Back to Journals » Drug Design, Development and Therapy » Volume 14
Salidroside Alleviates Cartilage Degeneration Through NF-κB Pathway in Osteoarthritis Rats
Authors Gao H, Peng L, Li C, Ji Q, Li P
Received 18 December 2019
Accepted for publication 20 February 2020
Published 14 April 2020 Volume 2020:14 Pages 1445—1454
DOI https://doi.org/10.2147/DDDT.S242862
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Hui Gao,1,* Lu Peng,2,* Chao Li,1 Qinlong Ji,1 Ping Li3
1Department of Orthopaedics, Tinglin Hospital, Shanghai 201505, People’s Republic of China; 2Department of Orthopaedics, Hospital of Traditional Chinese Medicine, E’dong Healthcare Group, Huangshi 435000, People’s Republic of China; 3Department of Rehabilitation, Hanchuan People’s Hospital, Hanchuan, 431600, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ping Li
Department of Rehabilitation, Hanchuan People’s Hospital, NO.1 Renmin Avenue, Hanchuan City, Hubei Province, People’s Republic of China
Tel +86-18371202625
Email [email protected]
Introduction: Osteoarthritis (OA) is the most common disease, which seriously affects the daily life of the elderly. Currently, no traditional or drug therapy has been shown to explicitly block the progression of OA. Salidroside (Sal) is a bioactive component of Rhodiola rosea, which has many beneficial effects on human health. However, the role and mechanism of Sal in OA have not been reported.
Methods: We established an anterior cruciate ligament transection (ACLT)-induced OA Rat model. The rats were divided into five groups (n = 10): Control group; ACLT group; ACLT + Sal (12.5 mg/kg) group; ACLT + Sal (25 mg/kg) group; ACLT + Sal (50 mg/kg) group.
Results: The study showed that Sal could significantly promote the proliferation of chondrocytes in OA rats induced by ACLT and restore the histological alteration of cartilage. Besides, Sal upregulated the levels of Collagen II and Aggrecan, and downregulated the level of MMP-13. Furthermore, Sal could reduce the number of CD4+IL-17+ cells and decrease the levels of IL-17, IKBα and p65, while elevating the number of CD4+IL-10+ cells and the level of IL-10. The decrease of IL-17 further inhibited the dissociation of IKBα to p65, thus reducing the release of TNF-α and VCAM-1. Taken together, Sal alleviates cartilage degeneration through promoting chondrocytes proliferation, inhibiting collagen fibrosis, and regulating inflammation and immune responses via NF-κB pathway in ACLT-induced OA Rats.
Discussion: Collectively, our study investigates the role and mechanism of Sal in OA, which lays a foundation for the application of Sal in OA.
Keywords: osteoarthritis, salidroside, cartilage degeneration, collagen fibrosis, inflammation, immune responses, NF-κB
Introduction
Osteoarthritis (OA) is a chronic arthritis that often occurs in the hip, knee and elbow joints. According to the World Health Organization (WHO), the prevalence of OA is 9.6% in men over 60 years old and 18% in women.1 OA is characterized by degenerative changes of articular cartilage and secondary osteoporosis.2,3 The main manifestations of articular cartilage injury caused by OA are loss of cartilage matrix, decrease of chondrocyte, degeneration and exfoliation of cartilage.4 OA is also caused by the complex interaction of many factors such as inflammation.5 Currently, no traditional or drug therapy has been shown to explicitly block the progression of OA.6–8 Surgical intervention may be beneficial when mechanical malformations are present. However, the benefits are limited to advanced OA, and are not considered a long-term solution.9 Therefore, it is very important to explore new therapeutic methods for the treatment and prevention of OA.
