Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Safety and Efficacy of Subcutaneous Daratumumab in Systemic AL Amyloidosis
Authors Hughes MS, Lentzsch S
Received 1 September 2023
Accepted for publication 4 December 2023
Published 28 December 2023 Volume 2023:19 Pages 1063—1074
DOI https://doi.org/10.2147/TCRM.S325859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Michael Sang Hughes, Suzanne Lentzsch
Department of Hematology-Oncology, Columbia University Irving Medical Center, New York, NY, USA
Correspondence: Suzanne Lentzsch, Department of Hematology-Oncology, Columbia University Irving Medical Center, MH-6GN 435, 161 Fort Washington Ave, New York, NY, 10032, USA, Tel +1 212-305-5098, Email [email protected]
Introduction: Systemic AL amyloidosis, a plasma cell dyscrasia, is characterized by the production of misfolded immunoglobulin light chain. These misfolded proteins aggregate into amyloid fibrils and deposit throughout the body, resulting in widespread organ dysfunction and ultimately death. Achieving rapid and maximal elimination of the plasma cell clone is crucial to long-term survival. Daratumumab, an anti-CD38 monoclonal antibody delivered intravenously, has been swiftly incorporated into standard first-line treatment regimens. A novel formulation of daratumumab has been developed that can be injected subcutaneously.
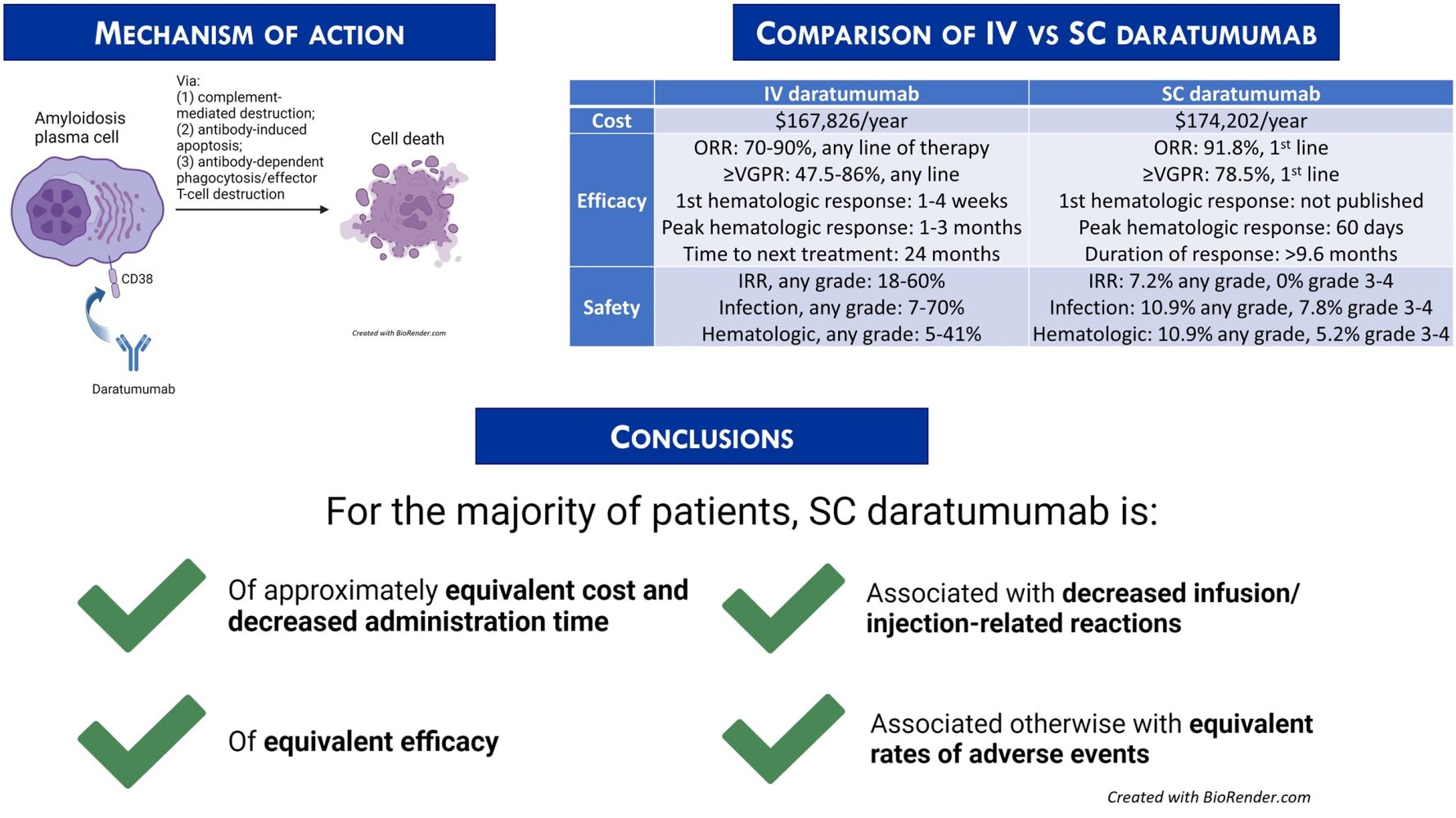
Areas Covered: As a retrospective qualitative review of prior publications involving daratumumab, this work briefly summarizes the existing data regarding the safety and efficacy of subcutaneous (SC) daratumumab, compared to intravenous (IV) daratumumab. SC daratumumab appears to deliver the same disease benefit as IV daratumumab to patients with decreased infusion-related reactions (IRRs), decreased time for administration, and similar rates of adverse events (AEs) intrinsically related to daratumumab.
Expert Opinion: SC daratumumab is preferred over IV daratumumab, but the clinical situation ultimately should determine route of administration. Further investigation into cost-effectiveness benefit is warranted.
