Back to Journals » Drug Design, Development and Therapy » Volume 18
Safety and Effectiveness of a Biosimilar Recombinant Human Growth Hormone in Children Requiring Growth Hormone Treatment: Analysis of Final Data from PATRO Children, an International, Post-Marketing Surveillance Study
Authors Loche S, Kanumakala S, Backeljauw P, Schwab KO, Lechuga-Sancho AM, Esmael A, Urosevic D, Boldea A, Zabransky M
Received 13 September 2023
Accepted for publication 29 January 2024
Published 2 March 2024 Volume 2024:18 Pages 667—684
DOI https://doi.org/10.2147/DDDT.S440009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Sandro Loche,1 Shankar Kanumakala,2 Philippe Backeljauw,3 Karl Otfried Schwab,4 Alfonso M Lechuga-Sancho,5– 7 Altaher Esmael,8 Dragan Urosevic,9 Anca Boldea,8 Markus Zabransky8
1Endocrinologia Pediatra e Centro, Screening Neonatale, Ospedale Pediatrico Microcitemico “A. Cao”, Cagliari, Italy; 2University Hospitals Sussex NHS Trust, Royal Alexandra Children’s Hospital, Brighton, UK; 3Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA; 4Department of Pediatrics, University Medical Center, Freiburg, Germany; 5Servicio de Pediatría, Hospital Universitario Puerta del Mar, Cádiz, Spain; 6Departamento Materno Infantil y Radiología, Universidad de Cádiz, Cádiz, Spain; 7Instituto de Investigación e Innovación Biomédica de Cádiz (INiBICA), Cádiz, Spain; 8HEXAL AG (a Sandoz company), Holzkirchen, Germany; 9Novartis Sandoz Biopharmaceutical AG, c/o HEXAL AG, Basel, Switzerland
Correspondence: Sandro Loche, Endocrinologia Pediatra e Centro, Screening Neonatale, Ospedale Pediatrico Microcitemico “A. Cao”, Via Jenner, 09121 Cagliari, Italy, Email [email protected]
Purpose: Omnitrope® (somatropin) was approved as a biosimilar recombinant human growth hormone (rhGH) in 2006. Here, we report final data from the PAtients TReated with Omnitrope® (PATRO) Children study, a post-marketing surveillance study designed to monitor the long-term safety and effectiveness of this treatment in pediatric patients.
Methods: The study population included all pediatric patients treated with Omnitrope® (biosimilar rhGH), administered via daily injection, in routine clinical practice. The primary objective was to assess long-term safety, with effectiveness assessed as a secondary objective.
Results: In total, 7359 patients were enrolled and treated in the PATRO Children study; 86.0% were treatment-naïve at baseline. Growth hormone deficiency was the most frequent indication (57.9%), followed by patients born small for gestational age (SGA; 26.6%). The mean (SD) duration of exposure to biosimilar rhGH was 3.66 years (2.39). A total of 16,628 adverse events (AEs) were reported in 3981 (54.1%) patients, most of which were mild/moderate. AEs suspected to be treatment related occurred in 8.3% of patients, most frequently headache (1.6%), injection-site pain (1.1%), or injection-site hematoma (1.1%). The incidence rate (IR) of type 2 diabetes mellitus was 0.11 per 1000 person-years (PY) across all patients, and 0.13 per 1000 PY in patients born SGA. The IR of newly diagnosed primary malignancies was 0.22 per 1000 PY across all patients. In the 6589 patients included in the effectiveness population, a sustained catch-up growth was observed across all indications. After 5 years of treatment, height SDS increased from baseline by a median (range) of +1.79 (– 3.7 to 6.2) in treatment-naïve patients and +0.73 (– 1.4 to 3.7) in pretreated patients.
Conclusion: This final analysis of the PATRO Children study indicates that biosimilar rhGH is well tolerated and effective in real-world clinical practice. These data are consistent with the well-characterized safety profile of rhGH treatment in pediatric patients.
Plain Language Summary: Why was the study done?Injections of growth hormone medicine can be used to increase height velocity in children with growth disorders.It is important to monitor the safety of long-term growth hormone treatment, especially regarding the risk of cancer and diabetes.
What did the researchers do?PATRO Children was a study designed to look at the safety of using a growth hormone medicine for long periods of time.This study included children with several different growth disorders who were treated with a growth hormone medicine called Omnitrope®, which is administered via daily injections, and followed them throughout their treatment.The researchers aimed to evaluate the risk of specific safety concerns in these patients, including cancer and diabetes.