Salidroside (Sal) is a bioactive component derived from Rhodiola rosea. Studies have suggested that Sal has various pharmacological effects, including anticancer,10 anti–inflammation,11 anti-fatigue12 and anti-apoptosis13,14 and enhanced immune response.15 Extensive evidences indicated that Sal ameliorated various diseases, such as diabetic kidney disease,13 asthma16 and Parkinson’s disease.17 For example, Kallscheuer et al found that Sal had a positive therapeutic effect on Huntington’s disease.18 Qin et al declared that Sal was a platinum sensitizer to improve the efficacy of chemotherapy for liver cancer.19 In OA, Liu et al found that Sal alleviated the inflammatory injury induced by LPS through regulating miR-145.20 Similarly, Zhang and Zhao et al reported that Sal inhibited OA inflammation induced by IL-1β through inhibiting the activation of NF-κB pathway in vitro.21 However, the role and possible molecular mechanism of Sal in OA have not been reported in animal models.
Inflammatory cytokines are associated with the pathogenesis of OA.22 Helper T cells (CD4+T cells) and Treg cells participate in the immune balance of the body.23–25 Th17 (CD4+IL-17+) is a kind of CD4+T cells secreting IL-17 is an important pro-inflammatory cytokine, which can stimulate the production of various inflammatory cytokines, such as IL-6, IL-8 and VCAM-1. CD4+IL-10+ is a regulator T cell that can secrete anti–inflammatory cytokine IL-10. Nuclear factor-kappa B (NF-κB), a regulatory protein, mediates the production of various inflammatory factors. NF-κB plays a key role in the pathogenesis of OA.26 Zeng et al found that the low-expression of FOXM1 alleviated IL-1β-induced inflammation by inactivating the NF-κB pathway in OA.26 Besides, Choi et al believed that the NF-κB pathway may be involved in Arg-II-induced OA cartilage injury by up-regulating the levels of MMP3 and MMP13.27
In this study, we elucidated the deeper molecular mechanism of Sal in OA, which laid the foundation for Sal as a clinical drug of OA.
Materials and Methods
Animal Model
Animal experiments were conducted according to the NIH Guide for the Care and Use of Laboratory Animals, and were approved by The Affiliated Hospital of North Sichuan Medical College (SYXK(川)2018–075). A total of 50 female Sprague‑Dawley rats (12‑week‑old, 250‑300 g) were purchased from the Animal Center of the Affiliated Hospital of North Sichuan Medical College. The rats are placed in an environment with a constant temperature of 25 ± 3°C and a humidity of 60%, and free access to food and water in a 12 h light/dark cycle. Bilateral knee anterior cruciate ligament transection (ACLT) was carried out as previously.28 Briefly, before ACLT, rats were anesthetized with pentobarbital sodium [0.1mL/100g intraperitoneally (Ip), 40 mg/kg]. After sufficient disinfection, the medial skin of the patellar ligament was incised and the joint capsule was opened to dislocate the patellar bone. Flexing the knee joint exposed the anterior cruciate ligament and cut the anterior cruciate ligament. The Lachman test was used to confirm ACLT.29 After rinsing with sterile saline, the wound was closed layer by layer and disinfected, and penicillin was injected intramuscularly to prevent infection. After successful modeling, the rats were divided into five groups (n = 10): Control group (Healthy rats without any treatment); ACLT group (Rats were modeled as described above), ACLT model; ACLT + Sal (12.5 mg/Kg) group, OA rats were given 12.5 mg/kg Sal; ACLT + Sal (25 mg/kg), OA rats were given 25 mg/kg of Sal; ACLT + Sal (50 mg/kg), OA rats were given 50 mg/kg of Sal.30 After 6 weeks, the rats were anesthetized with pentobarbital sodium (0.1 mL/100 g IP, 40 mg/kg) and sacrificed. The synovium was immediately taken out and embedded in paraffin for subsequent experiments.
Histological Analysis
Synovial tissue sections were dewaxed with 4% xylene and dehydrated with gradient alcohol.31 For H&E staining, tissues were stained with H&E staining kit (toluidine blue, Keygen Biotech. Nanjing, China). For Masson, tissues were stained with Masson trichrome staining kit (toluidine blue, Keygen Biotech. Nanjing, China). For Safranin O staining, tissues were stained with Safranin O solution (ScyTek, Logan, UT, USA). All staining was performed according to the manufacturer’s instruction, and the morphological changes were observed with an optical microscope (BX51; Olympus Corp., Tokyo, Japan).
