Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Safety Profile of Musculoskeletal Contrast-Enhanced Ultrasound with Sulfur Hexafluoride Contrast Agent
Authors Fischer C , Kunz P, Strauch M, Weber MA , Doll J
Received 18 October 2019
Accepted for publication 9 March 2020
Published 14 April 2020 Volume 2020:16 Pages 269—280
DOI https://doi.org/10.2147/TCRM.S235235
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Christian Fischer,1,* Pierre Kunz,1,* Marten Strauch,1 Marc-André Weber,2 Julian Doll1
1Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Ultrasound Center, HTRG - Heidelberg Trauma Research Group, Heidelberg University Hospital, Heidelberg 69118, Germany; 2Institute of Diagnostic and Interventional Radiology, Pediatric Radiology and Neuroradiology, University Medical Center Rostock, Rostock 18057, Germany
*These authors contributed equally to this work
Correspondence: Julian Doll
Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Ultrasound Center HTRG – Heidelberg Trauma Research Group Heidelberg University Hospital, Schlierbacher Landstrasse 200a, Heidelberg 69118, Germany
Tel +49 6221 5636388
Fax +49 6221 5629213
Email [email protected]
Purpose: Muscle, bone and tendon regeneration depend on the microperfusion of the corresponding tissue which can be quantified with contrast-enhanced ultrasound (CEUS) using sulfur hexafluoride contrast agent (SonoVue®). This study investigated the incidence of adverse events (AEs) in musculoskeletal patients and gives an overview of musculoskeletal CEUS applications.
Patients and Methods: Based on 13 studies in a standardized monocentric setting, a total of 2268 CEUS examinations in 764 patients were performed and AEs due to the administration of sulfur hexafluoride contrast agent were classified as either mild, moderate or severe.
Results: No fatal events occurred. AEs were reported in three cases, of which only one was classified as severe and two as mild. The total rate of all AEs was 0.13% and 0.04% for severe AEs.
Conclusion: The present analysis confirms the safety of musculoskeletal CEUS using sulfur hexafluoride contrast agent with a lower rate of AEs than that reported for other contrast agents even in elderly patients with concomitant diseases.
Keywords: contrast-enhanced ultrasound, CEUS, sulfur hexafluoride, musculoskeletal, contrast agents, adverse events
Introduction
Contrast-enhanced ultrasound (CEUS) allows insight into microperfusion of various tissues by ultrasonic waves. CEUS has become popular in recent years and is already integrated into the clinical routine in internal medicine. Since the first guidelines in 2004, CEUS has been particularly used in the context of supporting diagnostics of liver, spleen, as well as kidney cancer and lesions.1–3
Especially Celli et al provided results with high sensitivity, specificity and accuracy regarding the diagnosis between benign and malignant liver lesions.4 The review of Rettenbacher confirmed these results and suggested that CEUS is the imaging modality of choice for differentiation of focal liver lesions and may replace more expensive imaging techniques like MRI.5
The current 2017 guidelines of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) introduced CEUS for non-hepatic applications. Concerning the musculoskeletal area of application, its use has especially been described in inflammatory joint diseases;6 however, CEUS can also be applied to assess the microperfusion as a surrogate parameter for the vitality of bone and muscle tissue.7,8
Previous reports on the applicability of sulfur hexafluoride contrast agents (SonoVue®, Bracco Imaging, Milan, Italy) in internal medicine demonstrated a complication rate as low as 0.0086%.1,9
In contrast, this study regarding safety analysis at an university trauma and orthopedic ultrasound center focused exclusively on musculoskeletal indications for CEUS, specifically highlighting its applicability and benefits in trauma and orthopedic surgery.