Keywords: plasma cell dyscrasia, daratumumab, AL amyloidosis, adverse events, AE
Graphical Abstract:
Introduction
Systemic immunoglobulin light chain (AL) amyloidosis, classified as a plasma cell dyscrasia, is comprised of a typically small clonal expansion of CD38+ plasma cells that, due to somatic mutations in the variable light chain (VL) gene, produces and releases into the extracellular compartment significant amounts of monoclonal immunoglobulin light chains (LC) that misfold and form amyloidosis.1–4 It is unknown why such misfolded, kinetically unstable LC are released by the plasma cell. Studies of the molecular stress induced by these abnormal LC, however, suggest that cellular quality control is simply overwhelmed.5,6
The misfolded LC circulate in the extracellular compartment in a soluble globular configuration.7 Via a mechanism that has yet to be elucidated fully, at a critical concentration these LC undergo further conformational change and arrange into β-pleated sheets.3 Amyloid oligomers quickly form and provide nucleation sites for further aggregation. Fibrils precipitate out of the peripheral blood and deposit into tissues, with mild-moderate specificity for organs depending on VL amino acid sequence.2,8 These fibrils, upon Congo red staining of specimen, are visible under polarized light microscopy with apple-green birefringence and form the basis for diagnosis of AL amyloidosis. Amyloid fibril deposition is currently thought to be the primary driver of cellular damage and organ dysfunction, via direct replacement of tissue parenchyma as well as direct cytotoxicity.9
Though the incidence of AL amyloidosis has been estimated at 8–12 cases per 106 individuals per year, with approximately 4000 per year in the United States, it is the most common amyloidosis subtype and continues to be underdiagnosed.10,11 Clinical manifestations arising from such a widespread pathophysiologic process are protean, and may lead to diagnostic dilemmas.11,12 Signs and symptoms of AL amyloidosis can be generally categorized into neuropathy, cardiomyopathy, malabsorption, renal insufficiency, and rarely coagulopathy. Dysautonomia and/or enteric nervous system neuropathy are frequent presentations of disease; so too is inexorably progressive diastolic heart failure with a classically thickened interventricular septum. Gastrointestinal amyloid deposition, depending on location, can result in malnourishment in critical macro- or micronutrients and may result in repetitive hemorrhage. Renal amyloid burden can result in severe tubular dysfunction and nephrotic-range proteinuria. Amyloid deposition in vascular endothelium increases vessel fragility, and amyloid-related interference in the conversion of factor X to activated factor X is well described. Without intervention, the disease is invariably deadly: 5 year overall survival in the modern day is estimated at 35%, and patients with any cardiac involvement have a median OS of 2.6 years.13
The current therapeutic approach for AL amyloidosis rests on two primary assessments: the status of the plasma cell clone, and the pre-existing amyloid organ burden. At this time, there are no FDA-approved treatments to reduce amyloid burden. Two engineered monoclonal antibodies have proceeded through early-phase clinical trials14,15 and are in Phase 3 trials (birtamimab: NCT04973137; anselamimab: NCT04504825, NCT04512235). Treatments for AL amyloidosis have historically attempted to eliminate the PC clone as rapidly as possible, thus minimizing further fibril aggregation. Regimens at first consisted of melphalan and prednisone.16 Autologous stem cell transplant (ASCT) was integrated into the therapeutic approach soon thereafter, with confirmed long-term survival benefit.17 Proteasome inhibitors were introduced in 2009 and increased survival.18 Nevertheless, rates of at least very good partial response (VGPR) remained at approximately 50% after first-line cyclophosphamide, bortezomib, and dexamethasone.19 One-third of those who achieve hematologic complete response also ultimately relapse.20
The introduction of the anti-CD38 monoclonal antibody daratumumab revolutionized the treatment landscape for AL amyloidosis and plasma cell dyscrasias more generally (Figure 1). Two formulations have been developed: intravenous (IV) and subcutaneous (SC). We herein provide a focused review of the safety and efficacy of SC daratumumab compared to IV daratumumab. Given the recent advent of the SC formulation and thus the relatively sparse data available, we describe the use of SC daratumumab in both myeloma and AL amyloidosis.
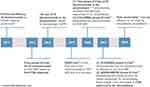 |
Figure 1 Summary timeline of major events involving daratumumab. |
Daratumumab in Plasma Cell Dyscrasias
CD38 is a 48 kDa transmembrane glycoprotein with numerous adhesion and signaling functions. It is expressed highly and uniformly on the surface of plasma cells, and is not typically expressed at such levels on other cells; it has thus been a prime target for directed therapies.21,22 Daratumumab is an IgG1-kappa fully humanized monoclonal antibody that was initially administered intravenously. It binds to CD38 and exerts its effect via three primary mechanisms: (1) Fcγ receptor-mediated crosslinking, which induces programmed cell death through both antibody-dependent andindependent mechanisms; (2) complement-dependent cytotoxicity; and (3) antibody-dependent cytotoxicity or phagocytosis.23–29 While daratumumab has been primarily deployed in plasma cell dyscrasias, it may have therapeutic benefit in multiple other settings. There have been reports of efficacy in relapsed/refractory T-cell acute lymphoblastic leukemia and in the management of advanced B-cell malignancies.30,31 Daratumumab may also be of clinical benefit in the non-malignant space: it has been considered for a role in desensitization protocols in patients with significant HLA presensitization prior to solid organ transplantation; refractory Sjögren’s disease; and immune-mediated thrombocytopenia.32–34
Recently, a formulation of daratumumab containing recombinant hyaluronidase in solution was developed. The hyaluronidase depolymerizes hyaluronan. Hyaluronan depolymerization locally and transiently increases permeability of the extracellular matrix that comprises human SC tissue. This allows for SC injected daratumumab to slowly and safely achieve peak and trough peripheral blood levels similar to an IV daratumumab infusion.35
Intravenous Daratumumab
Administration and Cost
Standard dosing of IV daratumumab consists of 16mg/kg infused into a peripheral vein weekly for eight doses, then every two weeks for doses 9–16, then every month thereafter. Initial infusion is recommended to last seven hours; second infusion lasts four hours; and subsequent infusions last 3.25–3.5 hours.23 Cost is $7057 for the drug alone per infusion in 2020 US dollars.36 Estimated total cost of administering IV daratumumab over 52 weeks, with 23 expected infusions, is US $167,826.37 For context, the mean cost of cyclophosphamide per 100mg infusion in 2023 US dollars is $17.942, for an estimated total cost per 6 regimen cycles at dosing 300mg/m2 in the average 1.91m2 individual of $1,291.82.38 The mean cost of bortezomib per 0.1mg injection in 2023 US dollars is $2.289, for an estimated total cost per 6 regimen cycles at dosing 1.3mg/m2 in the average 1.91m2 individual of $1364.06.39 Financial assistance programs are available via the pharmaceutical manufacturer Janssen and charitable organizations.