Western Blotting
Proteins isolated from synovial tissues were separated by 10% SDS-PAGE, transferred to PVDF membrane (Millipore, MA, USA) and sealed with 5% skim milk. Then, the membranes were reacted with primary antibodies overnight at 4°C. The primary antibodies were as follows: PCNA (#2586, 1:1000, Cell Signaling Technology, USA), IKBα (#4812, 1:1000, CST, USA), P65 (#8242, 1:1000, CST, USA), TNF-α (#11948, 1:1000, CST, USA), VCAM-1 (#39036, 1:1000, CST, USA), caspase-3 (#9665, 1:1000, CST, USA), caspase-9 (#9508, 1:1000, CST, USA), Lamin A (#86846, 1:1000, CST, USA), Ki-67 (sc-23900, 1:1000 Santa Cruz Biotechnology, USA), Collagen II (sc-47764, 1:1000, SCB, USA), Aggrecan (sc-166951, 1:1000, SCB, USA), MMP-13 (sc-515284, 1:1000, SCB, USA). After washing with PBS, the membranes were incubated with anti-rabbit IgG (#7074, 1:2000, CST, USA) for 1 h and visualized with ECL kit (Perkin Elmer Cetus, Foster City, CA, USA).
RT-qPCR
The total RNA isolated from synovial tissues were reverse-transcribed into cDNA by the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher). Then, RT-qPCR was performed with BeyoFast™ SYBR Green qPCR Mix (D7260, Beyotime, Shanghai, China) by using the Bio-Rad CFX96 PCR System (Bio-Rad, CA, USA). Primers were as follows: β-actin,5ʹ-TCACCGAGCGCGGCT-3ʹ (sense) and 5ʹ-TAATGTCACGCACGATTTCCC-3ʹ (antisense); Collagen, 5ʹ-TGACGCTAAGCTCGACGCG-3ʹ (sense) and 5ʹ-CCATGCAATCGCCATTAGC-3ʹ (antisense); Aggrecan, 5ʹ-ACGTGGCACGTTGCAATGCT-3ʹ (sense) and 5ʹ-TACGTACGTGGCATACGC-3ʹ (antisense); MMP-13, 5ʹ-TGCATGCCACTGCACGTACG-3ʹ (sense) and 5ʹ-GACTACTTGCAACGTACGC-3ʹ (antisense). β-actin was employed as an internal reference. The fold change is evaluated by the equation 2−ΔΔCt.
Enzyme-Linked Immuno Sorbent Assays (ELISA)
The serum was extracted and stored at –80°C for analysis. Inflammatory cytokine (interleukin-17, IL-17) and immunomodulatory factor (interleukin-10, IL-10) were detected by ELISA kits. (Bio-Swamp, Shanghai, China). The OD value was examined at 450 nm with a microplate reader (BioTek Epoch, Winooski, VT, USA).
Flow Cytometry
Single-cell isolated from synovial tissues were grown in RPMI 1640 medium (Sigma, St. Louis, MO, USA) containing 10% FBS (Whittaker, Walkersville, MD, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA). After induction with 50 ng/mL PMA (Sigma, St. Louis, MO, USA), 1 μg/mL ionomycin (Sigma, St. Louis, MO, USA) and brefeldin A (BD Bioscience) for 5 h, the cells were incubated with Anti-CD4-PC7, fixed with CytoFix/Cyto Perm buffer (BD Bioscience) and stained with Anti–IL-17-PE or Anti–IL-10-PE (BD Bioscience), respectively. The number of CD4+IL-17+ and CD4+IL-10+ cells were examined by FACS Canto (BD Biosciences).
