Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Safety and efficacy of cervical disc arthroplasty in preventing the adjacent segment disease: a meta-analysis of mid- to long-term outcomes in prospective, randomized, controlled multicenter studies
Authors Latka D , Kozlowska K, Miekisiak G , Latka K , Chowaniec J, Olbrycht T, Latka M
Received 28 November 2018
Accepted for publication 23 February 2019
Published 28 March 2019 Volume 2019:15 Pages 531—539
DOI https://doi.org/10.2147/TCRM.S196349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Dariusz Latka,1,2 Klaudia Kozlowska,3 Grzegorz Miekisiak,1,2 Kajetan Latka,2 Jacek Chowaniec,2 Tomasz Olbrycht,2 Miroslaw Latka3
1Department of Anatomy, Institute of Medicine, University of Opole, Opole, Poland; 2Department of Neurosurgery, University Hospital in Opole, Opole, Poland; 3Department of Bioengineering, Institute of Biomedical Engineering, Technical University of Wroclaw, Wroclaw, Poland
Objectives: Cervical disc arthroplasty (CDA) has become an alternative treatment for cervical radiculopathy and myelopathy. This technique preserves appropriate motion at both the index and adjacent disc levels and consequently may prevent adjacent segment degeneration (ASD). The authors performed a meta-analysis to compare the safety and efficacy of CDA to those of the gold standard, anterior cervical discectomy and fusion (ACDF). Both surgical and clinical parameters were employed to verify the hypothesis that CDA can reduce the risk of ASD.
Methods: The meta-analysis comprised high-quality randomized controlled trials that compared CDA and ACDF treatments of cervical degenerative disc disease. Included papers reported data for at least one of the following outcomes: 1) surgical parameters, 2) questionnaire clinical indices (pre- and postoperative values), and 3) complication rates at 24 months; in addition, for ASD we analyzed 60 month or longer follow-ups. We used mean differences (MDs) or ORs to compare treatment effects between CDA and ACDF.
Results: Twenty studies with 3,656 patients (2,140 with CDA and 1,516 with ACDF) met the inclusion criteria. CDA surgery, with mean duration longer than that of ACDF, was associated with higher blood loss. Visual analog scale neck pain score was significantly smaller for CDA (mean difference =-2.30, 95% CI [-3.72; -0.87], P=0.002). The frequency of dysphagia/dysphonia (OR =0.69, 95% CI [0.49; 0.98], P=0.04) as well as the long-term ASD rate for CDA was significantly smaller (OR =0.33, 95% CI [0.21; 0.50], P<0.0001).
Conclusion: A significantly lower probability of ASD reoperations in the CDA cohort after a 60-month or longer follow-up was the most important finding of this study. Despite the moderate quality of this evidence, the pooled data corroborated for the very first time that CDA was efficacious in preventing ASD.
Keywords: cervical disc arthroplasty, CDA, anterior cervical discectomy and fusion, ACDF, cervical degenerative disc disease, CDDD, meta-analysis, randomized controlled trial, RCT, cervical total disc replacement, CTDR
Introduction
Cervical degenerative disc disease (CDDD) has become a civilization disease and one of the significant causes of work-related disability.1 In the case of conservative treatment failure, surgery is the only alternative. For many decades, anterior cervical discectomy and fusion (ACDF) has been regarded as the gold standard. However, an analysis of long-term treatment results indicates that over 90% of patients who undergo ACDF develop significant degenerative changes in the adjacent spinal segments.2,3 Almost one-quarter of these changes are symptomatic and require surgery within a decade. The rate of reoperation is equal to 2.9% level/year.4 Degeneration may occur in its natural course but segment stiffening may exacerbate it. Clinical observations, especially in adolescent groups (traumatic fusions or Klippel-Feil syndrome) in which degeneration resulting from natural history is unlikely, indicate that stiffening may be a fundamental pathogenic factor causing degeneration of neighboring segments.5 Clinical evidence has led to the formulation of the concept of adjacent segment degeneration (ASD). The development of cervical disc arthroplasty (CDA) was driven by the expectations that preservation of motion at both index and adjacent disc levels would minimize the ASD risk. To some extent, these expectations were fueled by the success of hip joint arthroplasty, which replaced arthrodesis in the treatment of severe coxarthrosis.
Aims
Advantages and disadvantages of ACDF and CDA have been analyzed in several previous publications.6–12 The authors decided to design a meta-analysis that would assess the safety of both the methods and elucidate their long-term efficacy in ASD prevention.