What did the researchers find?A total of 7359 patients were enrolled from 304 sites around the world.Overall, 8.3% of patients had an adverse reaction that was considered to be related to growth hormone treatment.The most common adverse reaction considered related to growth hormone was headache, which occurred in 1.6% of patients.The risk of developing diabetes and cancer was low and comparable to what has been seen in previous studies in patients treated with growth hormone, and in the general population.
What do the findings mean?
The adverse reactions seen in this study were consistent with the well-characterized safety profile of growth hormone treatment in pediatric patients. This supports previous studies showing growth hormone treatment is not associated with an increased risk of cancer or diabetes in children.
Keywords: Omnitrope, PATRO Children, growth hormone, pediatrics, growth hormone deficiency, small for gestational age
Introduction
Recombinant human growth hormone (rhGH) replacement therapy has been used in children with growth hormone deficiency (GHD) since the 1950s, and has been shown to stimulate linear growth and increase growth rate.1,2 rhGH was first approved in 1985 for children with GHD, and since then, an accumulation of evidence has helped establish the efficacy, effectiveness, and safety of this therapy in this population.3 rhGH treatment has also been approved to treat children with other growth disorders, including short children born small for gestational age (SGA), Turner syndrome (TS), children with idiopathic short stature (ISS), Prader-Willi syndrome (PWS), chronic renal insufficiency (CRI), and Noonan syndrome.3,4
Following development that began in 1996, Omnitrope® (somatropin, Sandoz GmbH, Kundl, Austria; henceforth referred to as “biosimilar rhGH”) was the first biosimilar to be approved by the European Medicines Agency (EMA) in 2006. Approval was granted on the basis that the biosimilar rhGH matches the reference medicine (Genotropin® [somatropin], Pfizer Limited, Sandwich, United Kingdom) in terms of safety, efficacy, and quality.5 Biosimilar rhGH is administered via daily subcutaneous injections.5
To support and complement the efficacy and safety profile of biosimilar rhGH in infants, children, and adolescents demonstrated in clinical studies, the PAtients TReated with Omnitrope® (PATRO) Children study was initiated to provide real-world safety and efficacy data for biosimilar rhGH in pediatric patients.6,7 PATRO Children is an observational, multicenter, international, longitudinal study conducted as part of the risk management plan for biosimilar rhGH. Interim data from this study have been consistent with those seen in clinical studies, and suggest biosimilar rhGH is well tolerated and effective in real-world clinical practice.8–10 Here, we present the final analysis of the PATRO Children safety and effectiveness data of biosimilar rhGH treatment. This analysis followed agreement from the EMA to close this study based on the wealth of evidence accumulated to date with biosimilar rhGH, with a final database lock in July 2020.
Materials and Methods
The design of the study has been described previously in detail.8 Eligible patients were male or female infants, children, or adolescents receiving biosimilar rhGH whose parents/carers provided informed consent. Patients who had received treatment with another rhGH product before starting biosimilar rhGH therapy were also eligible for inclusion; these patients were described as “pretreated”. The patients who had not received treatment with another rhGH product before starting biosimilar rhGH therapy were described as “treatment-naïve”. Decisions to prescribe biosimilar rhGH were at the discretion of the treating physician and independent of participation in this study. The frequency of visits was at the discretion of the treating physician, with no specific tests or assessments required as part of the study. All adverse events (AEs), including serious AEs (SAEs) were monitored and recorded for the complete duration of biosimilar rhGH treatment. Particular emphasis was placed on long-term safety; diabetogenic risk; risk of malignancies; treatment-related AEs that are unexpected and/or unique to patients with PWS; and the occurrence and potential clinical impact of anti-rhGH antibodies. Long-term effectiveness outcomes were assessed as a secondary objective, including height standard deviation score (SDS) and height velocity SDS, derived from country-specific reference tables from the general population.11 All assessments and laboratory tests were carried out locally, except anti-rhGH assessments, which could be assessed locally (all countries) or in a central laboratory upon request from the treating physician (Germany, Sweden, and Taiwan only).