Efficacy
IV daratumumab was initially deployed in multiple myeloma, demonstrating encouraging overall response and survival benefit in a Phase I/II trial reported in 2015.21 Daratumumab was granted initial FDA approval in 2015, and swiftly moved into first-line therapy in multiple combinations for both ASCT-eligible and -ineligible patients.23,40–44
IV daratumumab was applied in systemic AL amyloidosis soon after its introduction into the myeloma therapeutic space. The Mayo group reported the first-in-human use for AL amyloidosis in 2016.45 One patient with multiply refractory Mayo 2012 stage IIIb AL amyloidosis suffering from amyloid-related rectal hemorrhage and secondary transfusion-dependent anemia achieved for the first time hematologic VGPR and within months thereafter saw the resolution of his transfusion dependence. A second patient with AL amyloidosis with primarily renal involvement received daratumumab after his disease progressed post-ASCT and CyBorD: he achieved hematologic complete remission within three months. The Stanford group reported the impact of daratumumab in a retrospective cohort of 25 heavily pretreated AL amyloidosis patients soon thereafter.46 Overall response rate (ORR) was 76%, with 60% achieving at least VGPR. Median time to maximum response was one month. Only two of 25 patients experienced hematologic progression during the study duration; median follow-up time was not reported.
Two simultaneous Phase II trials conducted soon after the publication of such retrospective data confirmed the striking response rates and impressive time to maximal response associated with IV daratumumab. The Boston University Amyloidosis Center reported an ORR of 90%, with 86% achieving at least VGPR; median time to first response was four weeks, and median time to deepest response was three months.47 Only three of 22 patients at time of publication progressed, at a median of 3 months from daratumumab initiation. The French AL amyloidosis network reported ORR of 70%, with at least VGPR in 47.5%.48 Reported median time to any response was one week, with most patients achieving their deepest responses within three months. Organ response after hematologic response was confirmed in both studies, with a delay between hematologic complete response and first organ response consistent with prior findings.49
Safety
Severe adverse events (AEs) attributable to IV daratumumab occur in a small but nontrivial proportion of both multiple myeloma and AL amyloidosis patient populations. In the multicenter phase II SIRIUS trial investigating daratumumab monotherapy in multiple myeloma, anemia, thrombocytopenia, and neutropenia occurred at any Common Terminology Criteria for Adverse Events (CTCAE) grade in 33%, 25%, and 23% of patients, respectively.50 Grade 3–4 anemia was noted at an incidence of 24%, thrombocytopenia 19%, and neutropenia 12%. Nonsevere (grade 1–2) fatigue (40%) and nausea (29%) were common. Five percent of patients experienced a grade 3 infusion-related reaction (IRR); 42% of patients experienced an IRR of any grade. The IRRs were characterized by sinopulmonary symptoms, bodily chills, and vomiting. IV daratumumab-associated AEs in subsequent Phase III studies largely recapitulated AEs seen in the SIRIUS trial. Of note, however, the representative CASSIOPEIA trial40 reported an any-grade sinopulmonary infection incidence of up to 38%, with 4% grade 3–4 pneumonia. Despite pre- and post-infusion supportive medications, IV daratumumab infusions were delayed or simply skipped in nine of 440 (2%) patients due to IRRs, and they were interrupted in 93 of 440 (21%) for a median of 3.75 months due to the same. In pooled analysis, IRRs occurred in 37% of patients upon initial administration of IV daratumumab.23 Daratumumab-induced interference with standard indirect antiglobulin testing was noted in the majority of patients throughout these trials. Immunogenicity has been observed in less than 1% of the population.23
Reported IV daratumumab-associated AE incidence remained much the same in the AL amyloidosis sphere (Table 1). The overwhelming majority of studies investigating daratumumab in AL amyloidosis employed the IV formulation, as they were mostly conducted prior to the development and use of SC daratumumab. We detail studies of particular interest and seminal prospective studies below.
While no patients required adjustment of diuretic dosing for infusion-related heart failure events in the Stanford group’s initial retrospective study, 15 of 25 patients (60%) experienced grade 1–2 IRRs after initial administration of IV daratumumab.46 Two of 25 were hospitalized for infections, type and grade unspecified. One patient required at least one transfusion of red blood cells. There were no cases of leukopenia or thrombocytopenia. Overall, four of 25 patients (16%) required daratumumab discontinuation due to assorted grade 3–4 AEs: pneumothorax, decompensated heart failure, and infections.