Materials and methods
Search strategy
The neurosurgeons independently carried out a comprehensive Internet literature search of the following English databases: PubMed, Medline, EMBASE, Cochrane Central Register of Controlled Trials – Issue 1, 2016 – Scopus, OvidSP, and Google Scholar. The queries were last updated in February 2018. The search terms contained a combination of the following keywords: “cervical arthroplasty,” “cervical disc replacement,” “total disc replacement,” “anterior cervical fusion,” “CDA,” “TDR,” “TDA,” “ACDF.”
Study selection
The first stage of selection was based on the publication’s title and screening of its abstract. The reviewers then independently assessed the eligibility of the content of the article. Any disagreement was resolved through discussion. All papers non-compliant with the recommendations contained in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-P checklist; Supplementary materials), duplicates, and studies with incomplete data were excluded from further analysis.13
Eligibility criteria
The inclusion criteria consisted of several parameters: 1) the publication was an English description of a prospective, controlled, multicenter, randomized controlled trial (RCT), and reported data of at least 2-year follow-up; 2) the publication compared the outcomes of ACDF and CDA treatments of patients over 18 years of age (regardless of gender) at one or two levels of the spine with radiculopathy and/or myelopathy; 3) clinical evaluation included at least one of the following indicators: neck disability index (NDI), visual analog scale (VAS) for neck and/or arm pain, reoperation rate at the operated and adjacent levels, surgical parameters (blood loss, surgery time, and hospital stay), and rate of adverse effects.
Data collection
Each paper was described using author, year of publication, country of origin, number of operating centers, number of CDA and ACDF groups, type of CDA prosthesis, number of implantation levels, and a period of observation. Some of the studies were extensions beyond the original observation period.
Quality of evidence assessment and risk of bias
The risk of bias was assessed with a six-item scale recommended by the Cochrane Back Review Group.13,14 We used the following appraisal parameters: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of results assessor, incomplete data, and selective result reporting. Risk of bias in all areas was defined as low, high, or unclear. The quality of evidence for each outcome was rated according to the Grades of Recommendation, Assessment, Development, and Evaluation approach. Several study criteria were assessed: design, quality, consistency, and directness. Publication bias was assessed using funnel plots.
Extraction of summary measures
Differences in treatment effects between CDA and ACDF were estimated using mean difference (MD) and its 95% CI for continuous measures or OR and its 95% CI for dichotomous measures (rate CDA/ACDF). The following outcome measures were used: surgical parameters (operation time, blood loss, and length of hospital stay), NDI, VAS for neck and arm, and complication rates (number of adverse events, adjacent segment disease, and reoperations at the index level). For questionnaire clinical indices, we compared postoperative values in addition to differences between pre- and postoperative values. Both dichotomous and continuous parameters were compared 24 months after the operation. In addition, the OR of reoperations due to ASD was calculated for 60 months or longer follow-ups.
Statistical analysis
The data analysis was performed using R (version 3.3.2; https://www.r-project.org/) and a “meta” package using a fixed-effects model.15 The chi-squared and Higgin’s I2 tests were used to evaluate heterogeneity across studies.
Cut-off values of 25%, 50%, and 75% were used to label heterogeneity as low, moderate, or high, respectively.16 If I2 was >50%, a subgroup analysis was attempted with respect to the type of prosthesis and/or the number of implantation levels in order to find the source of heterogeneity. The significance threshold for all statistical tests was set to 0.05.
Supplementary data
The forest plots of the following parameters determined at 24 months are available in Supplementary materials: operative time, hospital stay, blood loss, NDI, VAS neck and arm pain scores, adjacent segment disease, reoperations at index level, adverse effects, dysphagia/dysphonia. The supplementary data also include the forest plot of changes in NDI and VAS neck and arm pain scores over 24 months.
Results
Study search and characteristics
Twenty articles with 3,656 patients and nine types of prostheses (2,140 with CDA and 1,516 with ACDF) met the inclusion criteria. The follow-up duration in every study was at least 24 months. Four studies reported outcomes for 60 months and four up to 84 months. Figure 1 illustrates the study selection process. Figure 2 presents study and patient characteristics.