Data Collection and Study Populations
Data collection started in September 2007. Patient data were entered into an electronic case report form (eCRF) at each routine visit and subsequently into a web-based electronic data collection system. eCRFs were reviewed by a data management team and centralized monitoring was performed by a contract research organization. The safety population consisted of all patients who received at least one dose of biosimilar rhGH. Patients for whom neither a visit date nor a start date of biosimilar rhGH treatment had been documented, were not included. The effectiveness population was a subset of the safety set, and consisted of all patients with a documented height measurement at baseline visit (start of biosimilar rhGH treatment) and at least one dated height measurement under biosimilar rhGH treatment. Data from patients who discontinued treatment before reaching adult height were retained and included in all analyses.
Results
Patient Characteristics
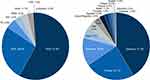
In this assessment (final database lock July 23, 2020), 7359 patients from 304 sites in 15 countries had been enrolled into the PATRO Children study (Figure 1). These patients made up the safety population. GHD was the most frequent indication (57.9% of patients), followed by patients born SGA (26.6% of patients). Most (86%) patients were treatment-naïve at baseline, and 1161 (15.8%) patients were pubertal at baseline. Characteristics of enrolled patients are shown in Table 1.
 |
Table 1 Patient Characteristics at Baseline |
Biosimilar rhGH Treatment
Mean (standard deviation [SD]) baseline doses for each indication are shown in Table 1. The mean (SD) duration of exposure to biosimilar rhGH was 3.66 (2.39) years. The mean (SD) duration of exposure for each indication in years was: GHD 3.56 (2.32), TS 3.54 (2.38), CRI 2.77 (2.08), SGA 3.86 (2.47), PWS 4.97 (2.86), ISS 3.22 (1.94), other 3.66 (2.32). Overall, the study collected data from 26,957 person-years (PY) of treatment experience.
Treatment Discontinuation
Primary reasons for treatment discontinuation are shown in Table 2. In patients who discontinued prior to study closure, the most frequent primary reason for discontinuation was having reached adult height or due to bone age maturity (n = 891; 12.1%). Lost to follow-up (n = 593; 8.1%), site closure (n = 591; 8.0%), and patient reached near-adult height (n = 589; 8.0%) were the next most frequent primary reasons for discontinuation. Discontinuation due to AEs was the primary reason for discontinuation in 140 (1.9%) patients.
 |
Table 2 Reasons for Treatment Discontinuation |
Adverse Events
A summary of reported AEs and SAEs is shown in Table 3. A total of 16,628 AEs were reported in 3981 (54.1%) patients (147.68 per 1000 PY) and 2063 SAEs in 1074 (14.6%) of patients (incidence rate [IR]: 39.84 per 1000 PY). Most AEs were mild or moderate and were resolved completely in 80.7% of patients (n = 3213/3981), and without any change to biosimilar rhGH dose in 95.6% of patients (n = 3808/3981). A similar trend was seen with SAEs, which resolved completely in 74.8% of patients (n = 803/1074), and without any change to biosimilar rhGH dose in 92.2% of patients (n = 990/1074). AEs suspected to be related to biosimilar rhGH occurred in 614 patients (8.3%; n = 835 events; IR: 22.78 per 1000 PY). The most frequent AEs with a suspected relationship to biosimilar rhGH were headache (1.6% of patients), injection-site pain (1.1% of patients), injection-site hematoma (1.1% of patients), and arthralgia (0.6% of patients). SAEs suspected to be related to biosimilar rhGH occurred in 84 patients (1.1%; n = 113 events; IR: 3.12 per 1000 PY). The most frequent SAEs with a suspected relationship to biosimilar rhGH were hypothyroidism (0.2% of patients) and sleep apnea syndrome (0.1% of patients).
 |
Table 3 Summary of AEs and SAEs |
In the 235 patients with PWS, AEs suspected to be related to biosimilar rhGH occurred in 39 patients (16.6%; n = 56 events; IR: 33.38 per 1000 PY). All were expected AEs in children with PWS; there were no unexpected or unique AEs in these patients. The most frequent AEs with a suspected relationship to biosimilar rhGH in patients with PWS were sleep apnea syndrome (6.4% of patients), tonsillar hypertrophy (2.1% of patients), and adenoidal hypertrophy (1.7% of patients). SAEs suspected to be related to biosimilar rhGH occurred in 22 patients with PWS (9.4%; n = 34 events; IR: 18.83 per 1000 PY). The most frequent SAEs with a suspected relationship to biosimilar rhGH in patients with PWS were sleep apnea syndrome (n = 11), adenoidal hypertrophy (n = 4), and tonsillar hypertrophy (n = 4).