The Boston University phase II trial found an 18% grade 1–2 IRR incidence; an 18% grade 3–4 respiratory infection rate; and a 9% incidence of grade 1–2 anemia.47 Ten of 22 patients (45%) ultimately required premature discontinuation of IV daratumumab due to varied grade 3–4 AEs. The French AL amyloidosis network phase II trial reported an IRR incidence of 42.5%, with nine grade 1–2 and 3 (cutaneous rash) grade 3–4.48 Twenty-eight of 40 patients (70%) experienced infection of any grade, with 22 suffering bronchitis and nine suffering pneumonia. Five percent of patients had anemia of any grade. No patients discontinued treatment due to AEs; data on delays or interruptions in treatment were not published. No adverse effects lasted significantly past discontinuation of daratumumab for any reason. Daratumumab does not appear to interfere with metabolism of drugs via the CYP system. Preclinical studies suggest that daratumumab may depress fetal immunity and decrease fetal bone density.23,35
Subcutaneous Daratumumab (Daratumumab-Hyaluronidase)
Administration and Cost
Daratumumab-hyaluronidase is FDA- and EMA-approved for injection into the SC tissue of the abdomen weekly for eight doses, then every two weeks for doses 9–16, then every month thereafter, in combination with cyclophosphamide, bortezomib, and dexamethasone.35,51 The FDA indication does not include Mayo stage IIIB disease at this time. Median duration of administration was five minutes per injection in a recent study.52 Projected cost is US $7574 (2020), and US $174,202 for 23 projected administrations over a 52 week period.53 Of note, SC edema in patients with heart failure can theoretically impair dispersal of drug; to our knowledge, there are no published data examining this phenomenon. As with the IV formulation, financial assistance programs are available via the pharmaceutical manufacturer and charitable organizations.
Efficacy
The PAVO trial was the first instance of prospectively studied single-agent SC daratumumab administration in humans.54 Fifty-three patients with relapsed/refractory multiple myeloma in total received the drug, 45 at an 1800mg dosage irrespective of weight. ORR was 42.2% for those treated with 1800mg per dose, and the pharmacokinetic profile of 1800mg SC daratumumab recapitulated that of IV daratumumab.
The COLUMBA noninferiority trial established SC daratumumab as a viable therapeutic option in relapsed/refractory multiple myeloma.55 Five hundred twenty-two patients with multiply relapsed/refractory multiple myeloma accrued from 10/2017 to 12/2018 were divided into SC (n = 263) and IV (n = 259) open-label treatment arms. Patients who had been previously exposed to anti-CD38 agents; undergone recent ASCT; had meningeal involvement; significant obstructive lung disease; or significant cardiac disease were excluded. Patients received a median of six cycles in each group. ORR was 41% for SC daratumumab and 37% for IV daratumumab, meeting noninferiority thresholds. There were no differences between SC and IV daratumumab efficacy in subgroup analysis, or among differing body weights. SC daratumumab by pharmacokinetic analysis reached a trough dose equivalent to IV daratumumab, qualifying as adequate exposure. At median follow-up of 7.5 months, progression of disease had been observed in 51% of patients in each arm. Median progression-free survival was 5.6 months for SC daratumumab vs. 6.1 months for IV daratumumab; median time to next treatment was 9.7 months for SC daratumumab and 8.7 months for IV daratumumab. Median duration of response had not been reached in either arm; median overall survival had not been reached in either arm. SC daratumumab was thus concluded to be at least noninferior to IV daratumumab in the relapsed/refractory multiple myeloma setting.
The open-label, international phase II PLEIADES study investigated the effect of SC daratumumab in multiple combinations across first- and second-line therapy, and constitutes the body of prospective evidence thus far for SC daratumumab use in newly diagnosed multiple myeloma.52 199 patients were assigned to three treatment arms: daratumumab, bortezomib, lenalidomide, and dexamethasone (D-VRd) in newly diagnosed transplant-eligible multiple myeloma; daratumumab, bortezomib, melphalan, and prednisone (D-VMP) in newly diagnosed transplant-ineligible myeloma; and daratumumab, lenalidomide, and dexamethasone (D-Rd) as second-line therapy for relapsed/refractory disease. High-risk cytogenetic disease was appropriately represented in the study population. ORR for the D-VRd arm at 3.9 months median follow-up was 97%, with 71.6% achieving at least VGPR and 11.6% achieving CR. ORR for the D-VMP arm at 14.3 months median follow-up was 89.6%, with 77.6% achieving at least VGPR and 47.8% achieving CR. ORR for the D-Rd arm at 14.7 months median follow-up was 93.8%, with 78.5% achieving at least VGPR and 38.5% achieving CR. The results are equivalent to previously published results from trials of IV daratumumab-containing regimens in newly diagnosed multiple myeloma.
Based on the encouraging data from multiple myeloma, the phase III ANDROMEDA trial included SC daratumumab in the upfront treatment of AL amyloidosis.56 From May 2018 to August 2019, 388 patients with newly diagnosed AL amyloidosis were randomized in a 1:1 ratio to a control arm (n = 193) receiving six cycles of cyclophosphamide, bortezomib, and dexamethasone (VCd), or to an experimental arm (n = 195) receiving six cycles of SC daratumumab with VCd and 18 injections of SC daratumumab maintenance thereafter. The study was not blinded. Notably, patients with Mayo stage IIIB cardiac AL amyloidosis were excluded from the study. Multiorgan dysfunction was present in the majority of patients. At a median follow-up of 11.4 months, 91.8% of patients achieved an overall response. VGPR was acheived by 78.5% of patients, and 53.3% achieved CR. Almost three quarters (70.5%) of patients achieved a difference in free light chain levels less than 20mg/L. Median time on treatment was 9.6 months, but median duration of response was not reached at the time of presentation. Time to best hematologic response was 60 days. Hazard ratio for major organ deterioration, hematologic progression, or death was 0.58 (95% CI: 0.36–0.93), favoring SC daratumumab-VCd. Time to next treatment was significantly prolonged as well (HR 0.39, 95% CI 0.27–0.56). Overall survival data were immature at time of publication. Only 9.8% of patients were eligible for ASCT; there was no noticeable difference in overall survival post-transplant. A notable proportion of patients who achieved hematologic complete remission with SC daratumumab-VCd ultimately exhibited cardiac response (41.5%) and renal response (53%) at six months. These findings suggest that SC daratumumab is at least as effective as IV daratumumab in newly diagnosed AL amyloidosis, though there has been no head-to-head trial.