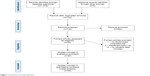  | Figure 1 Flowchart of study selection. |
Risk of bias
In the majority of papers, the risk of bias was unclear or low. In four articles, the number of low-risk points was at least equal to unclear points. At least one point of high risk of bias was marked in four papers. Figure 3 summarizes the assessment of risk of bias.
  | Figure 3 Risk of bias assessment: |
Analysis of surgical parameters
Nine studies with 2,353 patients (CDA =1,302, ACDF =1,051) were pooled to evaluate operative time of two surgical techniques. The mean operative time for ACDF was significantly shorter than that for CDA (MD =0.23, 95% CI [0.09; 0.36], P<0.001; I2=83.56%; 95% CI [70.33%; 90.89%]) (Figure S1). Subgroup with the Mobi-C prothesis could be a potential source of high heterogeneity (I2=57.0%). Furthermore, there were significant differences between subgroups (P<0.001). The asymmetry of funnel plot was not observed. The quality of this evidence was moderate.
There was no significant difference in length of hospital stay between CDA and ACDF groups (MD =−0.92, 95% CI [−0.11; 0.08], P=0.05, I2=49.78%, 95% CI [0.0%; 77.58%]) (Figure S2). The comparison was based on eight studies with 2,202 patients (CDA =1,221, ACDF =981).
Asymmetry in the funnel plot was not observed. Subgroup analysis based on type of prosthesis revealed statistically significant differences (P=0.02). However, the analysis did not identify the source of heterogeneity. The quality of this evidence was very low.
Nine studies with 2,778 patients (CDA =1,522, ACDF =1,256) provided data for blood loss. The blood loss for ACDF was significantly lower than that of CDA (MD =9.23, 95% CI [5.35; 13.12], P<0.0001, I2=1.54%, 95% CI [0.0%; 65.34%]) (Figure S3). There was no asymmetry in the funnel plot, and the quality of this evidence was moderate.
Analysis of clinical parameters at 24 months
Five studies with 1,635 patients (CDA =843, ACDF =792) reported data for NDI scores at 24 months. NDI scores were lower in CDA group, albeit the quality of evidence was low (MD =−0.85, 95% CI [−1.89; 0.18], P=0.11, I2=0.0%, 95% CI [0.0%; 66.73%]) (Figure S4). The source of heterogeneity was unknown. The funnel plot was symmetrical.
Four studies with 1,426 patients (CDA =740, ACDF =686) presented data for VAS neck pain score at the 24-month follow-up. The score for CDA was significantly lower than that of ACDF (MD =−2.30, 95% CI [−3.72; −0.87], P=0.002, I2=0.0%, 95% CI [0.0%; 74.96%]) (Figure S5). The source of heterogeneity was unknown. The asymmetry of funnel plot was not observed. The quality of this evidence was moderate.
The outcome of four studies comprising 1,426 patients (CDA =740, ACDF =686) showed that VAS arm pain score was lower for CDA (MD =−1.05, 95% CI [−2.41; 0.30], P=0.13, I2=0.0%, 95% CI [0.0%; 0.0%]) (Figure S6). The source of heterogeneity was unknown. The funnel plot was symmetrical. The quality of this evidence was low.
The meta-analysis of ten studies with 3,844 patients (CDA =2,021, ACDF =1,823) showed that patients who undergo CDA are at lower risk, statistically non-significant, to develop ASD in comparison with those who undergo ACDF (OR =0.68, 95% CI [0.44; 1.05], P=0.08, I2=5.76%, 95% CI [0.0%; 69.44%]) (Figure S7). The source of heterogeneity was unknown. The asymmetry of funnel plot was not observed. The quality of this evidence was low.
Eight studies with 2,921 patients (CDA =1,556, ACDF =1,365) were pooled to evaluate rate of reoperations at index level. Patients who undergo CDA are at statistically non-significant lower risk to undergo reoperation in comparison with those who undergo ACDF (OR =0.66, 95% CI [0.41; 1.06], P=0.09, I2=0.0%, 95% CI [0.0%; 60.39%]) (Figure S8), with a moderate quality of evidence. The source of heterogeneity was unknown. The funnel plot was symmetric.
Twelve studies with 4,383 patients (CDA =2,349, ACDF =2,034) provided data for adverse effects. There was no significant difference in risk for both groups (OR =0.87, 95% CI [0.56; 1.35], P=0.54, I2=75.23%, 95% CI [53.90%; 86.70%]) (Figure S9). Subgroup analysis with respect to type of prosthesis did not reveal the source of heterogeneity. The asymmetry of funnel plot was absent.
Nine studies with 3,369 patients (CDA =1,815, ACDF =1,554) reported the rate of dysphagia/dysphonia. Patients who undergo CDA are at a statistically significant lower risk for developing dysphagia/dysphonia in comparison with those who undergo ACDF. The OR for this parameter was significantly lower in the CDA group (OR =0.69, 95% CI [0.49; 0.98], P=0.04, I2=0.0%, 95% CI [0.0%; 57.38%]) (Figure S10). The source of heterogeneity was unknown. The asymmetry of funnel plot was not observed. The quality of this evidence was moderate.