The contrast agent, which is commonly used for CEUS examinations, consists of millions of sulfur hexafluoride bubbles with a phospholipid shell, an echogenic, poorly soluble gas, which oscillates measurably upon collision with ultrasonic waves.9 Sulfur hexafluoride contrast agent microbubbles are almost as large as erythrocytes and therefore remain intravascular in the capillaries after injection. Sulfur hexafluoride contrast agent presents a high reflectivity, allowing the visualization of tissue microcirculation.10 The average in vivo half-life of these microbubbles is 12 minutes (range, 2–33 minutes).11 Sulfur hexafluoride contrast agent is not excreted by the kidneys. Therefore, it can be used in patients with renal dysfunction, when contrast agents for computed tomography (CT) or magnetic resonance imaging (MRI) are contraindicated. Sulfur hexafluoride contrast agent does not interact with other organ systems, apart from the lungs and the liver through which the microvesicles are extracted from the body within 10 to 15 minutes by exhaling12 and the phospholipid shell is metabolized by the liver. Furthermore, sulfur hexafluoride contrast agent does not deposit in the human body like the MRI contrast agent Gadolinium, which, after serial application, has been identified in human and animal brain, liver, skin and bone.13 In addition, sulfur hexafluoride contrast agent has a lower side-effect profile than the MRI contrast agent gadolinium.14
Contraindications for MRI like implanted cardiac pacemakers or defibrillator systems, insulin pumps, cochlear implants and metal splinters or vascular clips made of ferromagnetic material, tattoos with metal-containing pigments, non-removable piercings made of magnetic materials, allergic reactions or incompatibility to gadolinium and economic aspects (high expenditure of time, increasing costs) do not apply for CEUS examinations. Moreover, CEUS is easily accessible, quick to use, well tolerated by patients and allows a real-time diagnostic workup.15
Since 2012, musculoskeletal CEUS examinations were applied to assess microperfusion of tendon, muscle,16,17 as well as osseous perfusion of healing bone,18,19 infected non-unions and its surrounding tissue20,21 for various clinical issues. Among multiple interactions and relationships, a strong correlation could be demonstrated between CEUS-based muscle perfusion of the deltoid or supraspinatus muscle with shoulder function before and after surgery, potentially allowing an outcome prediction for eg tendon integration after rotator cuff repair by preoperative CEUS. Furthermore, CEUS-based quantification of osseous microperfusion allowed the identification of infections in fracture non-unions and is related to the healing process of long-bone fractures.
Considering perfusion as one of the most important parameters for muscle functionality and bone healing, our goal was to provide a more detailed, safety-focused profile on the diagnostic value of sulfur hexafluoride contrast agent application in CEUS examinations especially for musculoskeletal issues in trauma and orthopedic surgery. To our knowledge, this is the largest monocentric database of musculoskeletal CEUS examinations. This comprehensive database with more than 2200 contrast agent applications, including demographic data such as individual concomitant diseases (eg cardiac, pulmonary, renal, metabolic and drug-related concomitant diseases), risk factors and biometric data of each patient, allowed us to create a side-effect analysis with regard to concomitant diseases of individual patients (Table 1). Adverse events (AEs) caused by the contrast agent and complications during the application were also registered.
 |
Table 1 Overview of the Incidence of Comorbidities Categorized into Organ-Related and Drug-Associated Groups |
The aim of this study was to evaluate the safety profile of sulfur hexafluoride contrast agent associated with musculoskeletal indications in a clinical setting and to summarize CEUS as a potential diagnostic modality for manifold indications/applications.
Patients and Methods
Patient Population and Study Protocol
This monocentric register study is based on data of a DEGUM (German Society of Ultrasound in Medicine) accredited university ultrasound center affiliated with a center for trauma and orthopedic surgery. The study was conducted in accordance with the declaration of Helsinki in its current form, approved by the Ethics Committee Heidelberg (S-428/2017) and registered at the German Clinical Trials Register (DRKS00017406).
All individuals had participated in CEUS studies of the register, were informed about side effects (eg joint and flank pain), approved the respective study protocol and had given their written informed consent. Data of study patients who underwent musculoskeletal CEUS examinations with sulfur hexafluoride contrast agent between 2012 and 2019 were included. Register information included, among other data, patient characteristics (age, sex, BMI, smoker status, ASA score, indications for surgery), any complications during or after the examination as well as potentially existing individual concomitant diseases. All participants were informed that any study relevant data were stored and monitored using pseudonyms and will be published after the final analysis.