AEs of Interest Possibly Related to Biosimilar rhGH Treatment
A summary of reported new cases of the development of type 1 and 2 diabetes mellitus (T1D, T2D), along with new and pre-existing malignancies, is shown in Table 4. T1D was diagnosed in three patients, all born SGA, and in one of these patients was reported to be suspected as related to biosimilar rhGH treatment. T2D was diagnosed in three patients: one patient with GHD (suspected as related to biosimilar rhGH treatment), one patient born SGA (not suspected as related to biosimilar rhGH treatment), and one patient with PWS (suspected as related to biosimilar rhGH treatment). The IRs for developing T1D or T2D across all indications was 0.11 per 1000 PY (same IR for both T1D and T2D). The IR for developing T2D was 0.13 per 1000 PY in patients born SGA and 0.86 per 1000 PY in patients with PWS. A small proportion of patients developed glucose metabolism disorders: 82 events in 81 (1.10%) patients with an IR of 3.00 per 1000 PY across all indications (Table 5).
 |
Table 4 Summary of Occurrence of T1D, T2D, and Malignancies |
 |
Table 5 Incidence of Glucose Metabolism Disorders |
A total of 10 new malignancies were diagnosed in seven patients during the study (GHD, n = 6; TS, n = 1; Table 4). No relationship with biosimilar rhGH treatment was suspected for any of these malignancies. One further patient experienced a benign pituitary tumor and a pineal gland cyst; it was unknow if the newly occurring neoplasm was malignant. One patient with TS had two events of new primary malignancies: a malignant astrocytoma, followed by neoplasm progression in the fourth year after biosimilar rhGH treatment. One patient with GHD experienced three events of new malignancies: neuroendocrine tumor, followed by two secondary malignancies (metastases to the liver and peritoneum). One patient with GHD and history of medulloblastoma and radiation treatment developed papillary thyroid cancer, which was regarded as a second primary malignancy. Newly diagnosed primary malignancies, each reported in a single patient with GHD, included acute lymphocytic leukemia, neuroblastoma, myelodysplastic syndrome, and testicular germ cell cancer. The IR of newly diagnosed malignancies was 0.26 per 1000 PY across all indications. The IR of newly diagnosed primary malignancies was 0.22 per 1000 PY across all indications.
Nine malignancies in seven (0.095%) patients (all with GHD and treatment-naïve) were considered recurrences or progressions of pre-existing malignancies that occurred during this study period (Table 4). Recurrences or progressions of pre-existing malignancies were suspected to be related to biosimilar rhGH treatment in two patients: one patient experienced progression of a pilomyxoid astrocytoma 2.3 years after the start of biosimilar rhGH treatment that resolved with sequelae, and one patient with a history of pituitary enlargement (thickness of pituitary stalk) was diagnosed with a central nervous system (CNS) germinoma (progressive thickness of pituitary stalk) 3 years after the start of biosimilar rhGH treatment. The IR was 0.26 per 1000 PY for patients who had recurrences or progression of a pre-existing malignancy and were treatment-naïve.
In total, six (0.1%) patients with GHD died during this study. None of these deaths were considered to be related to biosimilar rhGH treatment (Table 6).
 |
Table 6 Summary of Deaths Across All Indications |
AEs Leading to Discontinuation
AEs leading to discontinuation were documented in 141 (1.9%) patients. Two additional patients discontinued the study due to an AE, but the respective AE was not documented. In 76 patients (53.9% of those who discontinued therapy), the AE leading to discontinuation was considered as related to biosimilar rhGH treatment. Of these AEs, the most frequent were no response to treatment (15 patients), injection-site pain (nine patients), and headache (six patients).
AEs of Special Interest
AEs of special interest were documented in three patients: cerebral hemorrhage (one patient with GHD), intracranial aneurysm (one patient with GHD), subarachnoid hemorrhage and intraventricular hemorrhage (two AEs, both reported in one patient with TS); all were considered as not related to biosimilar rhGH treatment.
