Back to Journals » Journal of Inflammation Research » Volume 15
SAHA Alleviates Diarrhea-Predominant Irritable Bowel Syndrome Through Regulation of the p-STAT3/SERT/5-HT Signaling Pathway
Authors Shen J, Zhang B, Chen J, Cheng J, Wang J, Zheng X, Lan Y, Zhang X
Received 27 July 2021
Accepted for publication 22 November 2021
Published 10 March 2022 Volume 2022:15 Pages 1745—1756
DOI https://doi.org/10.2147/JIR.S331303
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jian Shen,1 Bimeng Zhang,2 Jianjie Chen,3 Jiazheng Cheng,1 Jiali Wang,1 Xianhui Zheng,1 Yu Lan,1 Xiaowen Zhang1
1Department of Pediatrics, Shuguang Hospital Affiliated to Shanghai Traditional Chinese Medical University, Shanghai, 201203, People’s Republic of China; 2Department of Acupuncture and Moxibustion, Shanghai General Hospital (The First People’s Hospital Affiliated to Shanghai Jiaotong University), Shanghai, 200080, People’s Republic of China; 3Department of Internal Medicine, Shuguang Hospital Affiliated to Shanghai Traditional Chinese Medical University, Shanghai, 201203, People’s Republic of China
Correspondence: Jian Shen Department of Pediatrics, Shuguang Hospital Affiliated to Shanghai Traditional Chinese Medical University, Shanghai, 201203, People’s Republic of China Tel +86-21-53821650 Email [email protected]
Objective: Irritable bowel syndrome (IBS) is characterized by abdominal pain, bloating, and stool irregularity. However, its pathophysiological mechanisms, which trigger intestinal motility disorders and diarrhea leading to diarrhea-predominant IBS (D-IBS), remain largely unknown.
Methods: In the present study, we established a D-IBS rat model by mother–infant separation combined with restraint stress. Then we exposed the modelled rats to suberoylanilide hydroxamic acid (SAHA) treatment, followed by determination of their visceral sensitivity. Toluidine blue staining served to reveal the effects of SAHA treatment on mast cells of D-IBS model rats. Then we measured the expression of serotonin (5-hydroxytryptamine; 5-HT) and its receptors by ELISA.
Results: Construction of short hairpin RNA (sh)-serotonin transporter (SERT) lentivirus vectors verified the regulation of the 5-HT signaling pathway by phosphorylated (p)-STAT/SERT. SAHA treatment of D-IBS model rats reduced the fecal water content, electromyography integral change rate, abdominal withdrawal reflex score, and number of mast cells, as well as the expression of 5-HT type 3A (5-HT3AR), 3B receptor (5-HT3BR), and 4 receptor (5-HT4R) receptors. The treatment also elevated the expression of signal transducer and activator for transcription 3 (STAT3) and SERT. Activation of p-STAT3 may reverse the inhibitory effect of SAHA on the elevated visceral sensitivity of D-IBS model rats. Moreover, SAHA promoted the transcription of SERT through repression of the p-STAT3/5-HT signaling, thereby inhibiting the visceral sensitivity of D-IBS model rats.
Conclusion: This study highlights that SAHA treatment can alleviate D-IBS through regulation of the p-STAT3/SERT/5-HT signaling pathway.
Keywords: diarrhea type irritable bowel syndrome, SAHA, p-STAT3, SERT, 5-HT
Introduction
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by abdominal pain, bloating, and stool irregularity.1,2 As one of the most common gastrointestinal disorders, IBS has a prevalence rate of 10–15% worldwide, bringing a great negative impact on quality of life and work efficiency of affected individuals.3,4 An abnormal increase in visceral sensitivity is hypothesized to be one of the key pathophysiological mechanisms of IBS, which triggers intestinal motility disorders and diarrhea leading to diarrhea-predominant IBS (D-IBS).5 To date, there is an incomplete understanding of the mechanistic pathways leading to D-IBS, which has hindered the development of specific and effective therapies.6 Thus, identifying the underlying mechanism of visceral sensitivity should open new avenues for therapeutics targeting D-IBS.