Real world data on SC daratumumab in AL amyloidosis remain sparse, due to the recent introduction of the SC formulation into the treatment space. A recent retrospective cohort study of 22 patients with Mayo IIIB AL amyloidosis treated with SC daratumumab is, at the time of this review’s writing, the sole instance of clear and validated SC daratumumab deployment in a non-trial setting which investigated survival outcomes.57 Patients achieved hematologic at least VGPR at a rate of 88.2% in one month, 93.8% at three months, and 86.7% at six months from initiation of treatment. Sixty-three percent of evaluable patients reached an involved free light chain level below 20mg/L and a difference in free light chain levels below 10mg/L at three months from treatment initiation. Though further study is needed, such results suggest that SC daratumumab achieves efficacy comparable to rates reported in ANDROMEDA in an expanded AL amyloidosis population. Prospective investigations of SC daratumumab efficacy in Mayo 2004 stage IIIB patients are currently underway.
Patients treated with either SC or IV daratumumab may ultimately become refractory to treatment; a small minority do not respond at all. Data regarding potential mechanisms of delayed or upfront resistance are sparse in AL amyloidosis and remain vexed in the multiple myeloma space. In multiple myeloma, the recent discovery of XBP1 loss driving decreased CD38 expression suggests simple decreased target antigen expression as a pathway for tumor escape from daratumumab.58 A separate molecular study of a combined cohort of multiple myeloma and AL amyloidosis patients found that upfront resistance did not, however, depend on CD38 expression levels.59 Evidence otherwise exists to suggest that cytogenetics and the inflammatory profile of the bone marrow milieu play key roles in mediating anti-CD38 agent resistance.58,60 Research continues regarding mechanisms of daratumumab resistance in AL amyloidosis specifically.
Safety
AEs associated with SC daratumumab in large part mirror those seen with IV daratumumab, with one notable exception in later-phase investigations: the rate of IRRs.
SC daratumumab-related AEs reported in the PAVO study in relapsed/refractory multiple myeloma included anemia at 33%, upper respiratory tract infection (27%), pyrexia (27%), and diarrhea (27%).54 Neutropenia occurred at 15.6% incidence in the 1800mg dosing group. Seven of 45 (15.6%) experienced grade 3–4 anemia; 6.7% experienced grade 3–4 neutropenia and thrombocytopenia. Two patients experienced grade 3 influenza infections. Eleven of 45 patients (24.4%) experienced IRRs; all were grade 1–2 and occurred with the first administration of drug. No patients discontinued therapy due to AE. Pre- or post-administration medication regimens were not discussed.
SC daratumumab was shown to be at least equally well-tolerated compared to IV daratumumab in patients with relapsed/refractory multiple myeloma by the COLUMBA noninferiority trial.55 IRR rate was significantly decreased for SC (33 of 260 patients, 13%) vs. IV daratumumab (89 of 258 patients, 34%; OR 0.28 95% CI 0.18–0.44). Most IRRs occurred after the first dose: one patient in the SC arm and two in the IV arm had at least one delayed-onset IRR, all grade 1–2. Grade 3 IRRs occurred in only four patients due to SC daratumumab but 14 patients due to IV daratumumab; there were no higher-grade reactions. IRRs manifested as chills, pyrexia, and dyspnea. Median time to onset of IRR was 3.4 hours for the SC daratumumab group, significantly longer than that for the IV daratumumab group (1.5 hours). IRRs in IV daratumumab interrupted dosing for 79 of 258 patients (31%) and led to treatment discontinuation in two patients. There were no dosing interruptions or discontinuations associated with IRRs in the SC daratumumab arm. Grade 1–2 injection site reactions were noted in 18 patients in the SC arm. AE profiles were otherwise similar between SC and IV daratumumab: 13–14% of patients experienced grade 3–4 anemia; 8–13% experienced neutropenia; and 14% experienced thrombocytopenia. Three to four percent of patients developed grade 3–4 pneumonia. Pharmacokinetic evidence suggesting that patients with less body mass had about 60% higher mean maximum systemic concentrations of daratumumab with SC formulation than with IV formulation correlated with a slightly higher incidence of any AEs in the SC daratumumab arm vs. IV daratumumab arm (95% vs. 89%). Treatment discontinuations due to AEs were equivalent.
No new safety concerns with SC daratumumab were seen in the phase II PLEIADES trial, focused on multiple myeloma.52 Rates of grade 3–4 treatment-emergent AEs were comparable to those cited in prior studies. Grade 3–4 neutropenia occurred at 28–32% incidence (D-VRd, D-Rd arms); grade 3–4 thrombocytopenia occurred at maximum 43.3% rate (D-VMP arm). Grade 3–4 pneumonia occurred in 3–12.3% of cases. IRRs occurred in 7.5% of patients overall, with most occurring with the first administration of SC daratumumab. Only one incident of grade 3 IRR, decreased oxygen saturation, occurred. Median time to IRR was 4.4–6.9 hours across the three cohorts. Grade 1–2 injection-site reactions occurred in 7.5% of patients.
The SC daratumumab-related AE profile reported by the ANDROMEDA trial for newly diagnosed AL amyloidosis was consistent with the profile seen in prior studies across plasma cell dyscrasias.56 Clinically significant respiratory infections of any grade were increased in the SC daratumumab-VCd arm, occurring at 10.9% incidence compared to 6.4% in the control VCd arm. Grade 3–4 pneumonia occurred in 7.8% of SC daratumumab-VCd patients. Neutropenia of any grade also occurred in 10.9% of patients who received daratumumab-containing regimen, with 5.2% incidence of grade 3–4 toxicity. There was no other significant hematologic toxicity recorded. Diarrhea, peripheral edema, and lymphopenia were otherwise common, in line with prior reported AEs. Only 4.1% of patients who received SC daratumumab-VCd discontinued treatment due to AEs, similar to the 4.3% rate in the control arm. Grade 1–2 injection site reactions occurred in 7.2% of patients receiving SC daratumumab; there were no severe reactions.