Exclusion criteria for the application of CEUS were a history of recent myocardial infarction, intracardial right-left shunt, uncontrollable fluctuations of blood pressure, severe respiratory disease (pulmonary arterial hypertension (PAH), severe acute respiratory syndrome (SARS)), pregnancy and breast-feeding (missing studies, no evidence) or any known allergic reaction to sulfur hexafluoride contrast agent as well as other similar contrast agents (product characteristics of contrast agent22). Patients younger than 18 years were excluded.
Considering the contraindications of sulfur hexafluoride contrast agents and the current state of scientific knowledge, patients were not exposed to any additional health damage or additional risk.15 Once a patient showed an adverse event to the contrast agent, he was excluded from the study.
All patients who received CEUS examination while participating in 1 of 13 musculoskeletal CEUS studies conducted at our institution since 2012 were primarily included in the study. An overview of the clinical-radiologic examination timeline with follow-up examinations of every included study is listed in Table 2.
 |
Table 2 Overview of the Clinical-Radiologic Examination Timeline with Follow-Up Appointments of All Studies |
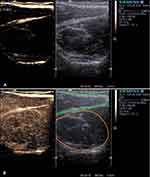
Muscular CEUS studies included perioperative perfusion assessment of the deltoid muscle in patients with cuff tear arthropathy or proximal humerus fractures, that were treated with reversed shoulder arthroplasty16 or PHILOS plating (proximal humeral internal locking system, DePuy Synthes®, Raynham, MA, U.S.).23 Another key aspect of muscular CEUS investigation has been the supraspinatus muscle microperfusion in patients with traumatic or degenerative supraspinatus tendon tear (Kunzet al 2019, data not yet published).17 Strong correlations could be demonstrated between pre- and postoperative muscle perfusion with shoulder function and the risk of postoperative retear17 (Figure 1). In studies involving the muscular system, the healthy contralateral sides were routinely examined as well to compare tissue perfusion. Patients, who were treated with a reversed shoulder arthroplasty, got an additional power Doppler examination to visualize the perfusion of the deltoid and teres minor muscle. The ultrasound examiner categorized the perfusion qualitatively from 1 (minimal perfusion) to 3 (maximum perfusion). A significantly weaker deltoid perfusion on the operated shoulder could be demonstrated.16
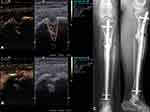
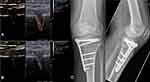
Regarding the detection of osseous microperfusion, patients with long-bone fractures, non-unions (Figure 2) as well as after high tibial open wedge osteotomy (Figure 3) in lower limb varus malalignment were examined to visualize and analyze the perfusion of fracture, non-union18–20 and osteotomy gaps. These studies intended to decode the process of bone regeneration with respect to the osseous microperfusion at different points of time and analyze the mechanisms of failed consolidation, especially in infected non-unions.18–20
CEUS Examinations and Evaluations
In general, a Siemens ACUSON S3000 (Siemens Healthineers, Erlangen, Germany) was used for all CEUS examinations. For the purpose of a device- and operator-dependence comparison study, additionally a Philips Epiq 7 (Philips Healthcare, Hamburg, Germany) was used.
All CEUS investigations were standardized for each conducted study and settings were adjusted according to the recommendations of the EFSUMB.6
The approved second-generation sulfur hexafluoride contrast agent was administered as a bolus via a peripheral venous catheter in the cubital vein of the patients´ forearm. The quantification of perfusion via disruption-replenishment kinetic loops with a constant venous infusion was not feasible for musculoskeletal issues and the measurement of quantitative and semiquantitative tissue parameters. It has been used only in cardiology so far,24 so that we chose to quantify via bolus administration.25 After the administration of sulfur hexafluoride contrast agent bolus, it always rinsed with 10 mL of 0.9% saline solution (NaCl). Subsequently, the CEUS examination in the Siemens-specific Cadence™ (Siemens Medical Solutions USA, Inc, Malvern, PA, USA) contrast pulse sequencing mode started at the relevant body region for the respective study. In each case, a 90– 120 seconds video was recorded in the dual view of contrast Cadence™ with conventional B-mode of the ultrasound device. Afterwards, the video clip was evaluated using the designated, commercially available VueBox® quantification software (Bracco Imaging, Milan, Italy) to provide quantitative information about muscle, tendon and osseous microperfusion.3,26
Application of Sulfur Hexafluoride Contrast Agent
The utilized sulfur hexafluoride contrast agent dose varied between the previously presented studies from 2.4 to 4.8 mL2,9 depending on the number of investigations and the issue (Table 3). For all ultrasound examinations in skeletal issues (non-union, osteotomies, fractures) the standard dose of 2.4 mL was established. In muscular applications, 2.4–4.8 mL per shoulder (4.8–9.6 mL in total) were applied at intervals of 15 minutes after complete elimination by the lungs,27 depending on whether the deltoid (reverse shoulder arthroplasty, proximal humerus fractures, device comparison) or supraspinatus (rotator cuff repair) muscle perfusion was investigated.