Analysis of changes of clinical parameters
Differences in NDI score before and after surgery (24-month follow-up) were evaluated using data from four studies with 1,122 patients (CDA =630, ACDF =492). The difference in NDI score was higher in the CDA cohort (MD =2.64, 95% CI [−1.82; 7.10], P=0.25, I2=80.94%, 95% CI [50.08%; 92.72%]) (Figure S11). The source of high heterogeneity was the subgroup with Bryan prosthesis (I2=86.50%). The asymmetry of funnel plot was not observed. The quality of this evidence was very low.
Three studies with 659 patients (CDA =388, ACDF =271) reported data for differences in VAS neck pain score before and after surgery (24-month follow-up). The difference in VAS neck pain scores was higher in CDA cohort group (MD =1.75, 95% CI [−0.93; 4.42], P=0.20, I2=0.0%, 95% CI [0.0%; 0.0%]) (Figure S12). The source of heterogeneity was unknown. The funnel plot was symmetric. The quality of this evidence was low.
The data from three studies with 659 patients (CDA =388, ACDF =271) showed that difference in VAS neck pain score was higher in ACDF (MD =−0.29, 95% CI [−2.73; 2.16], P=0.82, I2=0.0%, 95% CI [0.0%; 0.0%]) (Figure S13). The source of heterogeneity was unknown. An asymmetric funnel plot was not observed. The quality of this evidence was very low.
Analysis of ASD over 60 months
Five studies with 1,594 patients (CDA =956, ACDF =638) were pooled to evaluate the rate of ASD of two surgical techniques over a long-term observation. Patients who undergo CDA are at a statistically significant lower risk for developing ASD in comparison with those who undergo ACDF (OR =0.33, 95% CI [0.21; 0.50], P<0.0001, I2=0.0%, 95% CI [0.0%; 58.71%], Figure 4) with a moderate quality of supporting evidence. The source of heterogeneity was unknown. The asymmetry of funnel plot was not observed.
  | Figure 4 Forest plot of ASD at 60 months. |
Discussion
ACDF is a treatment of choice for CDDD.17 According to Cochrane Back and Neck, the creator of spine disease treatment standards, CDA is a viable alternative to ACDF, but it is not always the best treatment option.18 It is worth pointing out that ACDF leads to reduction in the range of motion at the site of spondylodesis and increased mobility at adjacent levels. Goffin et al used radiographic evidence from long-term observations of ACDF patients and demonstrated that degenerative changes at the level adjacent to the fusion occurred in as many as 92% of patients.2,19 Preservation of segmental movement at the indexed level was the design principle of CDA. It was believed that mobility would reduce ASD occurrence due to protection of adjacent segments from mechanical overload.20 The pathological consequences of such overload cannot be detected by RCTs with short or medium follow-up which make up majority of published studies.6–12,21–38 The most important meta-analyses concerning efficacy of CDA included only RCTs published before 2012.32,34,35 The rationale for this meta-analysis was the inclusion of the most recent RCTs with emphasis on long-term follow-up (60 months or longer).
The significantly lower probability of ASD reoperations in the CDA cohort after a 60-month follow-up is the most important study finding. Even though the quality of evidence is moderate, the pooled data corroborate for the very first time that CDA is efficacious in preventing ASD; these results contradict those from some earlier studies. Nunley et al showed that after 36 months, ASD risks after CDA and ACDF surgeries were equal.39 Therefore, it was hypothesized that some other factors could affect the occurrence of degeneration of the neighboring segment; these other factors include bone mineral density and the presence of co-existing degenerative changes in the lumbar spine. Verma et al also did not find significant differences in the frequency of ASD between CDA and ACDF.40 However, in their study, only the reoperation index without radiographic assessment was used to calculate ASD frequency. The results of their analysis could be affected by a significantly higher loss of patients in ACDF group during the follow-up period.
It is worth pointing out that the frequency of postoperative dysphagia/dysphonia was significantly lower in patients with mobile devices, which is at variance with the study published last year.36 Both the number of secondary surgical interventions at the index level and adverse events (excluding dysphagia/dysphonia) in CDA and ACDF groups were not statistically different, in agreement with the results of the previous studies.6,11,26,36,41 This is interesting because the previous generations of CDA prostheses (such as Bristol-Cummins) were known to cause subluxation, screw damage, and dysphagia.42 The recent advances in implant technology pave the way for improvement of safety and efficacy of CDA. In particular, modern implants are capable of maintaining optimal motion, disk height, and lordosis. They have also a longer life, cause less inflammation or osteolysis, and better mimic the function of natural intervertebral discs.43 The potential advantages of modern implants must be verified by RCTs.