No significantly different SC daratumumab-related AEs in AL amyloidosis patients were noted in the report by Chakraborty et al.57 There is one other instance of SC daratumumab use in real-world populations, which investigated short-term safety outcomes.61 Patients in this recently published multicenter Italian retrospective cohort study had either AL amyloidosis or multiple myeloma. After premedication with glucocorticoids, antihistamines, acetaminophen, and variably montelukast, 4% of the 189 subjects experienced mostly grade 1–2 IRRs. All clinically significant AEs occurred at less than 5% incidence in a comparison population of multiple myeloma patients with more frailty. At the time of manuscript writing, no cost-effectiveness or cost-comparison studies of SC vs. IV daratumumab have been published.
Summary and Conclusions
Systemic AL amyloidosis is a subset of plasma cell dyscrasias most prominently marked by the production of misfolded light chain immunoglobulins which aggregate and deposit as amyloid fibrils into bodily tissues, causing widespread organ dysfunction. It is an ultimately fatal disease and remains underdiagnosed. The standard-of-care therapeutic approach focuses on prevention of fibril generation by eliminating the plasma cell clone. Until recently, this has been accomplished with chemotherapy and, if the patient is healthy enough, ASCT.
The advent of the anti-CD38 monoclonal antibody, daratumumab, has fundamentally altered the treatment landscape for plasma cell dyscrasias and AL amyloidosis in particular. Daratumumab has swiftly been integrated into first-line combination regimens for AL amyloidosis: first as an IV infusion, and more recently as an SC injection. Daratumumab has been associated with an increased risk of sinopulmonary infections and increased risk of hematologic toxicities. IV daratumumab has been associated with long infusion times and grade 1–2 IRRs. These reactions, while typically easily managed with supportive medications, can cause distress to the patient and can cause delays in delivery of drug.
SC daratumumab appears to be as effective as IV daratumumab in plasma cell dyscrasias generally, with decreased IRRs, decreased time spent administering drug, decreased administered volume in patients who may not develop frank acute heart failure but who may have difficulty with diuresis, and no other new safety signals. From our clinical practice, the SC adipose layer in patients with AL amyloidosis can be so thin that SC injections are either too painful or indeed impossible; conversely, SC edema can be so significant that absorption and dispersal of drug is theoretically impaired. There are currently no published findings regarding cost effectiveness of SC vs. IV daratumumab. We note that given the findings presented, SC daratumumab appears likely to correlate with decreased time spent by patients in infusion centers, increased patient exposure to drug, and potentially increased treatment capacity in infusion centers. Overall, we find that SC daratumumab should be preferred over IV daratumumab, but the clinical situation should determine the route of administration. Further prospective investigations into cost-effectiveness, “real-world” experiences in non-trial populations, and patient-reported outcomes with SC daratumumab are warranted.
Disclosure
S.L. is a Consultant and/or Advisor for Adaptive Biotechnologies, Alexion Therapeutics, Bristol-Meyers-Squibb, Caelum Biosciences, Janssen Pharmaceuticals, Karyopharm Therapeutics, Oncopeptides AB, GSK, Abbvie, Janssen, Pfizer, and Takeda Pharmaceutical Company; receives research funding from Celgene, Inc., Sanofi, Zentali; received honoraria from Clinical Care Options and Regeneron Pharmaceuticals; and has Royalties/Patents with Caelum Biosciences. In addition, S.L. has a patent CAEL-101 with royalties paid to Columbia University. The authors report no other disclosures or conflicts of interest in this work.
References
1. Chakraborty R, Lentzsch S. Emerging drugs for the treatment of light chain amyloidosis. Expert Opin Emerg Drugs. 2020;25(3):299–317. doi:10.1080/14728214.2020.1803829
2. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–596. doi:10.1056/NEJMra023144
3. Merlini G. AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology Am Soc Hematol Educ Program. 2017;2017(1):1–12. doi:10.1182/asheducation-2017.1.1
4. Kourelis TV, Dasari S, Theis JD, et al. Clarifying immunoglobulin gene usage in systemic and localized immunoglobulin light-chain amyloidosis by mass spectrometry. Blood. 2017;129(3):299–306. doi:10.1182/blood-2016-10-743997
5. Oliva L, Orfanelli U, Resnati M, et al. The amyloidogenic light chain is a stressor that sensitizes plasma cells to proteasome inhibitor toxicity. Blood. 2017;129(15):2132–2142. doi:10.1182/blood-2016-08-730978
6. Genereux JC, Qu S, Zhou M, et al. Unfolded protein response-induced ER dj3 secretion links ER stress to extracellular proteostasis. EMBO J. 2015;34(1):4–19. doi:10.15252/embj.201488896
7. Kazman P, Absmeier RM, Engelhardt H, et al. Dissection of the amyloid formation pathway in AL amyloidosis. Nat Commun. 2021;12(6516). doi:10.1038/s41467-021-26845-0.
8. Rawat P, Prabakaran R, Kumar S, et al. Exploring the sequence features determining amyloidosis in human antibody light chains. Sci Rep. 2021;11(13785). doi:10.1038/s41598-021-93019-9.
9. Palladini G, Lavatelli F, Russo P, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107(10):3854–3858. doi:10.1182/blood-2005-11-4385
10. Kyle RA, Larson DR, Kurtin PJ, et al. Incidence of AL Amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin Proc. 2019;94(3):465–471. doi:10.1016/j.mayocp.2018.08.041
11. Ravichandran S, Lachmann HJ, Wechalekar AD. Epidemiologic and survival trends in Amyloidosis, 1987–2019. N Engl J Med. 2020;382(16):1567–1568. doi:10.1056/NEJMc1917321
12. Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97(6):818–829. doi:10.1002/ajh.26569
13. Staron A, Zheng L, Doros G, et al. Marked progress in AL amyloidosis survival: a 40-year longitudinal natural history study. Blood Cancer J. 2021;11(139). doi:10.1038/s41408-021-00529-w.