 |
Table 3 Overview of the Different Studies Divided into Muscular and Skeletal Applications with the Corresponding Consumption of Sulfur Hexafluoride Contrast Agent (SonoVue®) |
Repeated sulfur hexafluoride contrast agent injections were required in 65 out of 2268 CEUS examinations (2.9%) caused by increased movement during normal breathing excursions, coughing, sneezing, general agitation, lack of compliance or operating errors.26
Categorization of Adverse Events (AEs)
Only contrast agent-associated AEs were included in our analysis, whereas contrast agent-independent AEs and usual reactions at the injection site of the cubital vein (heat and pain or repositioning of the needle) were documented but not included in this evaluation.
Contrast agent-associated AEs were classified according to their degree of severity in either mild, moderate or severe:1
- Mild: No dis-/inability, no drug treatment/support
- Moderate: No dis-/inability, drug treatment/support
- Severe: (transient) dis-/inability, drug treatment/support.
Results
Patients
In 13 studies a total of 2268 musculoskeletal CEUS examinations during 1537 consultations were performed in 764 patients (Table 3) since 2012. Three hundred and thirty-six patients were women, 428 men; the mean age was 55.4 ± 17.5 years. For a better overview, we divided the studies into two groups – muscular and skeletal. Three hundred and twenty-eight (muscular, 171; skeletal, 157) patients were smokers or former smokers, 436 (muscular, 254; skeletal, 182) patients were non-smokers (Table 3).
Comorbidities of the study cohort included cardiovascular (arterial hyper-/hypotension, arrhythmia, coronary heart disease, myocardial infarction >6 months, cardiac insufficiency), pulmonary (bronchial asthma, obstructive sleep apnea syndrome (OSAS)), renal (renal insufficiency, hypokalemia), metabolic (NIDDM/IDDM, hyper-/hypothyroidism, dyslipidemia; non-specific hepatopathy) and drug-associated (penicillin, various antibiotics, contrast agents, iodine, analgesics, statins, local anaesthetics, muscle relaxants, Novalgin®) diseases.
Adverse Events
The total number of AEs in all studies – categorized as mild, moderate and severe – was three (Table 4). Every documented agent-associated AE took place within the first ten minutes after sulfur hexafluoride contrast agent administration during the CEUS examination and intervened if necessary.
 |
Table 4 Overview of Adverse Events (AEs) Divided into Mild, Moderate and Severe Depending on the Number of Patients, Mean Age and Contrast Agent Consumption of the Respective Studies |
One of these reactions to the contrast agent was severe, while the other two were mild (Table 5). This generally corresponds to a total rate of 0.13% (mild, moderate and severe AEs/examinations) for all reported AEs and a frequency rate of 0.04% for all severe AEs/examinations. In general, AEs occurred promptly and at first application. For these three AEs, there was no significant correlation between the AE and the mean volume of contrast agent per patient, the mean volume of contrast agent per consultations, as well as the seriousness of side effects (Table 4). Participants who did not show any adverse reactions at the first application of the contrast agent also did not do so in study-dependent multiple applications and at follow-up appointments.
 |
Table 5 Overview and Description of AE Cases |
Extravasation of sulfur hexafluoride contrast agent because of cannula malpositioning was strictly prevented and not reported.
Description of Mild AE Cases
Two AE cases (Case 1, 2) demonstrated a mild degree of severity and were self-limiting, so that patients did not require any medical support (Table 5). A direct association between the occurrence of side effects and administration of sulfur hexafluoride contrast agent was not evident, because they were similar to symptoms reported after intravenous injection of placebos.28 Typical mild side effects reported by Piscalgia et al1 include moderate hypotension, headache, dizziness, discomfort, nausea, vomiting, sensation of warmth and itching.