Statistical Analysis
SPSS 21.0 (Chicago, Illinois, USA) was used for statistical analysis (n=5). The measured data are expressed as mean ± standard deviation (x ± s), and the data consistent with the normal distribution were analyzed by t-test. Multiple groups of data were analyzed by one-way ANOVA. The count data are expressed as a percentage or ratio and verified by chi-square test. P < 0.05 was considered to be statistically significant.
Results
Sal Improved Cartilage Injury by Promoting Chondrocytes Proliferation in OA Rats
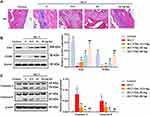
To investigate the role of Sal in cartilage injury, H&E staining and Western blotting were performed. As shown in Figure 1A, the normal chondrocytes were arranged neatly, with clear tidal lines and complete matrix in the control group. However, in the ACLT-induced OA group, chondrocytes were severely damaged and exfoliated. Sal treatment (12.5, 25 and 50 mg/kg) dose-dependently ameliorated cartilage injury (Figure 1A). Besides, Western blotting showed that chondrocytes proliferation was obviously inhibited in the ACLT-induced OA group. Interestingly, Sal (12.5, 25 and 50 mg/kg) treatment dose-dependently promoted the proliferation of chondrocytes compared to the ACLT-induced OA group (Figure 1B). In addition, ACLT also induced chondrocytes death in ACLT-induced OA group, and Sal treatment (12.5, 25 and 50 mg/kg) suppressed chondrocytes death in a dose-dependent manner (Figure 1C). Thus, we declared that Sal improved cartilage injury by promoting ACLT-induced chondrocytes proliferation in OA rats.
Sal Alleviated Cartilage Injury by Inhibiting the Proliferation of Collagen Fibers in OA Rats
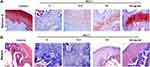
Safranin O staining and Masson staining were used to further confirm the healing effect of Sal on cartilage alteration. As shown in Figure 2A, Safranin O staining showed that the cartilage area of the control group was red and the bone area was blue. However, in OA group, the cartilage tissues were severely damaged and the red area almost disappeared. Intriguingly, Sal treatment (12.5, 25 and 50 mg/kg) dose-dependently ameliorated cartilage injury in cartilage tissue (Figure 2A). Besides, Masson staining showed that the collagen fibers were blue, while muscle fibers were red in the control group. However, in OA group, the blue areas of collagen fibers are markedly enlarged. Similarly, Sal treatment (12.5, 25 and 50 mg/kg) dose-dependently reduced the accumulation of collagen fibers and inhibited fibrosis in cartilage tissue (Figure 2B). The results of Safranin O staining were consistent with H&E staining. Therefore, this study demonstrated that Sal alleviated cartilage injury by inhibiting the proliferation of collagen fibers in ACLT-induced OA rats.
Sal Alleviated Cartilage Injury by Regulating the Levels of Extracellular Matrix Proteins (Collagen II, Aggrecan, MMP-13) in OA Rats
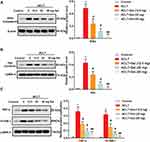
RT-qPCR showed that Collagen II and Aggrecan were decreased and MMP-13 was increased in OA group. However, different doses of Sal (12.5, 25 and 50 mg/kg) remarkedly increased the levels of Collagen II and Aggrecan, while decreasing MMP-13 level (Figure 3A). Consistent with these results, Western blotting showed that Sal dose-dependently increased the levels of Collagen II and Aggrecan, while decreasing the level of MMP-13 (Figure 3B). Taken together, these results demonstrated that Sal alleviated cartilage injury by regulating the levels of extracellular matrix proteins (Collagen II, Aggrecan, MMP-13) in ACLT-induced OA rats.
Sal Ameliorated Cartilage Injury by Regulating Inflammation and Immune Responses in OA Rats
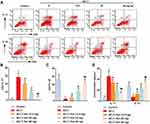
ELISA assay showed that the level of IL-17 in peripheral blood was increased, while the level of IL-10 was decreased compared to the control group (Figure 4D). Interestingly, treatment with different doses of Sal (12.5, 25 and 50 mg/kg), the level of IL-17 was dose-dependently decreased, while the level of IL-10 was increased. Besides, flow cytometry analysis further confirmed that ACLT increased the number of CD4+IL-17+ cells (Th17), and decreased the number of CD4+IL-10+ cells. Sal treatment (12.5, 25 and 50 mg/kg) reduced the number of CD4+IL-17+ cells and increased the number of CD4+IL-10+ cells to varying degrees (Figure 4A–C). Totally, these results indicated Sal ameliorated cartilage injury by regulating inflammation and immune responses in OA rats.