The intestinal neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) has demonstrated multiple effects on IBS, including stimulating intestinal secretion and colonic motility.7 Of note, 5-HT is mainly synthesized in enteric neurons of the gastrointestinal tract, where the serotonin transporter (SERT) mediates its reuptake, as in the central nervous system. Besides, postprandial increases in 5-HT are reported in D-IBS patients8 5-HT antagonists (such as the 5HT3 antagonist Alosetron) have approval for women with severe D-IBS, despite the risk of causing severe constipation.9
Accumulating evidence has suggested that histone deacetylase (HDAC) inhibitors play causative roles in multiple clinical disorders.10 While there are few studies on HDAC inhibitors in IBS, Moloney et al have demonstrated that the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) can normalize the visceral hypersensitivity caused by early life stress.11 This finding provides evidence justifying further efforts to develop HDAC inhibitor for treating IBS. Besides, there is prior evidence for the involvement of signal transducer and activator of transcription 3 (STAT3) in the pathophysiology of IBS.12 Moreover, SERT expression is related to the phosphorylation level of STAT3.13 Importantly, there is a report of reduced SERT mRNA expression in the intestinal mucosa of patients with D-IBS.14 Yang et al demonstrated that treatment with SAHA could decrease the expression of the non-coding mRNA miR-17-92 cluster through inhibition of tyrosine phosphorylation of STAT3 in patients with hepatoma.15 Based on the above-mentioned research, we set about to study the underlying mechanism concerning the role of SAHA in treating a D-IBS rat model, focusing on the involvement of SERT, STAT3 and 5-HT receptors.
Methods
Animal Modeling
One hundred and twenty-six healthy rats (female; 250–300 g; Hunan SJA Laboratory Animal Co., Ltd., Human, China) aged 6–8 weeks were raised in the SPF animal laboratory in separate cages with humidity set and 60–65%, and the temperature at 22–25°C. The rats had free access to food and water under a 12-hour light/dark cycle. The rats were acclimated for one week before experiments, and were observed for their health status before commencement of the experiment. The animal experiments were approved by the Animal Ethics Committee of Shuguang Hospital Affiliated to Shanghai Traditional Chinese Medical University (approval number: 2019–054) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Extensive efforts were made to ensure minimal suffering of the included animals.
The D-IBS model was established by the method of mother infant separation combined with restraint stress. In brief, rat pups of postnatal age 2–21 days were separated from their Dams for three hours every day. The young rats in the control and stressed groups were weaned at the age of 22 days, and their mothers were killed while under anesthesia. After mother–infant separation, rats in each group were raised normally to an age of 49, and in the interval of 50–59 days were subjected to restraint stress for two hours every day. Here, the young rats’ front upper limbs, front shoulders, and lower limbs were restrained such that they could not scratch their heads and faces; rats were able move forward and backward a little. The control group had no interventions until the age of 60 days.
After the model establishment, the rats were treated with different dosages of SAHA (Vorinostat, MK-0683, Zolinza®; high dose: 90 mg/kg, medium dose: 45.5 mg/kg, low dose: 22.8 mg/kg). Pinaverium bromide (Dicetel, Abbott) is recommended at a dose of 300–350 g/day for the treatment of IBS. Furthermore, Garcinone D (a natural xanthone from mangosteen; Shanghai FuSheng Industrial Co., Ltd., Shanghai, China; 10 μM) can increase phosphorylation signal transduction and transcriptional activation of phosphorylated (p)-STAT3.
The rats in the model group were further divided into low (22.8 mg/kg), medium (45.5 mg/kg), and high SAHA dose (90 mg/kg) groups, a pinaverium bromide group (serving as a positive control), a Garcinone D group, a SAHA + Garcinone D group, a short hairpin RNA against negative control (sh-NC) group (lentivirus carrying sh-NC) and a SAHA + sh-SERT group. There were six rats for each treatment group.
Construction of sh-SERT Lentiviral Vector
In this experiment, lentivirus carrying shRNAs against SERT (sh-SERT) and empty vector (sh-NC) were constructed. After 48 hours of transduction, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to detect the efficiency of sh-SERT. The lentivirus was purchased from Shanghai GenePharma Co., Ltd (Shanghai, China) and supplied at a concentration of 50 ng/mL. The successfully modeled rats were injected with the lentivirus carrying sh-NC and sh-SERT. In this procedure, the rats were weighed, and injected with a virus stock solution with an initial titer of 109 TU/mL diluted to 107 TU/mL at a dose of 20 μL/100 g through a tail vein. This procedure was performed once a day for three days.
Visceral Sensitivity Test
After the model establishment, we tested the visceral sensitivity of rats in each group. The rats were fasted with water only for 24 hours before the test, whereupon a F700 Small Animal Anesthesia Machine (EZ, USA) was used to obtain isoflurane anesthesia. Next, electrodes were implanted into the obliquus abdominis lateralis at a position 1.5 cm above the inguinal ligament. A liquid paraffin-coated gasbag and catheter were inserted carefully into the end of the balloon through the anus, extending about 2 cm from the anal edge. The external part of the anal canal was fixed to the tail root with medical tape. The gasbag was then inflated with 2 mL air using a syringe to ensure that the air bag could expand smoothly in the colon. The rats were then placed in a small cage, allowing little room for movement back and forth, without turning around. The gasbag was connected to an electronic constant pressure device through a catheter, the electrode was connected with an electrophysiological recorder through a wire, and the electromyographic (EMG) activity of the rat abdominal external oblique muscle was recorded on a computer.