Insulin-Like Growth Factor I Values
Insulin-like growth factor I (IGF-I) values were categorized as below, within, or above normal range, according to the local laboratory reference ranges. In all treatment-naïve patients with available data, 67.3% of patients had IGF-I levels within normal range at baseline, rising to 80.9% of patients after 1 year of treatment (Figure 2). Concurrently, the number of patients with IGF-I levels below normal dropped from 31.7% at baseline to 4.5% after 1 year of treatment, and 2.4% after 2 years. Beyond 2 years of treatment, the proportion of patients within the different IGF-I categories (low, normal, high) was broadly stable, with 74.1–78.4% of patients within the normal range at each subsequent time point up to Year 8. Patterns were generally similar across the different indications, with the proportion of patients with IGF-I levels below normal dropping from baseline to 1 year, and the proportion of patients within the different IGF-I categories broadly stable from 2 years onwards.
Anti-rhGH Antibody Assessments
Overall, only a low number of anti-rhGH antibody assessments were carried out in real-world clinical practice. In all 31 patients with pre-baseline assessments, the results were negative. In one out of 114 patients with an anti-rhGH antibody assessment at baseline, the result was positive. The patient had GHD and was treatment-naïve. All three post-baseline assessments over 1.5 years were negative in this patient. The patient had a height SDS score of –3.254 at baseline and achieved a height SDS of –1.021 after 5 years of treatment at 9 years old, before study discontinuation due to “physician decision”. In all 77 patients with assessments after the start of biosimilar rhGH treatment, the results were negative.
Effectiveness
Efficacy was assessed in the 6589 patients included in the effectiveness population. A sustained catch-up growth across all indications was observed as early as 6 months after initiation of biosimilar rhGH treatment compared with baseline, in both treatment-naïve and pretreated patients. In treatment-naïve patients, height SDS increased from baseline by a median (range) of +0.65 (–1.5 to 3.3) at 1 year and +1.79 (–3.7 to 6.2) at 5 years. In treatment-naïve patients, height velocity SDS (peak centered; PC) increased from –3.02 (–11.9 to 12.0) at baseline to +3.85 (–8.7 to 16.5) after 1 year and +1.18 (–9.0 to 11.7) after 5 years. Mean change in height SDS compared with the previous year in treatment-naïve patients is shown by indication in Figure 3. Mean change in height velocity SDS compared with the previous year in treatment-naïve patients is shown by indication in Figure 4. As can be seen in these figures, the greatest yearly increase in height SDS and height velocity SDS was observed from baseline to the first year of treatment.
In pretreated patients, height SDS increased from baseline by a median (range) of +0.24 (–3.8 to 2.9) at 1 year and +0.73 (–1.4 to 3.7) at 5 years. Height velocity SDS (PC) increased from –0.80 (–9.0 to 12.8) at baseline to +1.57 (–6.3 to 14.6) after 1 year and +0.49 (–5.6 to 11.8) after 5 years. Overall, a total of 1628 (24.7%) patients reached adult height according to the physician’s opinion (22.7% of treatment-naïve patients and 36.9% of pretreated patients). The overall median (range) baseline height SDS was –2.54 (–6.6 to 2.9). The overall median (range) adult height SDS was –1.50 (–9.3 to 2.7). The mean (SD) age when reaching adult height was 15.87 (1.60) years in treatment-naïve patients and 15.98 (1.81) years in pretreated patients. For boys, this was 16.54 (1.21) years in treatment-naïve patients and 16.62 (1.77) years in pretreated patients, while for girls, this was 15.44 (1.66) years in treatment-naïve patients and 15.26 (1.57) years in pretreated patients. Figure 5 shows the difference between the projected height and targeted height (PH–TH; target height derived from the height of the patient’s mother and father) for the effectiveness population. At baseline, PH–TH was −11.73 cm; after 8 years’ treatment, PH–TH was 0.12 cm.
 |
Figure 5 Mean difference between projected height and targeted height by visit (effectiveness population). Abbreviation: PH–TH, projected height minus targeted height. |
Discussion
The PATRO Children post-marketing surveillance study provides data on the long-term safety and effectiveness of biosimilar rhGH treatment in children in a real-life clinical setting. Overall, 26,957 PY of safety data in 7359 patients were documented during PATRO Children from 2006 to 2020. Based on the current analysis, biosimilar rhGH is well tolerated and effective in both treatment-naïve patients and pretreated patients who were indicated for rhGH therapy. No signs of an increased diabetogenic potential, increased risk of new or recurrent malignancies, or anti-rhGH antibody-induced lack or loss of efficacy has been observed across all the indications. There were no reports of any unexpected AEs in patients with PWS.