It comes as no surprise that this meta-analysis corroborated that the length of CDA surgery was longer than that of ACDF and was associated with greater blood loss. The longer traction on soft neck structures is an apparent disadvantage of CDA.
There are several limitations to this meta-analysis: 1) the review was restricted to papers published in English; 2) studies included in the meta-analysis involved 1- or 2-level CDAs and nine different types of prostheses; 3) the small number of included studies in some meta-analyses; and 4) the quality of evidence varied from very low to moderate.
Conclusion
The most important finding of this study is the significantly lower probability of ASD reoperations in the CDA cohort after a 60-month follow-up. Despite the moderate quality of this evidence, the pooled data corroborated for the very first time that CDA is efficacious in preventing ASD; these results contradict those from some earlier studies. At present, both the number of secondary surgical interventions at the index level and adverse events in CDA and ACDF are comparable. Due to ongoing improvements in implant technology, further RCTs are required to identify patients who could benefit from CDA treatment as well as practical clinical trials, which are specifically designed to confirm the proper indications for CDA, because it is clear that only a defined subset of patients requiring cervical surgery are valid candidates for disc arthroplasty.
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Hoy D, March L, Woolf A, et al. The global burden of neck pain: estimates from the Global Burden of Disease 2010 Study. Ann Rheum Dis. 2014;73:1309–1315. doi:10.1136/annrheumdis-2013-204431 | ||
Goffin J, Van Calenbergh F, Van Loon J. Intermediate follow-up after treatment of degenerative disc disease of the with the Bryan Cervical Disc Prothesis: single level and bi-level. Spine. 2013;28:2673–2678. doi:10.1097/01.BRS.0000099392.90849.AA | ||
Hunter LY, Braunstein EM, Bailey RW. Radiographic changes following anterior cervical fusion. Spine. 1980;5:399–401. | ||
Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. | ||
Gore DR. Roentgenographic findings in the cervical spine in asymptomatic persons: a ten year follow up. Spine. 2001;26:2463–2466. | ||
Fallah A, Akl EA, Ebrahim S, et al. Anterior cervical discectomy with artrhroplasty versus arthrodesis for single-level cervical spondylosis: a systematic review and metaanalysis. PLoS One. 2012;7(8):e43407. doi:10.1371/journal.pone.0043407 | ||
Gao Y, Liu M, Li T, Huang F, Tang T, Xiang Z. A meta-analysis comparing the results of cervical disc arthroplasty with anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical disc disease. J Bone Joint Surg Am. 2013;95:555–561. doi:10.2106/JBJS.K.00599 | ||
Ma Z, Ma X, Yang H, Guan X, Li X. Anterior cervical discectomy and fusion versus cervical arthroplasty for the management of cervical spondylosis: a meta-analysis. Eur Spine J. 2017;26(4):998–1008. doi:10.1007/s00586-016-4779-7 | ||
Rao MJ, Nie SP, Xiao BW, Zhang GH, Gan XR, Cao SS. Cervical disc arthroplasty versus anterior cervical discectomy and fusion for treatment of symptomatic cervical disc disease: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2015;135(1):19–28. doi:10.1007/s00402-014-2122-5 | ||
Ren C, Song Y, Xue Y, Yang X. Mid- to longterm outcomes after cervical disc arthroplasty compared with anterior discectomy and fusion: a systematic review and meta-analysis of randomized controlled trials. Eur Spine J. 2014;23(5):1115–1123. doi:10.1007/s00586-014-3220-3 | ||
Shangguan L, Ning GZ, Tang Y, Wang Z, Luo ZJ, Zhou Y. Discover cervical disc arthroplasty versus anterior cervical discectomy and fusion in symptomatic cervical disc diseases: a meta-analysis. PLoS One. 2017;12(3):e0174822. doi:10.1371/journal.pone.0174822 | ||
Xie L, Liu M, Ding F, Li P, Ma D. Cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) in symptomatic cervical degenerative disc diseases (CDDDs): an updated meta-analysis of prospective randomized controlled trials (RCTs). Springerplus. 2016;5(1):1188. doi:10.1186/s40064-016-2851-8 | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097 | ||