The experimental recordings were made when the rats had woken up and acclimated for 30 minutes. First, we obtained a stable 30 second baseline EMG recording of the abdominal external oblique muscle and calculated the mean baseline score. The gasbags in the colons of the rats were then pressurized in stages to 20, 40, 60, and 80 mmHg, with each stage lasting 30 seconds and spaced in intervals of 180 seconds. The behavior changes of rats were observed, and the abdominal withdrawal reflex (AWR) score and EMG activity of the rat abdominal external oblique muscle were monitored and recorded. Visceral sensitivity was evaluated using the AWR score and the EMG score relative to the baseline.
Determination of Fecal Water Content
After the model establishment, the rats in each group were placed in a metabolism cage (CLAMS Small Animal Metabolism System, Columbus, USA) for 24 hours. The rat feces were then collected and weighed quickly while wet. Next, the feces were dried and reweighed, and the fecal water content was calculated as (fecal wet weight – fecal dry weight)/fecal wet weight × 100%.
Specimen Preparation
After indicated administration, the rats were fasted for 24 hours before sample collection. The rats were anesthetized by intraperitoneal injection with 0.3% pentobarbital sodium. The abdominal cavity was opened surgically, and the distal colon about 1 cm from the anal margin was quickly resected. One section was fixed in 4% paraformaldehyde solution, and the other section was cut into two parts longitudinally, washed with normal saline, and frozen in liquid nitrogen. The lumbosacral spinal cord (L5-S2 segment) of rats was quickly removed after spraying the spine with ice-cold saline and then frozen in liquid nitrogen for later analysis.
Quantitative Measurement of 5-HT in Colon and Spinal Cord
The content of 5-HT in colon and spinal cord of rats was determined by enzyme-linked immunosorbent assay (ELISA) following methods in the ELISA kit and calculated in relation to tissue protein content measured using a bicinchoninic acid (BCA) assay kit.
Hematoxylin and Eosin (H&E) Staining
HE Staining Kit (PT001, Shanghai Bogoo Biological Technology Co., Ltd., Shanghai, China) was used for tissue staining. In brief, a 2–3 segment or rat colon was removed, washed with normal saline, fixed in 4% paraformaldehyde for 30–50 minutes, dehydrated, cleared, soaked in wax, embedded, and sliced into 5-μm-thick sections. The tissue sections were dried in a 45°C incubator, dewaxed, and washed for 5 minutes with distilled water. Afterwards, the sections were stained with hematoxylin for 5 minutes, washed under running water for 3 seconds, differentiated by 1% hydrochloric acid ethanol for 3 seconds, stained by 5% eosin for about 3 minutes, dehydrated, cleared, sealed and observed under an inverted microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Detection of Colonic Mast Cells
Toluidine blue (Shanghai Yuanye Biological Technology Co., Ltd., Shanghai, China) staining was used to detect mast cells in connective tissue of the colon of rats. Under a light microscope, the mast cells cytoplasm was purple red, the nucleus was blue, and the cell shape was round or oval or irregular. Smaller cells had less cytoplasm and clear borders; larger cells had more cytoplasm, with unclear borders, and purple-red particles were scattered around the nucleus. Under the guidance of experienced pathologists, mast cells were counted in three randomly selected visual fields.
Immunohistochemistry
Paraffin sections were dewaxed, hydrated with gradient alcohol and washed under tap water for 2 minutes. Next, the sections were treated with methanolic H2O2 for 20 minutes and subjected to antigen retrieval. Afterwards, the sections were sealed with normal goat serum sealing solution (c-0005, Shanghai Haoran Biotechnology Co., Ltd., Shanghai, China) at room temperature for 20 minutes and incubated with rabbit monoclonal antibody tryptase-β (xy-D781Mu01, 1:200; Peprotech, Rocky Hill, NJ, USA) overnight at 4°C. After three washes in phosphate-buffered saline (PBS), the sections were incubated with goat anti-rabbit IgG (ab6785, 1:1000, Abcam, Cambridge, UK) at 37°C for 20 minutes and with the streptomycin-ovalbumin working solution labeled with horseradish peroxidase (0343–10000u, Imunbio Biotechnology Co., Ltd., Beijing, China) at 37°C for 20 minutes. The sections were developed with the addition of 3,3ʹ-diaminobenzidine tetrahydrochloride (DAB; ST033, Guangzhou WeiJia Technology Co., Ltd., Guangzhou, China), counterstained with hematoxylin (PT001, Bogoo) for 1 minute, blued in 1% ammonia, dehydrated with alcohol, cleared in xylene, and sealed with neutral resin. Subsequently, the sections were observed and photographed under a microscope. One hundred cells were counted in each of five randomly selected high-power fields of view from each section, and the IHC positive rate was calculated.