The safety profile of biosimilar rhGH is consistent with the interim results from PATRO Children previously reported,8–10 and from other observational studies of long-term rhGH therapy.12–19 Data presented here are also comparable with previous registry data accumulated with the reference medicine. Nonetheless, there are challenges comparing data between these studies due to differences in observational time periods and data collection.
In PATRO Children, 16,628 AEs were reported in 3981 patients (54.1%; 147.68 per 1000 PY) and 2063 SAEs in 1074 patients (14.6%; 39.84 per 1000 PY). AEs suspected to be related to biosimilar rhGH occurred in 614 patients (8.3%; n = 835 events; 22.78 per 1000 PY) and SAEs suspected to be related to biosimilar rhGH were reported in 84 patients (1.1%; n = 113 events; 3.12 per 1000 PY). GeNeSIS (the Genetics and Neuroendocrinology of Short Stature International Study) was carried out to monitor the safety and effectiveness of rhGH in pediatric patients and included 22,294 patients in the safety population from 1999 to 2015.13 As in PATRO Children, this study required reporting of all AEs, irrespective of whether a causal relationship with rhGH treatment was suspected. GeNeSIS reported SAEs in 567 (2.5%) patients, and assessed SAEs as causally related to rhGH in 82 patients (0.4%; IR not reported). KIGS (Kabi/Pfizer International Growth Database) was created to determine the long-term safety and treatment outcomes of pediatric rhGH therapy (Genotropin®). At data lock in 2012, it included 83,803 children with growth disorders from 52 countries.20 As in PATRO Children and GeNeSIS, KIGS required reporting of all AEs, irrespective of whether a causal relationship with rhGH treatment was suspected. SAEs were reported in 3108 (3.7%) patients, and assessed SAEs as causally related to rhGH treatment in 607 patients (0.7%; IR not reported). The International Cooperative Growth Study NutropinAq® European Registry (iNCGS) included 3493 patients in the safety population from 2005 to 2016.21 This study aimed to only collect information on SAEs (related and unrelated) and non-serious related AEs, limiting comparisons with PATRO Children. In iNCGS, 27 patients (0.8%; n = 30 events; IR not reported) reported treatment-related SAEs.21 A combined analysis of data collected from 676 clinics participating in two multicenter longitudinal observational studies: the NordiNet International Outcome Study (Europe; 2006–2016) and the ANSWER Program (USA; 2002–2016) included 37,702 patients and 130,476 PY of exposure to rhGH treatment.22 This combined analysis only reported SAEs (related and unrelated) and AEs considered possibly/probably related to rhGH treatment, again, limiting comparisons with PATRO Children. There were 190 (15.3% of all events) SAEs considered possibly/probably related to rhGH treatment in NordiNet/ANSWER.
Rates of SAEs and treatment-related SAEs observed in PATRO Children are slightly higher compared with those reported previously from the GeNeSIS, KIGS, iNCGS, and NordiNet/ANSWER studies. This likely reflects differences between the designs of the studies. As the primary objective of PATRO Children was to collect data on safety, it was handled like an interventional study in terms of collecting AEs and SAEs, which may have led to slightly higher rates. In addition, the patient populations were not completely comparable between these studies, with a higher proportion of patients born SGA and a lower proportion of patients with ISS in PATRO Children compared with the GeNeSIS and KIGS studies.
In PATRO Children, seven patients were diagnosed with 10 new malignancies during biosimilar rhGH treatment, none of which were suspected to be related to biosimilar rhGH treatment. Nine malignancies in seven patients were considered recurrences or progressions of pre-existing malignancies, two of which were suspected as related to biosimilar rhGH treatment. Since it is known that there is an increased risk of a second neoplasm in patients treated with somatropin after their first neoplasm, and this is included in the product label,1 these new and recurrent malignancies warrant further discussion.