Sal Ameliorated Cartilage Injury by the Regulation of NF-κB Pathway in OA Rats
To clarify the potential molecular mechanism of Sal in cartilage repair, Western blotting was performed. As shown in Figure 5A–C, ACLT induction increased the level of IKBα compared with the control group. Increased IKBα then dissociated to NF-κB p65, and further promoted the release of TNF-α and VCAM-1. However, Sal treatment (12.5, 25 and 50 mg/kg) inhibited the up-regulation of IKBα induced by ACLT, and then decreased the level of NF-κB p65, TNF-α and VCAM-1. Collectively, this study demonstrated that Sal ameliorated cartilage injury by the regulation of NF-κB pathway in OA rats.
Discussion
As the most common arthropathy, OA seriously affects the daily life of the elderly.32 Articular cartilage degeneration is the leading cause of OA. The main drugs for the treatment of OA are acetaminophen, NSAIDs, celecoxib and VA692.33–35 Recently, studies on the treatment of OA have focused on Chinese herbal extracts. Evidence from Wang et al revealed that Agkistrodon, an ethanol extract of Agkistrodon acutus, improved chondrogenic injury of OA rats by enhancing cell survival, restraining cell apoptosis and abnormal expression of collagen II and MMP13.36 As reported by Zeng et al, curcumin, a component extracted from Curcuma longa, alleviated inflammation of OA by down-regulating the level of MMP3, inhibiting cell proliferation and inducing cell apoptosis.37 At this point, we elucidated the role of Sal in OA in vivo. The results demonstrated Sal has a significant therapeutic effect on OA rats. Our results were consistent with previous research in vitro.20,21
Salidroside is the bioactive component of Rhodiola rosea, which has various beneficial effects on human health. Numerous studies have mentioned that Sal was involved in the treatment of many diseases, such as non-alcoholic steatohepatitis38 and Alzheimer’s disease.39 During OA, chondrocytes undergo massive apoptosis. Previous studies have shown that Sal promoted chondrocyte proliferation, inhibited IL-1β-induced apoptosis, and removed reactive oxygen species (ROS) and nitric oxide (NO) from chondrocytes.40 Similarly, this study found that Sal promoted the proliferation of chondrocytes in OA rats and inhibited the expression of apoptotic proteins (cleaved caspase 3 and cleaved caspase 9). The main factors for the progression of OA are chronic inflammation and progressive structural changes in joint tissues. Among them, inflammatory cytokines and proteases are the two main factors that change the microenvironment of the cell by regulating the composition of the extracellular matrix of cartilage, and further interfere with cell function. It is worth noting that Sal inhibited the production of matrix metalloproteinases induced by IL-1β in human OA chondrocytes, and had a protective effect on LPS-induced ATDC5 cell damage.20,21 According to reports, Sal had an inhibitory effect on IL-1β-induced OA inflammation by inhibiting the activation of the NF-κB pathway in vitro.21 Similarly, this study found that Sal reduced the number of CD4 + IL-10 + cells in OA rats and increased the number of CD4 + IL-10 + cells, thereby regulating the inflammation and immune response in OA rats. The fibrosis of articular cartilage, which is dominated by type Ⅲ collagen deposition, can affect the local cellular metabolism and biomechanical characteristics of joints during OA. Based on this, the present study found that Sal inhibited collagen fibrosis and reduced cartilage degeneration in OA rats by NF-κB pathway.