Tashani OA, El-Tumi H, Aneiba K. Quality of systematic reviews: an example of studies comparing artificial disc replacement with fusion in the cervical spine. Libyan J Med. 2015;10:e28857. doi:10.3402/ljm.v10.28857 | ||
Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. | ||
Higgins JP, Thompson JG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7420.895 | ||
Gore DR, Sepic SB. Anterior cervical fusion for degenerated or protruded disc A review of one hundred forty six patients. Spine. 1984;9:667–671. | ||
Boselie TF, Willems PC, van Mameren H, de Bie R, Benzel EC, van Santbrink H. Arthroplasty versus fusion in single-level cervical degenerative disc disease: a Cochrane Review. Spine. 2013;38(17):E1096–E1107. doi:10.1097/BRS.0b013e3182994a32 | ||
Goffin J, Van Callenbergh F, Vantomme N. Long term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79–85. | ||
Le H, Thongtrangan I, Kim DH. Historical review of cervical arthroplasty. Neurosurg Focus. 2004;17:E1. doi:10.3171/foc.2004.17.3.1 | ||
Anderson PA, Nassr A, Currier BL, et al. Evaluation of adverse events in total disc replacement: a meta-analysis of FDA summary of safety and effectiveness data. Global Spine J. 2017;7(1 Supl):76S–83S. doi:10.1177/2192568216688195 | ||
Li GL, Hu JZ, Qu J, Guo LY, Zai FL. Anterior cervical discectomy with arthroplasty versus anterior cervical discectomy and fusion for cervical spondylosis. J Clin Neurosci. 2015;22(3):460–467. doi:10.1016/j.jocn.2014.09.010 | ||
Luo J, Gong M, Huang S, Yu T, Zou X. Incidence of adjacent segment degeneration in cervical disc arthroplasty versus anterior cervical decompression and fusion meta-analysis of prospective studies. Arch Orthop Trauma Surg. 2015;135(2):155–160. doi:10.1007/s00402-014-2125-2 | ||
Muheremu A, Niu X, Wu Z, Muhanmode Y, Tian W. Comparison of the short- and long-term treatment effect of cervical replacement and anterior cervical disk fusion: a meta-analysis. Eur J Orthop Surg Traumatol. 2015;25(1):S87–S100. doi:10.1007/s00590-014-1469-1 | ||
Shriver MF, Lubelski D, Sharma AM, Steinmetz MP, Benzel EC, Mroz TE. Adjacent segment degeneration and disease following cervical arthroplasty: a systematic review and meta-analysis. Spine J. 2016;16(2):168–181. doi:10.1016/j.spinee.2015.10.032 | ||
Tan W, Zhou C, Guo D, et al. Treatment of single-level cervical spondylosis: cervical disk arthroplasty versus anterior cervical decompression and fusion. Orthopedics. 2017;40(1):e23–e34. doi:10.3928/01477447-20161213-01 | ||
Wu AM, Xu H, Mullinix KP, et al. Minimum 4-year outcomes of cervical total disc arthroplasty versus fusion: a metaanalysis based on prospective randomized controlled trials. Medicine. 2015;94(15):e665. doi:10.1097/MD.0000000000000874 | ||
Xing D, Ma XL, Ma JX, Wang J, Ma T, Chen Y. A meta-analysis of cervical arthroplasty compared to anterior cervical discectomy and fusion for single-level cervical disc disease. J Clin Neurosci. 2013;20(7):970–978. doi:10.1016/j.jocn.2012.03.046 | ||
Xu B, Ma J, Tian J, Ge L, Ma X. Indirect meta-analysis comparing clinical outcomes of total cervical disc replacements with fusions for cervical degenerative disc disease. Sci Rep. 2017;7:1740. doi:10.1038/s41598-017-01865-3 | ||
Yang B, Li H, Zhang T, He X, Xu S. The incidence of adjacent segment degeneration after cervical disc arthroplasty (CDA): a meta analysis of randomized controlled trials. PLoS One. 2012;7(4):e35032. doi:10.1371/journal.pone.0035032 | ||
Yao Q, Liang F, Xia Y, Jia C. A metaanalysis comparion total disc arthroplasty with anterior cervical discectomy and fusion for the treatment of cervical degenerative diseases. Arch Orthop Trauma Surg. 2016;136(3):297–304. doi:10.1007/s00402-015-2337-0 | ||
Yin S, Yu X, Zhou S, Yin Z, Qiu Y. Is cervical disc arthroplasty superior to fusion for treatment of symptomatic cervical disc disease? A metaanalysis. Clin Orthop Relat Res. 2013;471:1904–1919. doi:10.1007/s11999-013-2830-0 | ||