Case 1 reported from a 78-year-old woman who was scheduled for reverse shoulder arthroplasty (RSA) due to post-traumatic glenohumeral arthritis. The preoperative consultation revealed a medication-controlled hypertension and hypothyroidism as well as hyperlipoproteinemia. Case 2 was a 34-year-old athletic woman who underwent HTOWO due to varus malalignment of her leg. No concomitant diseases were known. Phenomena that were observed in both cases were combinations of mild symptoms dominated by the sensation of warmth, dizziness and discomfort (1 to 2 minutes after intravenous application). Case 1 additionally reported on nausea (5 to 10 minutes after intravenous administration) while the second patient complained of subsequent itching and headache (3 minutes after iv-administration) (Table 5).
Description of Moderate AE Cases
No moderate AEs could be observed during our CEUS examinations.
Description of Severe AE Cases
One of the three AE cases (Case 3) was classified as severe in a 53-year-old man with a tibial shaft fracture. The preoperative survey of the internal medicine council showed a medication-controlled hypertension and hyperlipoproteinemia as well as a myocardial infarction more than 6 months ago due to arteriosclerotic alteration in a coronary artery which was treated with a coronary stent.
Immediately (within 1 minute) after sulfur hexafluoride contrast agent application (2.4 mL), the patient reacted with an increased blood pressure, tachycardia and dyspnea. Sweating and nausea followed immediately afterwards. The patient’s reaction was initially rated as anaphylactic, so that prednisolone (250 mg) and dimetindene (4 mg) were administered intravenously. In addition, he received volume therapy with 500 mL Ringer’s solution. His condition, especially hypertension and dyspnea, stabilized after about 40 minutes without any further intervention (Table 5).
Discussion
In this safety analysis for the clinical application of sulfur hexafluoride contrast agent in musculoskeletal indications, the overall rate of reported AEs (mild, moderate and severe) was less than 0.13%. Related to all “severe AEs”, the reporting rate was less than 0.04%. Both results conform to the findings of Piscaglia and Tang and confirm our observations of a low incidence rate, similar to that of commonly used drugs such as analgetics or antibiotics29 and lower than those reported by contrast agents used for X-ray, CT or MRI (gadolinium, iodine; rate 0.33–12.66%).30,31 Moreover, we can confirm that the second-generation sulfur hexafluoride contrast agent has a very favorable side-effect profile when used in musculoskeletal applications and that musculoskeletal CEUS can be regarded as a safe examination tool.
Prior studies in internal medicine showed that contrast agents used for ultrasound diagnostics are associated with a low side-effect incidence of less than 0.01%.32,33 They are generally considered to be safe, very well tolerated and moreover, do not require further medical examination (with regard to renal or cardiac function) prior to their application.32,33 In 2006, Piscaglia et al retrospectively analyzed the safety of sulfur hexafluoride contrast agent in 23,188 cases in abdominal applications of 28 medical centers, the overall reporting rate of severe AEs was 0.0086%.1 In another study from 2017, Tang et al reported similar results on 30,222 cases, undergoing CEUS of abdominal (liver, gallbladder, pancreas, spleen, and kidney) and superficial (thyroid, breast, lymph nodes and prostate) organs with an overall incidence rate of severe AEs at 0.007%.11
The adverse events, which were monitored in our study patients during or immediately following CEUS, have been similarly described in other studies and are included in the sulfur hexafluoride contrast agent product insert. Typical mild side effects, as described in the sulfur hexafluoride contrast agent product insert or in our and former studies, were also observed after controlled randomized intravenous injection of placebos,4,28 including moderate hypotension, headache, discomfort, dizziness, nausea, vomiting, sensation of warmth and itching. Some of these AEs (including the two mild AEs) – such as nausea, discomfort, dizziness, hypotension, sensation of warmth – reported in our analysis may be due to a vasovagal reaction. Similar symptoms usually occurred previously and thus independently of the sulfur hexafluoride contrast agent application. This has most commonly been associated with the placement of intravenous access and pre- or postoperative pain.