Among the patients with new malignancies, one patient with TS had a newly diagnosed malignant astrocytoma and neoplasm progression. The other patients in the study with newly diagnosed malignancies were those with GHD. One patient experienced a medulloblastoma that led to GHD before treatment with biosimilar rhGH. The physician considered the development of papillary thyroid cancer in this patient as a consequence of radiation treatment of the medulloblastoma, and as such, a second primary cancer. A single patient had acute lymphocytic leukemia, which was treated with allogenic bone marrow transplantation prior to biosimilar rhGH treatment, and later developed a neuroendocrine tumor. Secondary to this, the patient had liver and peritoneal metastases. One patient had a history of pituitary disorder and was diagnosed with acute lymphocytic leukemia 2.3 years after starting biosimilar rhGH treatment. A single patient had a history of neurosecretory dysfunction that could have been related to the development of a malignant neuroblastoma prior to biosimilar rhGH treatment. One patient had secondary hypothyroidism reported as chronic illness and developed myelodysplastic syndrome 1.2 years after the start of biosimilar rhGH treatment. This patient was pretreated with another rhGH preparation (0.60 mg/day NutropinAq® [Roche, Basel, Switzerland]) and had an overall rhGH treatment duration of 34.6 months (2.88 years). This patient was the only rhGH-pretreated patient with a newly diagnosed malignancy. A single patient had cryptorchism and ectopic testis, which may have contributed to the development of testicular germ cell cancer, diagnosed during the second year of biosimilar rhGH treatment.
Recurrence or progression of pre-existing malignancies was reported only in patients with GHD. Cancer types included astrocytomas, medulloblastoma, Langerhans cell histiocytosis, papillary tumor of the pineal gland, and CNS germinoma. The two cases suspected as related to biosimilar rhGH treatment were progression of a pilomyxoid astrocytoma that occurred 2.3 years after the start of biosimilar rhGH treatment and resolved with sequelae, and a CNS germinoma diagnosed 3 years after the start of biosimilar rhGH treatment.
Overall, these data do not raise any new safety concerns regarding malignancies and are generally in line with reports from other long-term studies. Across all patients in PATRO Children, the IR was 0.26 per 1000 PY for newly diagnosed malignancies and 0.22 per 1000 PY for primary malignancies. In GeNeSIS, malignant primary neoplasms were reported in 14 patients (0.16 per 1000 PY), four of whom were considered as having a defined predisposition to cancer.13 In iNCGS, a total of 20 patients experienced a malignant or unspecified neoplasm; four of these were considered to be related to rhGH treatment (low grade astrocytoma, cholesteatoma, craniopharyngioma, and germ cell cancer; IR per 1000 PY not reported in publication).21 During the NordiNet/ANSWER studies, 56 patients reported 62 neoplasms, of which 22 (35.5%) were considered possibly rhGH treatment related, and three (4.8%) were considered probably rhGH treatment related (IR per 1000 PY not reported in publication).22 In SAGhE (Safety and Appropriateness of Growth hormone treatments in Europe), cancer incidence risk doubled; however, differences in presentation of data related to malignancies limit comparisons with PATRO Children.19 In KIGS, neoplasms of all-causality were reported in 0.7% of patients, and considered treatment related in 0.2% of patients (IR per 1000 PY not reported in publication).20
In PATRO Children, AEs of special interest were documented in two patients with GHD (cerebral hemorrhage and intracranial aneurysm) and in one patient with TS (subarachnoid hemorrhage and intraventricular hemorrhage); all were considered as not related to biosimilar rhGH treatment. In GeNeSIS, three cases of hemorrhagic stroke were reported, all in patients with considerable risk factors.13 Nonetheless, since findings from the French SAGhE study showed an increased risk of stroke, particularly hemorrhagic stroke in patients with GHD, ISS, or born SGA treated with rhGH,23 long-term monitoring of patients is important to further assess the cardiovascular safety of rhGH treatment. KIGS reported cerebral hemorrhage in seven patients, which was considered related to rhGH treatment in one patient, and intracranial hemorrhage was reported in three patients, which was considered related to rhGH treatment in two of these patients.20 There was one case of intraventricular hemorrhage and one case of subarachnoid hemorrhage, neither of which were considered related to rhGH treatment.20
In PATRO Children, the IRs for developing T1D or T2D were 0.11 per 1000 PY over all indications. The IR for developing T2D was comparable in patients born SGA (0.13 per 1000 PY). In the general untreated population, the International Diabetes Federation Atlas 10th edition reports incident cases per annum (1000s) for T1D in 0- to 14-year-olds as 108.3 worldwide, with rates of 24.7 reported in Europe, 18.7 in North America and Caribbean region, and 0.1 in Western Pacific.24 Recent global estimates for T2D in children (<20 years) from a systematic review reported IRs per country, ranging from 0.001 per 1000 PY in Germany to 0.907 per 1000 PY in Iran.25 In GeNeSIS, the incidence rates of T1D and T2D across all indications in patients treated with rhGH were 0.18 per 1000 PY and 0.17 per 1000 PY, respectively.13 The IR of T2D in patients born SGA was 0.35 per 1000 PY.13 These findings suggest that there is no meaningful increased risk of developing T1D or T2D due to treatment with rhGH in the general population, or developing T2D in patients born SGA.