qRT-PCR
TRIzol reagent (Invitrogen, Calsbad, CA, USA) was used to extract total RNA from the colon tissues of rats. The Nanodrop2000 micro-ultraviolet spectrophotometer (1011U, Nanodrop, USA) was used to detect the concentration and purity of total RNA. According to the instructions of the TaqMan MicroRNA Assay reverse transcription primer (4,427,975, Applied Biosystems, Foster City, CA, USA) PrimeScript RT Regent Kit (RR047A, Takara, Japan), the extracted RNA was reversely transcribed to complementary DNA (cDNA). The primers of 5-hydroxytryptamine type 3A receptor (5-HT3AR), 5-hydroxytryptamine type 3B receptor (5-HT3BR), 5-hydroxytryptamine 4 receptor (5-HT4R), SERT and STAT3 were designed and synthesized by TaKaRa (Table S1). The ABI 7500 quantitative PCR instrument (Applied Biosystems) was used for real-time fluorescent quantitative PCR detection of these genes. The relative transcription level of the target gene was calculated by the relative quantitative method (2−ΔΔCT method) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal reference primer.
Western Blot Analysis
Total protein was extracted from colon tissues and spinal cord tissues using enhanced radioimmunoprecipitation assay (RIPA) lysis buffer (Wuhan Boster Biological Technology, LTD, Wuhan, Hubei, China) containing protease inhibitor. A BCA protein quantitative Kit (Boster) was used to determine the protein concentration in conjunction with a fluorescence microplate reader (Molecular Devices Corporation, USA). The protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was sealed with 5% bovine serum albumin (BSA) at room temperature for 2 hours to block non-specific binding and then incubated overnight at 4°C with the diluted primary rabbit antibodies (5-HT3AR, ab13897, 1:2000; 5-HT3BR, ab115023, 1:2000; 5-HT4R, ab101910, 1:2000; STAT3, ab68153, 1:1000; p-STAT3, phosphorylation site: tyr-705, ab76315, 1:1000; SERT, ab130130, 1:2000). After washing with phosphate-buffered saline-Tween-20 (PBST) at room temperature, the membrane was incubated with goat anti-rabbit IgG (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) labeled with horseradish peroxidase (HRP) at room temperature for 1 hour. The membrane was developed by enhanced chemiluminescence (ECL; Thermo Fisher Scientific Inc., Waltham, MA, USA) and observed using the Bio-Rad ChemiDoc™ Gamma Imaging system. Image J analysis software was used to quantify the gray level of each band in Western blot image, and GAPDH (Rabbit, ab181602, 1:1000) was used as the internal control.
Statistical Analysis
SPSS 19.0 statistical software (IBM Corp. Armonk, NY, USA) was used for data processing. The measurement data are represented as mean ± standard deviation. The independent sample t-test was used to compare data between two groups, while one-way analysis of variance with Tukey’s post-hoc test was used to compare data among multiple groups. Bonferroni-corrected repeated measures analysis of variance was used for comparison of data of different concentrations (doses). A value of p < 0.05 was considered to be indicative of statistical significance.
Results
Successful Establishment of D-IBS Rat Models
Mother–infant separation is a severe life stress event in early life, which can lead to the occurrence of visceral hypersensitivity in rats after reaching adulthood. Since the mother–infant separation can better simulate the chronic hyperesthesia state of IBS, we combined this stress with subsequent restraint stress to induce the D-IBS model. Stress rats showed a significantly lower body weight, compared with the control group, but there were no significant differences in weight of the two groups of rats before and after the mother–infant separation (p > 0.05, Figure 1A). The fecal water content of the D-IBS model group was significantly higher than that of the control group. In addition, when the pressure of the gasbag was 40, 60 and 80 mmHg, the AWR and EMG scores in the model group was significantly higher than those in the control group (Figure 1B–D). After modeling, the rats were euthanized and the colon tissues were removed and colored by H&E staining to analyze the histopathological changes. The results showed no obvious pathological morphological changes of the colon mucosa of rats in the control and model groups (Figure 1E), which is in line with the disease characteristics of D-IBS. The aforementioned results demonstrated the successful establishment of the D-IBS rat model.
SAHA Reduces the Visceral Sensitivity of D-IBS Rats
HDACs can participate in epigenetic regulation of transcription, cell cycle and cell metabolism by catalyzing post-translational modifications of histones.16 HDAC inhibitors may potentially provide a way to alleviate visceral hypersensitivity related to irritable bowel syndrome.17 Compared with the control group, the fecal water content of the model group was significantly increased, but was significantly reduced after SAHA administration (Figure 2A). Compared with the control group, the AWR and EMG scores of the model group increased significantly at pressures of 40, 60, and 80 mmHg, but SAHA administration significantly decreased the rate of change of AWR score and EMG score (Figure 2B and C). The high and middle dose of SAHA exerted a better effect in reducing the increase in fecal water content, AWR score and EMG score of D-IBS rats, compared to the low dose of SAHA and the pinaverium bromide treatment.