In contrast, one patient showed symptoms of an anaphylactic-anaphylactoid reaction with tachycardia, hypertension and dyspnea. Of note, all three AEs took place within minutes after contrast agent administration. Among the three cases of AEs, two had similar concomitant diseases, including hypertension and metabolic disorder. But an immediate relation between the AEs and the patients´ clinical risk profiles could not be identified.
Although in our analysis AEs were only observed at initial application, Piscaglia et al described one AE after the fourth injection of sulfur hexafluoride contrast agent. This implies, in general, that the lack of any reaction during previous sulfur hexafluoride contrast agent administrations does not exclude the possibility of any reactions later in the follow-up examinations. Therefore, attention should be spent on possible later reactions in repeated CEUS examinations. For patients with similar risk profiles, either an extended observation about 30 minutes after CEUS examination should be performed, or these patients should not undergo a CEUS examination with sulfur hexafluoride contrast agent. If CEUS is indeed necessary, an efficient emergency protocol is needed for a timely treatment of patients with severe AEs. If discomfort occurs while contrast agent is injected, the injection should immediately be stopped.
This study was not designed to detect all possible reactions. Specific risks, such as symptoms or changes in electrocardiogram as well as changes in blood pressure or oxygen saturation were not routinely investigated during the examination. Possible microvascular effects and interactions of CEUS with the microbubbles were also out of range. A prospective analysis with a previously well-defined establishment of all individual risk factors of every patient would have been more accurate in classifying and detailing events, as well as estimating the risk of AEs caused by sulfur hexafluoride contrast agent.
Possible reactions with delayed occurrence (30–60 minutes after the examination and application of sulfur hexafluoride contrast agent) or after the patient’s discharge might have been missed, especially if patients did not consider it necessary to inform about afterwards or at subsequent follow-up appointments. Because of the retrospective design, patients with the delayed occurrence of reactions were not questioned for other possible causes (eg concurrent medication, food and physical contact). In contrast to that, it is very unlikely that any severe adverse event had been missed.
Strengths of this study include precisely defined study protocols with exactly defined sulfur hexafluoride contrast agent applications, the high number of exclusively musculoskeletal examinations and the fact that all CEUS examinations were supervised by the same DEGUM level III approved orthopedic and trauma surgeon. Another advantage is the monocentric design, which ensures high quality and awareness for possible reactions after sulfur hexafluoride contrast agent administration. These combinations provide valid data and minimal bias.
In musculoskeletal applications, CEUS represents a complementary investigative approach, which offers certain advantages in comparison to conventionally used diagnostic instruments such as DCE-MRI or DCE-CT.34 Contrary to MRI, CT and X-ray examinations, the cost-effective CEUS can be safely used in patients with renal impairment, without ionizing radiation or deposition of contrast agents in human body and fear of claustrophobia.15,35-37
Considering, among other things, the resolution and frame rate of CEUS, whose detail accuracy is superior to conventional MRI, CEUS may soon be able to support other time-consuming and cost-intensive medical investigations for various indications.36
Conclusions
This retrospective analysis showed that CEUS with sulfur hexafluoride contrast agent has a satisfying safety profile in musculoskeletal applications with less than 0.13% adverse events. In comparison, the rate of AEs of contrast agents used in radiologic examinations, CT and MRI (gadolinium, iodine) are higher or equal to that of the second-generation ultrasound sulfur hexafluoride contrast agent even in patients with higher age or multiple concomitant diseases.
Abbreviations
AE, adverse events; ASA score, American Society of Anesthesiologists score; BMI, body mass index; CEUS, contrast-enhanced Ultrasound; CT, computed tomography; DCE, dynamic contrast-enhanced; DEGUM, Deutsche Gesellschaft für Ultraschall in der Medizin; eg, exempli gratia; et al, et alii; HTOWO, high tibial open wedge osteotomy; RSA, reverse shoulder arthroplasty; MRI, magnetic resonance imaging; NIDDM/IDDM (non-)insulin-dependent diabetes mellitus; OSAS, obstructive sleep apnea syndrome; PAH, pulmonary arterial hypertension; SARS, severe acute respiratory syndrome.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors have revealed all financial and personal relationships to other persons and organizations that could inappropriately influence (bias) this work.