Young IA, Cleland JA, Michener LA, Brown C. Reliability, construct validity and responsiveness of the neck disability index, patient-specific functional scale and numeric pain rating scale in patients with cervical radiculopathy. Am J Phys Med Rehabil. 2010;89(10):831–839. doi:10.1097/PHM.0b013e3181ec98e6 | ||
Yu L, Song Y, Yang X, Lv C. Systematic review and meta-analysis of randomized controlled trials: comparison of total disc replacement with anterior decompression. Orthopedics. 2011;34(10):e651–e658. doi:10.3928/01477447-20110826-09 | ||
Zeichmeister I, Winkler R, Mad P. Artificial total disc replacement versus fusion for the cervical spine: a systematic review. Euro Spine J. 2011;20:177–184. doi:10.1007/s00586-010-1583-7 | ||
Zhao GS, Zhang Q, Zx QUAN. Mid-term efficiacy and safety of cervical disc arthroplasty versus fusion in cervical spondylosis: a systematic review and meta-analysis. Biomed Rep. 2017;6(2):159–166. doi:10.3892/br.2016.823 | ||
Zhong ZM, Zhu SY, Zhuang JS, Wu Q, Zhu Q. Reoperation after cervical disc arthroplasty versus anterior cervical decompresion and fusion – a meta-analysis. Clin Orthop Relat Res. 2016;474:1307. doi:10.1007/s11999-016-4707-5 | ||
Zhu Y, Zhang B, Liu H, Wu Y, Zhu Q. Cervical disc arthroplasty versus anterior cervical discectomy and fusion for incidence of symptomatic adjacent segment disease: a metaanalysis of prospective randomized controlled trials. Clinical. 2016;41(19):1493–1502. | ||
Nunley PD, Jawahar A, Kerr EJ, Gordon CJ, Cavanaugh DA. Factors effecting the incidence of symptomatic adjacent level disease in cervical spine after total disc artrhroplasty. 2- to 4-yrs follow up of three prospective randomized trials. Spine. 2012;37:445–451. doi:10.1097/BRS.0b013e31822174b3 | ||
Verma K, Gandhi SD, Maltenford M, Albert TJ, Hillibrand AS. Rate of adjacent segment disease in cervical disc arthroplasty versus single-level fusion: metaanalysis of prospective studies. Spine. 2013;38:2253–2257. doi:10.1097/BRS.0000000000000052 | ||
Chen C, Zhang X, Ma X. Durability of cervical disc arthroplasties and its influence factors: a systematic review and a network meta-analysis. Medicine. 2017;96(6):e5947. | ||
Pickett GE, Sekhon LH, Sears WR, Duggal N. Complications with cervical arthroplasty. J Neurosurg Spine. 2006;4:98–105. doi:10.3171/spi.2006.4.2.98 | ||
Cason GW, Herkowitz HN. Cervical intervertebral disc replacement. J Bone Joint Surg. 2013;95(3):279–285. doi:10.2106/JBJS.J.01042 | ||
Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fesler RG, Hacker RJ. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of the randomized, controlled clinical trial. Spine. 2009;34:101–107. doi:10.1097/BRS.0b013e31818ee263 | ||
Sasso RC, Anderson PA, Riew KD, Heller JG. Results of cervical arthroplasty compared with anterior discectomy and fusion: four year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am. 2011;93(18):1684–1692. doi:10.2106/JBJS.J.00476 | ||
Zhang X, Zhang X, Chen C, et al. Randomized, controlled, multicenter clinical trial comparing Bryan cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine. 2012;37(6):433–438. doi:10.1097/BRS.0b013e31822699fa | ||
Mummaneni PV, Burkus JK, Haid RV. Clinical and radiographic of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6(3):198–209. doi:10.3171/spi.2007.6.3.198 | ||
Burkus JK, Traynelis VC, Haid RW Jr, Mummanei PV. Clinical and radiographic analysis of an artificial cervical disc: 7 year follow-up from the Prestige prospective randomized controlled clinical trial: clinical article. J Neurosurg Spine. 2014;21(4):516–528. doi:10.3171/2014.6.SPINE13996 | ||
Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B. Results of the prospective, randomized, controlled multicenter Food and Administration investigational device exemption study of the Prodisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomaticervical disc disease. Spine J. 2009;9:275–286. doi:10.1016/j.spinee.2008.05.006 | ||