SAHA Alleviates the Visceral Sensitivity in D-IBS Rats by Inhibiting 5-HT Signaling Pathway
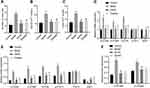
The enteric 5-HT signaling pathway plays an important role in IBS clinical symptoms.18 5-HT is one of the main active mediators for the activation and degranulation of mast cells, and degranulation of mast cells can itself regulate the local concentration of 5-HT.19 Here, upon combining the previous results, we selected a medium dose of SAHA (45.5 mg/kg) to treat D-IBS rats, and then performed toluidine blue staining to detect the number of mast cells in the colon tissues. Compared with the control group, the number of mast cells was significantly increased in the model group, which was negated after SAHA intervention (Figures 3A and S1A). ELISA data exhibited that, compared with the control group, the 5-HT content was significantly increased in the colon and spinal cord of the model group but was significantly reduced after the SAHA treatment (Figure 3B and C).
Among the many subtypes of 5-HT receptor, 5-HT3R and 5-HT4R are most closely related to the pathogenesis of D-IBS.20 Meanwhile, the 5-HT signaling pathway is regulated by SERT, making it a potential pharmacological target for the treatment of gastrointestinal diseases.21 In addition, phosphorylation of STAT3 can regulate the expression of SERT.13 Thus, we measured the expression of 5-HT3AR, 5-HT3BR, 5-HT4R, SERT and STAT by qRT-PCR and Western blot analysis in the rat colon tissues. Compared with the control group, the expression of 5-HT3AR, 5-HT3BR and 5-HT4R in the colon tissues of rats in the model group was significantly increased, but that of SERT was decreased, while the expression of STAT3 was unchanged. However, the expression of 5-HT3AR, 5-HT3BR and 5-HT4R was significantly reduced, SERT expression was elevated, and STAT3 expression did not change significantly after SAHA treatment (Figure 3D). Moreover, Western blot analysis results suggested that, compared with the control group, the phosphorylation level of STAT3 was increased in the model group, which was reversed following SAHA treatment. The Western blot findings of protein expression of 5-HT3AR, 5-HT3BR, 5-HT4R, SERT and STAT3 were consistent with those of qRT-PCR (Figures 3E and S2A). Furthermore, the protein expression of 5-HT3AR and 5-HT3BR was significantly increased in the spinal cord tissues of rats in the model group, but was significantly reduced after SAHA treatment (Figures 3F and S2B). In summary, the beneficial effect of SAHA on D-IBS model rats may occur via a reduction in the number of mast cells in the colon, inhibition of STAT3 phosphorylation, and the altered expression of several subtypes of 5-HT receptors.
SAHA Suppresses the 5-HT Signaling Pathway Activation by Regulating STAT3 Phosphorylation
To further verify whether SAHA affects the visceral sensitivity of D-IBS model rats by regulating STAT3 phosphorylation, we treated rats with Garcinone D and then observed histopathological changes of the rat colon tissues by toluidine blue staining. Compared with the model group, there were significantly mast cells in the SAHA group, but mast cells numbers were significantly increased in the Garcinone D group relative to the model group. In addition, in comparison to the SAHA group, significantly more mast cells were observed in the SAHA + Garcinone D group (Figures 4A and S1B). Moreover, the results of Western blot analysis showed that, compared with the model group, 5-HT3AR, 5-HT3BR, 5-HT4R expression, along with the phosphorylation level of STAT3, was reduced, while SERT expression was elevated in the colon tissues of rats in the SAHA treatment group. In contrast, 5-HT3AR, 5-HT3BR, 5-HT4R expression, along with the phosphorylation level of STAT3, was significantly elevated, but SERT expression was decreased in the Garcinone D group relative to the model group. Compared with the SAHA group, 5-HT3AR, 5-HT3BR, 5-HT4R expression, along with the phosphorylation level of STAT3, was significantly increased, whereas SERT expression was diminished in the SAHA + Garcinone D group (Figure 4B). The above data indicate that the activation of p-STAT3 can reverse the inhibitory effect of SAHA on the visceral sensitivity of D-IBS model rats and that SAHA can disrupt the 5-HT signaling pathway by regulating STAT3 phosphorylation.