We acknowledge the financial support of the Ruprecht-Karls-University of Heidelberg within the funding program Open Access Publishing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Piscaglia F, Bolondi L; Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32(9):1369–1375. doi:10.1016/j.ultrasmedbio.2006.05.031
2. Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60(3):324–330. doi:10.1016/j.ejrad.2006.06.022
3. Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17(8):1995–2008. doi:10.1007/s00330-007-0623-0
4. Celli N, Gaiani S, Piscaglia F, et al. Characterization of liver lesions by real-time contrast-enhanced ultrasonography. Eur J Gastroenterol Hepatol. 2007;19(1):3–14. doi:10.1097/01.meg.0000250585.53608.3c
5. Rettenbacher T. Focal liver lesions: role of contrast-enhanced ultrasound. Eur J Radiol. 2007;64(2):173–182. doi:10.1016/j.ejrad.2007.07.026
6. Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: update 2017 (Long Version). Ultraschall in der Medizin - Eur J Ultrasound. 2018;39(2):e2–e44. doi:10.1055/a-0586-1107
7. Adler RS, Johnson KM, Fealy S, et al. Contrast-enhanced sonographic characterization of the vascularity of the repaired rotator cuff: utility of maximum intensity projection imaging. J Ultrasound Med. 2011;30(8):1103–1109. doi:10.7863/jum.2011.30.8.1103
8. Mayevsky A, Manor T, Pevzner E, et al. Tissue spectroscope: a novel in vivo approach to real time monitoring of tissue vitality. J Biomed Opt. 2004;9(5):1028–1045. doi:10.1117/1.1780543
9. Bokor D. Diagnostic efficacy of SonoVue. Am J Cardiol. 2000;86(4):19G–24G. doi:10.1016/S0002-9149(00)00985-1
10. Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography. 2018;37(1):25–35. doi:10.14366/usg.17037
11. Tang C, Fang K, Guo Y, et al. Safety of sulfur hexafluoride microbubbles in sonography of abdominal and superficial organs: retrospective analysis of 30,222 cases. J Ultrasound Med. 2017;36(3):531–538. doi:10.7863/ultra.15.11075
12. Greis C. Ultrasound contrast agents as markers of vascularity and microcirculation. Clin Hemorheol Microcirc. 2009;43(1–2):1–9. doi:10.3233/CH-2009-1216
13. Guo BJ, Yang ZL, Zhang LJ. Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci. 2018;11:335. doi:10.3389/fnmol.2018.00335
14. Forghani R. Adverse effects of gadolinium-based contrast agents: changes in practice patterns. Top Magn Reson Imaging. 2016;25(4):163–169. doi:10.1097/RMR.0000000000000095
15. Chong WK, Papadopoulou V, Dayton PA. Imaging with ultrasound contrast agents: current status and future. Abdom Radiol (NY). 2018;43(4):762–772. doi:10.1007/s00261-018-1516-1
16. Fischer C, Krammer D, Hug A, et al. Dynamic contrast-enhanced ultrasound and elastography assess deltoid muscle integrity after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(1):108–117. doi:10.1016/j.jse.2016.04.012
17. Fischer C, Gross S, Zeifang F, Schmidmaier G, Weber MA, Kunz P. Contrast-enhanced ultrasound determines supraspinatus muscle atrophy after cuff repair and correlates to functional shoulder outcome. Am J Sports Med. 2018;46(11):2735–2742. doi:10.1177/0363546518787266
18. Fischer C, Haug T, Weber MA, Kauczor HU, Bruckner T, Schmidmaier G. Contrast-enhanced ultrasound (CEUS) identifies perfusion differences between tibial fracture unions and non-unions. Ultraschall in der Medizin. 2018.