NF-κB is an important inflammatory response regulator.41 Abnormal activation of NF-κB pathway is implicated in the degeneration of OA chondrocytes.42 NF-κB mediates the release of various inflammatory factors, such as IL-6, IL-1β, IFN-γ, TNF-α and MMPs. As reported by Wang et al, Umbelliferone (Umb) mitigated LPS-induced acute lung injury (ALI) by attenuating inflammatory cell infiltration and production of inflammatory cytokines, and enhancing antioxidant activity via inactivating TLR4/MyD88/NF-κB pathway.43 Besides, Hu et al declared that follistatin-like protein 1 elevated the levels of MMP-13, IL-1β, TNF-α and IL-6 by activating the NF-κB pathway in chondrocyte.44 We found that Sal treatment markedly decreased the levels of MMP-13 and TNF-α. MMP-13 was an important regulator that maintained the metabolic balance of chondrocytes. TNF-α induced the expression of ADAMTS-4 and ADAMTS-5, and degraded collagen and proteoglycan. Besides, we found that Sal treatment promoted the production of collagen II and aggrecan, which contributed to reduce the damage to cartilage structure and prevent the destruction of the cartilage matrix. Furthermore, our results showed that Sal reduced the number of CD4+IL-17+ cells, elevated the number of CD4+IL-10+ cells and suppressed the levels of IKBα and p65. These results demonstrated that Sal reduced IL-17 level and increased IL-10 level. The decrease of IL-17 further inhibited the dissociation of IKBα, and consequently reduced the production of TNF-α and VCAM-1. Taken together, Sal alleviated cartilage degeneration by promoting chondrocytes proliferation, inhibiting collagen fibrosis, and regulating inflammation and immune responses via NF-κB pathway in OA rats.
Conclusion
In conclusion, our results revealed the role and mechanism of Sal in OA in vivo. In the current study, we proved that Sal (12.5, 25 and 50 mg/kg) significantly promoted chondrocyte proliferation and inhibited chondrocyte apoptosis. Besides, Sal alleviated collagen fibrosis and modulated ACLT-induced inflammation and immune response in OA rats through the NF-κB pathway. Collectively, our study investigates the role and mechanism of Sal in OA, which lays a foundation for Sal as a clinical drug of OA.
Disclosure
Hui Gao and Lu Peng are co-first authors. The authors report no conflicts of interest in this work.
References
1. Li MH, Xiao R, Li JB, Zhu Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2017;25(10):1577–1587. doi:10.1016/j.joca.2017.07.004
2. Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33(11):1601–1610. doi:10.1002/(ISSN)1529-0131
3. Musumeci G, Loreto C, Leonardi R, et al. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31(3):274–284. doi:10.1007/s00774-012-0414-9
4. Litwic AE, Parsons C, Edwards MH, Jagannath D, Cooper C, Dennison EM. Comment on: inflammatory mediators in osteoarthritis: a critical review of the state-of-the art, prospects, and future challenges. Bone. 2018;106:28–29. doi:10.1016/j.bone.2016.08.001
5. Madry H, Luyten FP, Facchini A. Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):407–422.
6. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–1975. doi:10.1001/jama.2017.5283
7. Rillo O, Riera H, Acosta C, et al. PANLAR consensus recommendations for the management in osteoarthritis of hand, hip, and knee. J Clin Rheumatol. 2016;22(7):345–354. doi:10.1097/RHU.0000000000000449
8. Wise J.Steroid injections for knee osteoarthritis are not supported by study. BMJ. 2017;357:j2386.
9. Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2020;31.