14. Gertz MA, Cohen AD, Comenzo RL, et al. Results of the Phase 3 VITAL study of NEOD001 (Birtamimab) plus standard of care in patients with Light Chain (AL) Amyloidosis suggest survival benefit for mayo stage IV patients. Blood. 2019;134(Supplement_1):3166. doi:10.1182/blood-2019-124482
15. Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–995. doi:10.1200/JCO.2011.38.5724
16. Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336(17):1202–1207. doi:10.1056/NEJM199704243361702
17. Sidiqi MH, Aljama MA, Buadi FK, et al. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018;36(13):1323–1329. doi:10.1200/JCO.2017.76.9554
18. Reece DE, Sanchorawala V, Hegenbart U, et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a Phase 1 dose-escalation study. Blood. 2009;114(8):1489–1497. doi:10.1182/blood-2009-02-203398
19. Manwani R, Cohen O, Sharpley F, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134(25):2271–2280. doi:10.1182/blood.2019000834
20. Palladini G, Milani P, Foli A, et al. Presentation and outcome with second-line treatment in AL amyloidosis previously sensitive to nontransplant therapies. Blood. 2018;131(5):525–532. doi:10.1182/blood-2017-04-780544
21. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–1219. doi:10.1056/NEJMoa1506348
22. Deaglio S, Vaisitti T, Billington R, et al. CD38/CD19: a lipid raft-dependent signaling complex in human B cells. Blood. 2007;109(12):5390–5398. doi:10.1182/blood-2006-12-061812
23. DARZALEX [Package Insert]. Washington D.C: Food and Drug Administration; 2022.
24. Sanchez L, Wang Y, Siegel DS, et al. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. 2016;9(51). doi:10.1186/s13045-016-0283-0.
25. Van Wagoner CM, Rivera-Escalera F, Jaimes-Delgadillo NC, et al. Antibody-mediated phagocytosis in cancer immunotherapy. Immunol Rev. 2023;319(1):128–141. doi:10.1111/imr.13265
26. Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol. 2016;197(3):807–813. doi:10.4049/jimmunol.1501351
27. Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–321. doi:10.1080/19420862.2015.1007813
28. Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370(m3176). doi:10.1136/bmj.m3176
29. de Weers M, Tai Y-T, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. doi:10.4049/jimmunol.1003032
30. Prejzner W, Piekos O, Beldzinska K, et al. The role of daratumumab in relapsed/refractory CD38 positive acute leukemias-case report on three cases with a literature review. Front Oncol. 2023;13(1228481). doi:10.3389/fonc.2023.1228481.
31. Yang EH, Muhsen IN, Samarkandi H, et al. Role of anti-CD38 monoclonal antibodies in the treatment of adult immune hematological diseases. Hematol Oncol Stem Cell Ther. 2023;17(1):4–12. doi:10.56875/2589-0646.1108
32. Zhao D, Guo Z, Zhao G, et al. A novel daratumumab-based regimen for desensitization in highly HLA-presensitized patients awaiting kidney transplantation. Transpl Int. 2023;36(11771). doi:10.3389/ti.2023.11771.
33. Nocturne G, Marmontel O, Di Filippo M, et al. Efficacy of daratumumab in refractory primary Sjogren disease. RMD Open. 2023;9(3):e003464. doi:10.1136/rmdopen-2023-003464
34. Al-Samkari H, Neufeld EJ. Novel therapeutics and future directions for refractory immune thrombocytopenia. Br J Haematol. 2023;203(1):65–78. doi:10.1111/bjh.19078
35. DARZALEX FASPRO [Package Insert]. Washington D.C: Food and Drug Administration; 2020.
36. Medicare Part B ASP Drug Cost. HCPCS J-Code: J9145 (Daratumumab). Center for Medicare and Medicaid Services; 2020.
37. Gordan LN, Marks SM, Xue M, et al. Daratumumab utilization and cost analysis among patients with multiple myeloma in a US community oncology setting. Future Oncol. 2022;18(3):301–309. doi:10.2217/fon-2021-1072
38. Medicare Part B ASP Drug Cost. HCPCS J-Code: J9070 (Cyclophosphamide). Center for Medicare and Medicaid Services; 2023.
39. Medicare Part B ASP Drug Cost. HCPCS J-Code: J9041 (Bortezomib). Center for Medicare and Medicaid Services; 2023.
40. Moreau P, Hulin C, Perrot A, et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(10):1378–1390. doi:10.1016/S1470-2045(21)00428-9
41. Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582–1596. doi:10.1016/S1470-2045(21)00466-6
42. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–945. doi:10.1182/blood.2020005288
43. Dimopoulos MA, Oriol A, Nahi H, et al. Overall survival with daratumumab, lenalidomide, and dexamethasone in previously treated multiple myeloma (POLLUX): a randomized, open-label, Phase III trial. J Clin Oncol. 2023;41(8):1590–1599. doi:10.1200/JCO.22.00940
44. Sonneveld P, Chanan-Khan A, Weisel K, et al. Overall survival with daratumumab, bortezomib, and dexamethasone in previously treated multiple myeloma (CASTOR): a randomized, open-label, Phase III trial. J Clin Oncol. 2023;41(8):1600–1609. doi:10.1200/JCO.21.02734
45. Sher T, Fenton B, Akhtar A, et al. First report of safety and efficacy of daratumumab in 2 cases of advanced immunoglobulin light chain amyloidosis. Blood. 2016;128(15):1987–1989. doi:10.1182/blood-2016-06-722496
46. Kaufman GP, Schrier SL, Lafayette RA, et al. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130(7):900–902. doi:10.1182/blood-2017-01-763599
47. Sanchorawala V, Sarosiek S, Schulman A, et al. Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: results of a Phase 2 study. Blood. 2020;135(18):1541–1547. doi:10.1182/blood.2019004436
48. Roussel M, Merlini G, Chevret S, et al. A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood. 2020;135(18):1531–1540. doi:10.1182/blood.2019004369
49. Kaufman GP, Dispenzieri A, Gertz MA, et al. Kinetics of organ response and survival following normalization of the serum free light chain ratio in AL amyloidosis. Am J Hematol. 2015;90(3):181–186. doi:10.1002/ajh.23898
50. Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560. doi:10.1016/S0140-6736(15)01120-4
51. Darzalex. Amsterdam. Netherlands: European Medicines Agency; 2023.
52. Chari A, Rodriguez-Otero P, McCarthy H, et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy (PLEIADES): an open-label Phase II study. Br J Haematol. 2021;192(5):869–878. doi:10.1111/bjh.16980
53. Report submitted 6/5/2020 to the Vermont office of the attorney general for introduction of a new prescription drug to market. Vermont: Janssen Biotech, Inc; 2020.