Zigler JE, Delamarter RB. Five year results of the prospective, randomized, multicenter, Food and Drugs Administration investigational device exemption study of the ProDisc-L total replacement versus circumferential arthrodesis for the treatment of single-level degenerative disc disease. Clinical article. J Neurosurg. 2013;17(6):493–501. | ||
Janssen ME, Zigler JE, Spivak JM, Delamarter RB, Darden BV 2nd, Kopjar B. Prodisc-C total disc replacement versus anterior cervical discectomy and fusion for single level symptomatic cervical disc disease: seven year follow-up of the prospective randomized U.S. Food and Drug Administration Investigational Device Exemption Study. J Bone Joint Surg Am. 2015;97(21):1738–1747. doi:10.2106/JBJS.N.01186 | ||
Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, Gordon CR. Prostpective randomized multicenter study of cervical arthroplasty: 269 patients from the Kineflex-C artificial disc investigational device exemption study with minimum 2 yrs follow-up: clinical article. J Neurosurg Spine. 2011;15(4):348–358. | ||
Vaccaro A, Beutler W, Peppelman W, et al. Clinical outcomes with selectively constrained Secure-C cervical disc arthroplasty: two year results from the prospective, randomized, controlled multicenter investigational device exemption study. Spine. 2013;38:2227–2239. doi:10.1097/BRS.0000000000000031 | ||
Hisey MS, Zigler JE, Jackson R, et al. Prospective randomized comparison of one level Mobi-C cervical total disc replacement vs anterior cervical discectomy and fusion: results at 5 year follow up. Int J Spine Surg. 2016;10:10. doi:10.14444/3010 | ||
Davis RJ, Nunley PD, Kim KD, Hisey MS, Jackson RJ, Bae HW. Two level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine. 2015;22(1):15–25. doi:10.3171/2014.7.SPINE13953 | ||
Radcliff K, Lerner J, Yang C, Bernard T, Zigler JE. Seven-year cost-effectiveness of ProDisc-C total disc replacement: results from investigational device exemption and post-approval studies. J Neurosurg Spine. 2016;24(5):760–768. doi:10.3171/2015.10.SPINE15505 | ||
Jackson RJ, Davis RJ, Hoffman GA, et al. Subsequent surgery rates after cervical total disc replacement using a Mobi-C Cervical Disc Prothesis versus anterior cervical discectomy and fusion: a prospective randomized clinical trial with 5 year follow up. J Neurosurg Spine. 2016;24(5):734–745. doi:10.3171/2015.8.SPINE15219 | ||
Gornet MF, Burkus JK, Shaffrey ME, Argires PJ, Nian H, Harrell FE Jr. Cervical arthroplasty with PRESTIGE LP discus versus anterior cervical discectomy and fusion: a prospective, multicenter investigational device excemption study. J Neurosurg Spine. 2015;23:558–573. doi:10.3171/2015.1.SPINE14589 | ||
Gornet MF, Burkus JK, Shaffrey ME, Nian H, Harrel FE Jr. Cervical disc arthroplasty with Prestige LP Disc versus anterior cervical discectomy and fusion: seven years outcomes. In J Spine Surg. 2016;10:24. doi:10.14444/3024 | ||
Gornet MF, Lanman TH, Burkus JK, Hodges SD, McConnel JR, Dryer RF. Cervical disc arthroplasty at two levels with PRESTIGE LP disc versus anterior cervical discectomy and fusion: results of a prospective, multicenter pivotal clinical trial at twenty four months. J Neurosurg Spine. 2017;26(6):653–667. doi:10.3171/2016.10.SPINE16264 | ||
Phillips FM, Lee JYB, Geisler FH, Cappucino A, Chaput CD, DeVine JG. A prospective randomized controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. 2 years results from the US FDA IDE clinical trials. Spine. 2013;38:E907–E918. doi:10.1097/BRS.0b013e318296232f | ||
Phillips FM, Geisler FH, Gilder KM, Reah C, Howell KM, McAfee PC. Long-term outcomes of the US FDA IDE prospective randomized controlled clinical trial comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. Spine. 2015;40(10):674–683. doi:10.1097/BRS.0000000000000869 | ||
Skeppholm M, Lindgren L, Henriques T, Vavruch L, Loefgren H, Olerud C. The discover artificial disc replacement versus fusion in cervical radiculopathy – a randomized controlled outcome trial with 2 year follow up. Spine J. 2015;15(6):1284–1294. doi:10.1016/j.spinee.2015.02.039 |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


 low,
low,  high,
high,  unclear.
unclear.