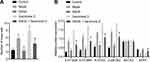
SAHA Reduces the Visceral Sensitivity in D-IBS Rats by Regulating the STAT3/SERT/5-HT Signaling
Through the construction of sh-SERT lentivirus vector intervention system, we further verified that SAHA affects the 5-HT signaling system by regulating p-STAT/SERT. The intervention efficiency of sh-SERT was confirmed by qRT-PCR, as shown by the decreased expression of SERT following SERT silencing (Figure 5A). Previous literature has indicated that the 5-HT3R plays a regulatory role in colonic secretion induced by acute stress.20 Western blot analysis results showed increased expression of SERT, reduced 5-HT3AR and 5-HT3BR expression, along with reduced phosphorylation level of STAT3, and no alterations in STAT3 expression in the colon tissues of rats in the SAHA group as compared with the sh-NC group. Compared with the SAHA group, the SAHA + sh-SERT group showed reduced expression of SERT, elevated 5-HT3AR and 5-HT3BR expression, yet unaltered expression of STAT3 and STAT3 phosphorylation levels (Figure 5B).
Toluidine blue staining results showed that, compared with the sh-NC group, the number of mast cells was significantly reduced in the SAHA group. Compared with the SAHA group, there were significantly more mast cells in the SAHA + sh-SERT group (Figures 5C and S1C). Moreover, immunohistochemistry showed significantly reduced expression of tryptase-β in the SAHA group, but an increased expression of tryptase-β in the SAHA + sh-SERT group compared with the sh-NC group (Figure 5D). In conclusion, SAHA can promote the transcription of SERT by inhibiting the phosphorylation of STAT3 and thus inhibit the 5-HT signaling pathway, thereby reducing the visceral sensitivity of D-IBS model rats.
Discussion
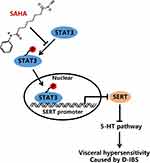
Previous evidence has shown that HDAC inhibitor SAHA could normalize visceral hypersensitivity induced by early life stress.11 Overall, we demonstrated that SAHA could promote the transcription of SERT by inhibiting STAT3 phosphorylation and the 5-HT signaling pathway, thereby reducing the visceral sensitivity of D-IBS rats and the resultant D-IBS progression (Figure 6). Thus, we feel encouraged that SAHA has the potential to serve as a new strategy for the treatment of D-IBS.
First, we found that SAHA could relieve diarrhea and improve the visceral hypersensitivity in D-IBS rats. Moreover, the efficacy SAHA was comparable to that of pinaverium bromide in improving visceral hypersensitivity, but might be superior to pinaverium bromide in relieving diarrhea. There is a report that gut microbiota changes are associated with the pathogenesis of IBS.22 Specifically, Fellows et al revealed that microbial-derived short-chain fatty acids (SCFA) are dominant determinants in intestinal microbiota-host crosstalk, and that inhibition of HDACs by SCFA is the key mechanism of this effect.23 We now speculate that the HDAC is SAHA may improve D-IBS, especially in relieving diarrhea, via effects on gut microbiota; testing this conjecture calls for further studies of gut fauna. Furthermore, stressful life events, anxiety and depression may contribute to IBS in children and adults.24 Of note, other work has shown that SAHA treatment can not only normalize visceral hypersensitivity but may also ameliorate anxiety reactions.11 This latter finding further provides us with a new potential mechanism for SAHA to improve D-IBS via central actions.
Further mechanistic investigations showed that SAHA administration significantly reduced the number of mast cells in the colon of D-IBS rats. A previous study showed a 75% increase in mast cell numbers in IBS patients, and biopsy lysates showed increased tryptase levels along with a more activated morphological/ultrastructural mast cell phenotype.25 Importantly, mast cells lying close to sensory nerve fibers exerted crucial effects on the development of visceral hypersensitivity in IBS through the local release of prostaglandin E2.26 In the current study, we also demonstrated that SAHA administration significantly reduced the expression of 5-HT, 5-HT3AR, and 5-HT3BR in colon. As reported by a recent study, 5-HT is an important mediator of all aspects of intestinal function, and has a particular role in IBS by stimulating intestinal secretion and colonic motility.7 Additionally, 5-HT affects various gut functions by interacting with its specific receptors, whereas SERT regulates these effects.27 Among the 5-HT related receptors, 5-HT3R and 5-HT4R are the most closely related to the pathophysiological development of D-IBS. 5-HT3R in intestinal tissue mainly acts to promote intestinal contraction, intestinal fluid secretion, peristalsis, and content transportation.28 5-HT4R mainly acts on the periphery of the gastrointestinal tract to alleviate indigestion, gastroesophageal reflux disease, gastroparesis, or IBS.29 Gunn et al also demonstrated that D-IBS patients have significant abnormalities in their 5-HT metabolism and signaling in the gut,7 thus providing some substantiation of our findings.