54. Usmani SZ, Nahi H, Mateos MV, et al. Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood. 2019;134(8):668–677. doi:10.1182/blood.2019000667
55. Mateos MV, Nahi H, Legiec W, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7(5):e370–e380. doi:10.1016/S2352-3026(20)30070-3
56. Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. doi:10.1056/NEJMoa2028631
57. Chakraborty R, Rosenbaum C, Kaur G, et al. First report of outcomes in patients with stage I IIb AL amyloidosis treated with Dara–VCD front-line therapy. Br J Haematol. 2023;201(5):913–916. doi:10.1111/bjh.18733
58. Maura F, Boyle EM, Coffey D, et al. Genomic and immune signatures predict clinical outcome in newly diagnosed multiple myeloma treated with immunotherapy regimens. Nat Cancer. 2023. doi:10.1038/s43018-023-00657-1
59. Seckinger A, Hillengass J, Emde M, et al. CD38 as immunotherapeutic target in light chain amyloidosis and multiple myeloma-association with molecular entities, risk, survival, and mechanisms of upfront resistance. Front Immunol. 2018;9(1676). doi:10.3389/fimmu.2018.01676.
60. van de Donk N, Usmani SZ. CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol. 2018;9(2134). doi:10.3389/fimmu.2018.02134
61. De Novellis D, Fontana R, Palmieri S, et al. Safety of subcutaneous daratumumab in anti-CD38 monoclonal antibody-naive patients with plasma cell disorders: a multicenter real-life experience. Target Oncol. 2023;18(6):885–892. doi:10.1007/s11523-023-01001-4
62. Abeykoon JP, Zanwar S, Dispenzieri A, et al. Daratumumab-based therapy in patients with heavily-pretreated AL amyloidosis. Leukemia. 2019;33(2):531–536. doi:10.1038/s41375-018-0262-2
63. Al Saleh AS, Ebraheem MS, Sidiqi MH, et al. Treatment and outcomes of patients with light chain amyloidosis who received a second line of therapy post autologous stem cell transplantation. Blood Cancer J. 2022;12(59). doi:10.1038/s41408-022-00655-z.
64. Chung A, Kaufman GP, Sidana S, et al. Organ responses with daratumumab therapy in previously treated AL amyloidosis. Blood Adv. 2020;4(3):458–466. doi:10.1182/bloodadvances.2019000776
65. Cohen OC, Brodermann MH, Blakeney IJ, et al. Rapid response to single agent daratumumab is associated with improved progression-free survival in relapsed/refractory AL amyloidosis. Amyloid. 2020;27(3):200–205. doi:10.1080/13506129.2020.1765768
66. Gounot R, Le Bras F, Dupuis J, et al. Daratumumab is safe and induces a rapid hematological response in light-chain amyloidosis with severe cardiac impairment. Leuk Lymphoma. 2021;62(4):979–983. doi:10.1080/10428194.2020.1850717
67. Kastritis E, Rousakis P, Kostopoulos IV, et al. Consolidation with a short course of daratumumab in patients with AL amyloidosis or light chain deposition disease. Amyloid. 2021;28(4):259–266. doi:10.1080/13506129.2021.1971192
68. Kimmich CR, Terzer T, Benner A, et al. Daratumumab for systemic AL amyloidosis: prognostic factors and adverse outcome with nephrotic-range albuminuria. Blood. 2020;135(18):1517–1530. doi:10.1182/blood.2019003633
69. Kimmich CR, Terzer T, Benner A, et al. Daratumumab, lenalidomide, and dexamethasone in systemic light-chain amyloidosis: high efficacy, relevant toxicity and main adverse effect of gain 1q21. Am J Hematol. 2021;96(7):E253–E257. doi:10.1002/ajh.26191
70. Lecumberri R, Krsnik I, Askari E, et al. Treatment with daratumumab in patients with relapsed/refractory AL amyloidosis: a multicentric retrospective study and review of the literature. Amyloid. 2020;27(3):163–167. doi:10.1080/13506129.2020.1730791
71. Milani P, Fazio F, Basset M, et al. High rate of profound clonal and renal responses with daratumumab treatment in heavily pre-treated patients with light chain (AL) amyloidosis and high bone marrow plasma cell infiltrate. Am J Hematol. 2020;95(8):900–905. doi:10.1002/ajh.25828
72. Sammartano V, Antonioli E, Buda G, et al. Daratumumab in AL amyloidosis: a real-life experience of the ”RTM” (Regional Tuscan Myeloma Network). J Pers Med. 2022;12(3):484. doi:10.3390/jpm12030484
73. Shragai T, Gatt M, Lavie N, et al. Daratumumab for relapsed AL amyloidosis-when cumulative real-world data precedes clinical trials: a multisite study and systematic literature review. Eur J Haematol. 2021;106(2):184–195. doi:10.1111/ejh.13535
74. Szalat RE, Gustine J, Sloan JM, et al. Predictive factors of outcomes in patients with AL amyloidosis treated with daratumumab. Am J Hematol. 2022;97(1):79–89. doi:10.1002/ajh.26399
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