10. Chen X, Wu Y, Yang T, et al. Salidroside alleviates cachexia symptoms in mouse models of cancer cachexia via activating mTOR signalling. J Cachexia Sarcopenia Muscle. 2016;7(2):225–232. doi:10.1002/jcsm.12054
11. Yang DW, Kang OH, Lee YS, et al. Anti-inflammatory effect of salidroside on phorbol-12-myristate-13-acetate plus A23187-mediated inflammation in HMC-1 cells. Int J Mol Med. 2016;38(6):1864–1870. doi:10.3892/ijmm.2016.2781
12. Ma C, Hu L, Tao G, Lv W, Wang H. An UPLC-MS-based metabolomics investigation on the anti-fatigue effect of salidroside in mice. J Pharm Biomed Anal. 2015;105:84–90. doi:10.1016/j.jpba.2014.11.036
13. Guo C, Li Y, Zhang R, et al. Protective effect of salidroside against diabetic kidney disease through inhibiting BIM-mediated apoptosis of proximal renal tubular cells in rats. Front Pharmacol. 2018;9:1433. doi:10.3389/fphar.2018.01433
14. Musumeci G, Loreto C, Carnazza ML, Martinez G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):307–313. doi:10.1007/s00167-010-1215-0
15. Zhao X, Lu Y, Tao Y, et al. Salidroside liposome formulation enhances the activity of dendritic cells and immune responses. Int Immunopharmacol. 2013;17(4):1134–1140. doi:10.1016/j.intimp.2013.10.016
16. Li H, Yang T, Wu R, Chen T, Sun Z, Yang L. Salidroside inhibits platelet-derived growth factor-induced proliferation and migration of airway smooth muscle cells. J Cell Biochem. 2019;120(4):6642–6650.
17. Zhou F, Ju J, Fang Y, et al. Salidroside protected against MPP(+) -induced Parkinson’s disease in PC12 cells by inhibiting inflammation, oxidative stress and cell apoptosis. Biotechnol Appl Biochem. 2019;66(2):247–253. doi:10.1002/bab.1719
18. Kallscheuer N, Menezes R, Foito A, et al. Identification and microbial production of the raspberry phenol salidroside that is active against huntington’s disease. Plant Physiol. 2019;179(3):969–985.
19. Qin Y, Liu HJ, Li M, et al. Salidroside improves the hypoxic tumor microenvironment and reverses the drug resistance of platinum drugs via HIF-1alpha signaling pathway. EBioMedicine. 2018;38:25–36. doi:10.1016/j.ebiom.2018.10.069
20. Liu M, Zhang J, Liu W, Wang W. Salidroside protects ATDC5 cells against lipopolysaccharide-induced injury through up-regulation of microRNA-145 in osteoarthritis. Int Immunopharmacol. 2019;67:441–448. doi:10.1016/j.intimp.2018.12.041
21. Zhang Y, Zhao Q. Salidroside attenuates interleukin-1beta-induced inflammation in human osteoarthritis chondrocytes. J Cell Biochem. 2018.
22. Papalia R, Vadala G, Torre G, et al. The cytokinome in osteoarthritis, a new paradigm in diagnosis and prognosis of cartilage disease. J Biol Regul Homeost Agents. 2016;30(4 Suppl 1):77–83.
23. Landuyt AE, Klocke BJ, Colvin TB, Schoeb TR, Maynard CL. Cutting edge: ICOS-deficient regulatory T cells display normal induction of Il10 but readily downregulate expression of Foxp3. J Immunol. 2019;202(4):1039–1044.
24. Ashfaq H, Soliman H, Saleh M, El-Matbouli M. CD4: a vital player in the teleost fish immune system. Vet Res. 2019;50(1):1.
25. Abbasi J. Can exercise prevent knee osteoarthritis? JAMA. 2017;318(22):2169–2171. doi:10.1001/jama.2017.16144
26. Zeng RM, Lu XH, Lin J, et al. Knockdown of FOXM1 attenuates inflammatory response in human osteoarthritis chondrocytes. Int Immunopharmacol. 2019;68:74–80. doi:10.1016/j.intimp.2018.12.057
27. Choi WS, Yang JI, Kim W, et al. Critical role for arginase II in osteoarthritis pathogenesis. Ann Rheum Dis. 2019;78(3):421–428. doi:10.1136/annrheumdis-2018-214282
28. Zhou J, Zhao Y, Wu G, Lin B, Li Z, Liu X. [Corrigendum] Differential miRNAomics of the synovial membrane in knee osteoarthritis induced by bilateral anterior cruciate ligament transection in rats. Mol Med Rep. 2019;20(6):5363.
