treatment Options
Treatment options vary between different SDEs. Some authors recommended craniotomy as the preferred surgical technique for SDE.16 However, in our case, the suppurative fluid collection had a runny brown-yellow content, which can be easily drained and radically irrigated through a burr hole, which is an alternatively a successful solution for the patient. The main purpose of burr hole drainage was decompression and obtaining the final culture report. After surgery, we could prescribe precise antimicrobial agents to treat SDE. There was thick residual subdural fluid collection 4 days after surgery (Figure 1B). However, after 52 days of effective antibiotic treatment, MR showed a significant reduction in SDE with only a trivial midline shift (Figure 1C).
Furthermore, the blood-brain barrier blocks the delivery of medications to the CNS.17 Additionally, a thick wall surrounds the SDE peripherally. Therefore, surgery not only provides an accurate diagnosis, efficiently removes and drains the infective abscess, but also breaks the blood-brain barrier and thick wall of SDE to increase the efficacy of systemic antibiotics significantly.16
As shown in Table 1, all patients had good outcomes regardless of the type of surgery applied. Although craniotomy remains the gold standard for the treatment of SDE,16 simple burr hole drainage is another decent option that is not inferior to traditional craniotomy. Furthermore, the advantages of minimally invasive burr hole surgery include shorter surgical time, smaller operative wounds, and less bleeding.18
Limitations
The current study has fundamental limitations as a case report.19 Although there were good short- and long-term outcomes following timely surgical decompression with burr hole drainage and effective antibiotic treatment, further randomized controlled trials should be conducted to confirm the efficacy of these treatments.
Conclusions
We present the clinical course of our exceptional case with a diagnosis of an uncommon SDE that was first diagnosed as SDH, followed by surgical and specific antibiotics, with good short- and long-term outcomes.
Salmonella SDE is a fatal condition requiring immediate recognition and prompt and correct combined management involving surgical and medical regimens. To prevent such severe infections, it is important to maintain good personal hygiene and avoid the consumption of unclean raw seafood.
Data Sharing Statement
All clinical and other information is available in the patient’s medical record in the Division of Neurosurgery, Department of Surgery, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation.
Institutional Review Board Statement
Approval from the institutional of Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation review board and ethics committee was obtained for the study (Protocol No.: 11-CR-048).
Informed Consent Statement
Written informed consent for publication of case details and accompanying images has been obtained from the patient.
Acknowledgments
We would like to express our gratitude to the peer reviewers who helped us to shape this manuscript.
Funding
This research received no external funding.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Sellick JA
2. Osborn MK, Steinberg JP. Subdural empyema and other suppurative complications of paranasal sinusitis. Lancet Infect Dis. 2007;7(1):62–67. doi:10.1016/S1473-3099(06)70688-0
3. Silverberg AL, DiNubile MJ. Subdural empyema and cranial epidural abscess. Med Clin North Am. 1985;69(2):361–374. doi:10.1016/S0025-7125(16)31048-3
4. Wu TJ, Chiu NC, Huang FY. Subdural empyema in children--20-year experience in a medical center. J Microbiol Immunol Infect. 2008;41(1):62–67.
5. Suthar R, Sankhyan N. Bacterial infections of the central nervous system. Indian J Pediatr. 2019;86(1):60–69. doi:10.1007/s12098-017-2477-z
6. Chattaway MA, Langridge GC, Wain J. Salmonella nomenclature in the genomic era: a time for change. Sci Rep. 2021;11(1):7494. doi:10.1038/s41598-021-86243-w
7. Chen KM, Lee HF, Chi CS, Huang FL, Chang CY, Hung HC. Obscure manifestations of Salmonella subdural empyema in children: case report and literature review. Childs Nerv Syst. 2011;27(4):591–595. doi:10.1007/s00381-010-1274-z
8. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL
9. Thurnher MM, Sundgren PC. Intracranial Infection and Inflammation. In: Hodler J, Kubik-Huch RA, von Schulthess GK, editors. Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging. Cham (CH): Springer; 2020:59–76.
10. Mortazavi MM, Quadri SA, Suriya SS, et al. Rare concurrent retroclival and pan-spinal subdural empyema: review of literature with an uncommon illustrative case. World Neurosurg. 2018;110:326–335. doi:10.1016/j.wneu.2017.11.082
11. Stanaway JD, Parisi A, Sarkar K; GBD. Non-typhoidal Salmonella invasive disease collaborators. the global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the global burden of disease study 2017. Lancet Infect Dis. 2019;19(12):1312–1324. doi:10.1016/S1473-3099(19)30418-9
12. Spadoni I, Zagato E, Bertocchi A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350(6262):830–834. doi:10.1126/science.aad0135
13. Dill SR, Cobbs CG, McDonald CK. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20(2):372–386. doi:10.1093/clinids/20.2.372
14. Elhadi N. Prevalence and antimicrobial resistance of Salmonella spp. in raw retail frozen imported freshwater fish to Eastern Province of Saudi Arabia. Asian Pac J Trop Biomed. 2014;4(3):234–238. doi:10.1016/S2221-1691(14)60237-9
15. Bhooshan P, Shivaprakasha S, Dinesh KR, Kiran M, Karim P. Chronic subdural empyema and cranial vault osteomyelitis due to Salmonella paratyphi A. Indian J Med Microbiol. 2010;28(1):60–62. doi:10.4103/0255-0857.58733
16. Kural C, Kırmızıgoz S, Ezgu MC, Bedir O, Kutlay M, Izci Y. Intracranial infections: lessons learned from 52 surgically treated cases. Neurosurg Focus. 2019;47(2):E10. doi:10.3171/2019.5.FOCUS19238
17. Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. doi:10.1016/j.jconrel.2017.12.015
18. Brouwer MC, van de Beek D. Epidemiology, diagnosis, and treatment of brain abscesses. Curr Opin Infect Dis. 2017;30(1):129–134. doi:10.1097/QCO.0000000000000334
19. Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;23(7):264. doi:10.1186/1756-0500-7-264
20. Jain KC, Mahapatra AK. Subdural empyema due to salmonella infection. Pediatr Neurosurg. 1998;28(2):89–90. doi:10.1159/000028627
21. Mahapatra AK, Pawar SJ, Sharma RR. Intracranial Salmonella infections: meningitis, subdural collections and brain abscess. A series of six surgically managed cases with follow-up results. Pediatr Neurosurg. 2002;36(1):8–13. doi:10.1159/000048342
22. Aliaga L, Mediavilla JD, López de la Osa A, López-Gómez M, de Cueto M, Miranda C. Nontyphoidal salmonella intracranial infections in HIV-infected patients. Clin Infect Dis. 1997;25(5):1118–1120. doi:10.1086/516101
23. Grosinger L, Lauter CB. Salmonella subdural empyema in a patient with brain metastasis. Rev Infect Dis. 1986;8(5):830–831. doi:10.1093/clinids/8.5.830a
24. Parkers PJ, Harland SP, Protheroe AS. Subdural empyema in an HIV positive patient. Br J Neurosurg. 1995;9(1):85–86. doi:10.1080/02688699550041818
25. Kan M, Kim T, Miyaichi T, et al. A case of Salmonella subdural empyema developed in chronic subdural hematoma. No Shinkei geka. Neurological Surgery. 1998;26(10):903–907.
26. Chandy MJ. Subdural Salmonella empyema in an adult. Neurol India. 2000;48(3):297.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.