Our results also demonstrated that SAHA administration led to reduced phosphorylation level of STAT3 but increased transcription of SERT. Furthermore, SAHA could regulate 5-HT signaling system through p-STAT3/SERT in D-IBS. The duration and spatial extent of 5-HT signaling is regulated by the SERT, which removes 5-HT from the interstitial space.18 Chen et al indicated that 5-HT and the N-methyl-D-aspartate receptor subunit NR2B in the brain-gut axis may play important roles in the development of IBS, where NR2B can participate in the formation and development of chronic visceral hyperalgesia.30 Additionally, a previous study has illustrated that the mRNA expression of SERT is significantly reduced in the rectal mucosa of IBS patients, irrespective of the presence of diarrhea or constipation.31 Pharmacologically targeting the expression of SERT in the gut may emerge as a therapeutic option for IBS.32 More importantly, the present findings of an interaction between SERT and the phosphorylation level of STAT3 confirmed a previous literature report.33
To conclude, we report that the HDAC inhibitor SAHA could enhance the transcription of SERT by inhibiting STAT3 phosphorylation and the 5-HT signaling system, thereby alleviating D-IBS in a rat model. We found that SAHA treatment not only relieved diarrhea but also ameliorated visceral sensitivity. However, we cannot exclude the possibility that SAGA may evoke adverse reactions or have differing efficiency in subtypes of IBS. We see a need to explore the mechanisms of SAHA extending beyond the 5-HT system. Finally, further research is still required to prove the translational feasibility of SAHA as a therapeutic strategy for IBS.
Data Sharing Statement
The datasets generated and/or analysed during the current study are available in the manuscript and supplementary materials.
Acknowledgments
This work was supported by Inheritance and Innovation of Traditional Chinese Medicine Talent Project - ‘One billion’ National Training Program for Innovative Key Talents of Traditional Chinese Medicine (No. 19Z-1-18), Clinical Study of Shenlingbaizhu Powder in Treating Infantile Chronic Diarrhea of Spleen Deficiency Type (No.201840008), Training of Overseas High-end Pediatric Training Team in Shanghai Children Health Service Capacity Building program-integrated Chinese and Western pediatrics (No. GDEK201704), Science and Technology Commission of Shanghai TCM Umbilical Therapy Standardized Solution Research for the Treatment of Infantile Diarrhea (No. 15401971100), Major National Science and Technology Projects during the 13th Five-year Plan (No. 2017ZX10305501-007), and Construction of Important and Weak Disciplines in Shanghai Health and Family Planning System – Pediatrics of Integrated Chinese and Western Medicine (No. 2016ZB0104-02).
Author Contributions
J.S., B.M.Z., and J.J.C. conceived and designed research; J.S., J.Z.C., and J.L.W. performed experiments; X.H.Z. and Y.L. analyzed data; J.L.W., B.M.Z., and X.W.Z. interpreted results of experiments; J.J.C. and X.H.Z. prepared figures; J.S., Y.L., and X.W.Z. drafted manuscript; J.S., B.M.Z., J.J.C., J.Z.C., J.L.W., X.H.Z., Y.L., and X.W.Z. edited and revised manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi:10.1038/nrdp.2016.14
2. Sultan S, Malhotra A. Irritable bowel syndrome. Ann Intern Med. 2017;166(11):ITC81–ITC96. doi:10.7326/AITC201706060
3. Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1(2):133–146. doi:10.1016/S2468-1253(16)30023-1
4. Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376(26):2566–2578. doi:10.1056/NEJMra1607547
5. Botschuijver S, Roeselers G, Levin E, et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology. 2017;153(4):1026–1039. doi:10.1053/j.gastro.2017.06.004
6. Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Aliment Pharmacol Ther. 2017;45(1):100–114. doi:10.1111/apt.13848
7. Gunn D, Garsed K, Lam C, et al. Abnormalities of mucosal serotonin metabolism and 5-HT3 receptor subunit 3C polymorphism in irritable bowel syndrome with diarrhoea predict responsiveness to ondansetron. Aliment Pharmacol Ther. 2019;50(5):538–546. doi:10.1111/apt.15420
8. Camilleri M. Intestinal secretory mechanisms in irritable bowel syndrome-diarrhea. Clin Gastroenterol Hepatol. 2015;13(6):
9. Camilleri M. Management options for irritable bowel syndrome. Mayo Clin Proc. 2018;93(12):1858–1872. doi:10.1016/j.mayocp.2018.04.032
10. Qiu X, Xiao X, Li N, Li Y. Histone deacetylases inhibitors (HDACis) as novel therapeutic application in various clinical diseases. Prog Neuropsychopharmacol Biol Psychiatry. 2017;72:60–72. doi:10.1016/j.pnpbp.2016.09.002