29. Fu SC, Cheng WH, Cheuk YC, et al. Effect of graft tensioning on mechanical restoration in a rat model of anterior cruciate ligament reconstruction using free tendon graft. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1226–1233. doi:10.1007/s00167-012-1974-x
30. Zhang X, Lai W, Ying X, et al. Salidroside reduces inflammation and brain injury after permanent middle cerebral artery occlusion in rats by regulating PI3K/PKB/Nrf2/NFkappaB signaling rather than complement C3 activity. Inflammation. 2019;42(5):1830–1842.
31. Castrogiovanni P, Di Rosa M. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int J Mol Sci. 2019;20(3):511. doi:10.3390/ijms20030511
32. Yennan P, Suputtitada A, Yuktanandana P. Effects of aquatic exercise and land-based exercise on postural sway in elderly with knee osteoarthritis. Asian Biomed. 2018;4(5):739–745. doi:10.2478/abm-2010-0096
33. Bannwarth B. Acetaminophen or NSAIDs for the treatment of osteoarthritis. Best Pract Res Clin Rheumatol. 2006;20(1):117–129.
34. de Boer TN, Huisman AM, Polak AA, et al. The chondroprotective effect of selective COX-2 inhibition in osteoarthritis: ex vivo evaluation of human cartilage tissue after in vivo treatment. Osteoarthritis Cartilage. 2009;17(4):482–488. doi:10.1016/j.joca.2008.09.002
35. Cheleschi S, Calamia V, Fernandez-Moreno M, et al. In vitro comprehensive analysis of VA692 a new chemical entity for the treatment of osteoarthritis. Int Immunopharmacol. 2018;64:86–100. doi:10.1016/j.intimp.2018.08.025
36. Wang C, Yan L, Yan B, et al. Agkistrodon ameliorates pain response and prevents cartilage degradation in monosodium iodoacetate-induced osteoarthritic rats by inhibiting chondrocyte hypertrophy and apoptosis. J Ethnopharmacol. 2019;231:545–554. doi:10.1016/j.jep.2018.12.004
37. Zeng JJ, Wang HD, Shen ZW, Yao XD, Wu CJ, Pan T. curcumin inhibits proliferation of synovial cells by downregulating expression of matrix metalloproteinase-3 in osteoarthritis. Orthop Surg. 2019;11(1):117–125. doi:10.1111/os.12412
38. Zheng T, Yang X, Li W, et al. Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid Med Cell Longev. 2018;2018:8597897.
39. Li Q, Wang J, Li Y, Xu X. Neuroprotective effects of salidroside administration in a mouse model of Alzheimer’s disease. Mol Med Rep. 2018;17(5):7287–7292. doi:10.3892/mmr.2018.8757
40. Wu M, Hu R, Wang J, et al. Salidroside suppresses IL-1beta-induced apoptosis in chondrocytes via phosphatidylinositol 3-kinases (PI3K)/Akt signaling inhibition. Med Sci Monit. 2019;25:5833–5840. doi:10.12659/MSM.917851
41. Feng D, Ling WH, Duan RD. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-kappaB in macrophages. Inflamm Res. 2010;59(2):115–121. doi:10.1007/s00011-009-0077-8
42. Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi:10.22203/eCM
43. Wang D, Wang X, Tong W, Cui Y, Li X, Sun H. Umbelliferone Alleviates lipopolysaccharide-induced inflammatory responses in acute lung injury by down-regulating TLR4/MyD88/NF-kappaB signaling. Inflammation. 2019;42(2):440–448. doi:10.1007/s10753-018-00953-4
44. Hu PF, Ma CY, Sun FF, Chen WP, Wu LD. Follistatin-like protein 1 (FSTL1) promotes chondrocyte expression of matrix metalloproteinase and inflammatory factors via the NF-kappaB pathway. J Cell Mol Med. 2019;23(3):2230–2237. doi:10.1111/jcmm.14155
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.