19. Krammer D, Schmidmaier G, Weber MA, Doll J, Rehnitz C, Fischer C. Contrast-enhanced ultrasound quantifies the perfusion within tibial non-unions and predicts the outcome of revision surgery. Ultrasound Med Biol. 2018;44(8):1853–1859. doi:10.1016/j.ultrasmedbio.2018.04.013
20. Fischer C, Preuss EM, Tanner M, et al. Dynamic contrast-enhanced sonography and dynamic contrast-enhanced magnetic resonance imaging for preoperative diagnosis of infected nonunions. J Ultrasound Med. 2016;35(5):933–942. doi:10.7863/ultra.15.06107
21. Doll J, Gross S, Weber MA, Schmidmaier G, Fischer C. The AMANDUS Project-advanced microperfusion assessed non-union diagnostics with contrast-enhanced ultrasound (CEUS) for the detection of infected lower extremity non-unions. Ultrasound Med Biol. 2019;45(9):2281–2288. doi:10.1016/j.ultrasmedbio.2019.05.007
22. Geleijnse ML, Nemes A, Vletter WB, et al. Adverse reactions after the use of sulphur hexafluoride (SonoVue) echo contrast agent. J Cardiovasc Med (Hagerstown). 2009;10(1):75–77. doi:10.2459/JCM.0b013e328319bfba
23. Fischer C, Frank M, Kunz P, et al. Dynamic contrast-enhanced ultrasound (CEUS) after open and minimally invasive locked plating of proximal humerus fractures. Injury. 2016;47(8):1725–1731. doi:10.1016/j.injury.2016.05.005
24. Jirik R, Nylund K, Gilja OH, et al. Ultrasound perfusion analysis combining bolus-tracking and burst-replenishment. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60(2):310–319. doi:10.1109/TUFFC.2013.2567
25. Hudson JM, Karshafian R, Burns PN. Quantification of flow using ultrasound and microbubbles: a disruption replenishment model based on physical principles. Ultrasound Med Biol. 2009;35(12):2007–2020. doi:10.1016/j.ultrasmedbio.2009.06.1102
26. Greis C. Technical aspects of contrast-enhanced ultrasound (CEUS) examinations: tips and tricks. Clin Hemorheol Microcirc. 2014;58(1):89–95. doi:10.3233/CH-141873
27. Morel DR, Schwieger I, Hohn L, et al. Human pharmacokinetics and safety evaluation of SonoVue, a new contrast agent for ultrasound imaging. Invest Radiol. 2000;35(1):80–85. doi:10.1097/00004424-200001000-00009
28. Nanda NC, Wistran DC, Karlsberg RP, et al. Multicenter evaluation of SonoVue for improved endocardial border delineation. Echocardiography. 2002;19(1):27–36. doi:10.1046/j.1540-8175.2002.00027.x
29. Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology. Arch Intern Med. 2001;161(1):15–21. doi:10.1001/archinte.161.1.15
30. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese committee on the safety of contrast media. Radiology. 1990;175(3):621–628. doi:10.1148/radiology.175.3.2343107
31. Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011;196(2):W138–143. doi:10.2214/AJR.10.4885
32. Jakobsen JA, Oyen R, Thomsen HS, Morcos SK. Safety of ultrasound contrast agents. Eur Radiol. 2005;15(5):941–945. doi:10.1007/s00330-004-2601-0
33. Dijkmans PA, Visser CA, Kamp O. Adverse reactions to ultrasound contrast agents: is the risk worth the benefit? Eur J Echocardiogr. 2005;6(5):363–366. doi:10.1016/j.euje.2005.02.003
34. Torzilli G. Adverse effects associated with SonoVue use. Expert Opin Drug Saf. 2005;4(3):399–401. doi:10.1517/14740338.4.3.399
35. Gulati M, King KG, Gill IS, Pham V, Grant E, Duddalwar VA. Contrast-enhanced ultrasound (CEUS) of cystic and solid renal lesions: a review. Abdom Imaging. 2015;40(6):1982–1996. doi:10.1007/s00261-015-0348-5
36. Ranganath PG, Robbin ML, Back SJ, Grant EG, Fetzer DT. Practical advantages of contrast-enhanced ultrasound in abdominopelvic radiology. Abdom Radiol (NY). 2018;43(4):998–1012. doi:10.1007/s00261-017-1442-7
37. Choi JW, Moon WJ. Gadolinium Deposition in the Brain: current updates. Korean J Radiol. 2019;20(1):134–147. doi:10.3348/kjr.2018.0356
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.