11. Moloney RD, Stilling RM, Dinan TG, Cryan JF. Early-life stress-induced visceral hypersensitivity and anxiety behavior is reversed by histone deacetylase inhibition. Neurogastroenterol Motil. 2015;27(12):1831–1836. doi:10.1111/nmo.12675
12. Li B, Rui J, Ding X, Chen Y, Yang X. Deciphering the multicomponent synergy mechanisms of SiNiSan prescription on irritable bowel syndrome using a bioinformatics/network topology based strategy. Phytomedicine. 2019;63:152982. doi:10.1016/j.phymed.2019.152982
13. Kong E, Sucic S, Monje FJ, et al. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci Rep. 2015;5:9009. doi:10.1038/srep09009
14. Foley S, Garsed K, Singh G, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140(5):1434–1443 e1431. doi:10.1053/j.gastro.2011.01.052
15. Yang H, Lan P, Hou Z, et al. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br J Cancer. 2015;112(1):112–121. doi:10.1038/bjc.2014.547
16. Zhang M, Wei W, Peng C, et al. Discovery of novel pyrazolopyrimidine derivatives as potent mTOR/HDAC bi-functional inhibitors via pharmacophore-merging strategy. Bioorg Med Chem Lett. 2021;49:128286. doi:10.1016/j.bmcl.2021.128286
17. Cao D-Y, Bai G, Ji Y, Karpowicz JM, Traub RJ. EXPRESS: histone hyperacetylation modulates spinal type II metabotropic glutamate receptor alleviating stress-induced visceral hypersensitivity in female rats. Mol Pain. 2016;12:174480691666072. doi:10.1177/1744806916660722
18. El-Ayache N, Galligan JJ. 5-HT3 receptor signaling in serotonin transporter-knockout rats: a female sex-specific animal model of visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2019;316(1):G132–G143. doi:10.1152/ajpgi.00131.2018
19. Park JH, Rhee PL, Kim HS, et al. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21(1 Pt 1):71–78. doi:10.1111/j.1440-1746.2005.04143.x
20. Zhao JM, Lu JH, Yin XJ, et al. Comparison of electroacupuncture and moxibustion on brain-gut function in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. Chin J Integr Med. 2015;21(11):855–865. doi:10.1007/s11655-015-2049-x
21. Zhang Y, Zhang H, Zhang W, Zhang Y, Wang W, Nie L. LncRNA XIST modulates 5-hydroxytrytophan-induced visceral hypersensitivity by epigenetic silencing of the SERT gene in mice with diarrhea-predominant IBS. Cell Signal. 2020;73:109674. doi:10.1016/j.cellsig.2020.109674
22. Zhuang X, Tian Z, Li L, Zeng Z, Chen M, Xiong L. Fecal microbiota alterations associated with diarrhea-predominant irritable bowel syndrome. Front Microbiol. 2018;9:1600. doi:10.3389/fmicb.2018.01600
23. Fellows R, Varga-Weisz P. Chromatin dynamics and histone modifications in intestinal microbiota-host crosstalk. Mol Metab. 2020;38:100925. doi:10.1016/j.molmet.2019.12.005
24. Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103(3):
25. Bednarska O, Walter SA, Casado-Bedmar M, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology. 2017;153(4):948–960 e943. doi:10.1053/j.gastro.2017.06.051
26. Grabauskas G, Wu X, Gao J, Li JY, Turgeon DK, Owyang C. Prostaglandin E2, produced by mast cells in colon tissues from patients with irritable bowel syndrome, contributes to visceral hypersensitivity in mice. Gastroenterology. 2020;158(8):2195–2207 e2196. doi:10.1053/j.gastro.2020.02.022
27. Coates MD, Tekin I, Vrana KE, Mawe GM. Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46(6):569–580. doi:10.1111/apt.14226
28. Asagarasu A, Matsui T, Hayashi H, et al. Discovery of a novel 5-HT(3) antagonist/5-HT(1A) agonist 3-amino-5,6,7,8-tetrahydro-2-{4-[4-(quinolin-2-yl)piperazin-1-yl]butyl}quinazolin −4(3H)-one (TZB-30878) as an orally bioavailable agent for irritable bowel syndrome. J Med Chem. 2010;53(21):7549–7563. doi:10.1021/jm1002292
29. Rebholz H, Friedman E, Castello J. Alterations of expression of the serotonin 5-HT4 receptor in brain disorders. Int J Mol Sci. 2018;19(11). doi:10.3390/ijms19113581
30. Chen MX, Chen Y, Fu R, Liu SY, Yang QQ, Shen TB. Activation of 5-HT and NR2B contributes to visceral hypersensitivity in irritable bowel syndrome in rats. Am J Transl Res. 2016;8(12):5580–5590.
31. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126(7):1657–1664. doi:10.1053/j.gastro.2004.03.013
32. Camilleri M, Andrews CN, Bharucha AE, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132(1):17–25. doi:10.1053/j.gastro.2006.11.020
33. Buckley MM, O’Halloran KD, Rae MG, Dinan TG, O’Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592(23):5235–5250. doi:10.1113/jphysiol.2014.279968
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.