Regarding baseline dose, this was generally similar across indications (~0.0300−0.0400 mg/kg/day), except for patients with PWS, where lower doses were observed (0.0235±0.0091 mg/kg/day [mean±SD]). This is likely due to earlier commencement of rhGH treatment in patients with PWS, or may suggest that treating physicians followed a more cautious approach in patients with PWS in general. This may have been due to concerns regarding the use of rhGH treatment in those with extreme obesity or disordered breathing.26 In addition, in patients with PWS, standard rhGH dosing regimens can be associated with high IGF-I levels; therefore, these patients may be treated with lower doses of rhGH to avoid elevated IGF-I. Nonetheless, the benefits of rhGH treatment in terms of improving body composition and normalizing adult height in children with PWS are well established.27,28
The reported effectiveness of biosimilar rhGH in PATRO Children is consistent with those from observational studies of other approved rhGH medicines.29–31 A substantial catch-up growth was observed with biosimilar rhGH treatment in both treatment-naïve and pretreated patients, which was sustained over approximately 12–13 years. Increase in height velocity was most pronounced during the first year of treatment. While data were limited because anti-rhGH antibody assessments were only performed in a small number of patients, there were no reports of anti-rhGH antibody-induced lack or loss of efficacy.
It is of interest that, in those who discontinued treatment, only 2.9% switched to another rhGH preparation, compared with 13.9% who had been pretreated with other rhGH products and switched to biosimilar rhGH. Future studies regarding satisfaction with different rhGH preparations and their devices would be helpful in making the most appropriate treatment choices for patients.
As with all observational studies, PATRO Children has its limitations. Data are collected according to routine clinical practice, rather than using strict standardized protocols in well-designed clinical trials, so there is the potential for missing data that risks skewing the results and conclusions drawn. In addition, most assessments and laboratory tests, including IGF-I measurements, were carried out locally, rather than centrally, which may lead to variations between centers. Furthermore, the mean duration of biosimilar rhGH treatment in PATRO Children is approximately 3 years. This is in line with iNCGS (reported as median 3.2 years) and NordiNet/ANSWER (reported as mean 3.5 years), but shorter than in GeNeSIS (reported as mean 4.9 years). Thus, since some AEs, such as malignancies, may take several years to develop, and increased risk of stroke was reported in SAGhE, continued patient follow-up is required.
Conclusion
In summary, data from the PATRO Children study indicate that biosimilar rhGH is a well-tolerated and effective therapy in its approved indications in children of different ages. Beyond clinical trials, biosimilar rhGH has generated more than 300 million patient-days of treatment experience across 80 approved countries worldwide (across approved rhGH indications) until January 2022.32 The growing clinical experience with EMA-approved biosimilars, including biosimilar rhGH, should offer additional reassurance to healthcare professionals and patients that these agents are as effective and well tolerated as others, thus widening patients’ access to life-changing treatments worldwide.
Abbreviations
AE, adverse event; BMI, body mass index; CNS, central nervous system; CRI, chronic renal insufficiency;eCRF, electronic case report form; EMA, European Medicines Agency; GeNeSIS, Genetics and Neuroendocrinology of Short Stature International Study; GHD, growth hormone deficiency; IGF-I, insulin-like growth factor I; iNCGS, International Cooperative Growth Study NutropinAq® European Registry; IR, incidence rate; ISS, idiopathic short stature; KIGS, Kabi/Pfizer International Growth Database; LCH, Langerhans cell histiocytosis; PATRO, PAtients TReated with Omnitrope®; PC, peak centered; PH–TH, projected height minus targeted height; PWS, Prader-Willi syndrome; PY, person-years; rhGH, recombinant human growth hormone; SAE, serious adverse event; SAF, safety analysis set; SAGhE, Safety and Appropriateness of Growth hormone treatments in Europe; SD, standard deviation; SDS, standard deviation score; SGA, small for gestational age; T1D, type 1 diabetes mellitus; T2D, type 2 diabetes mellitus; TS, Turner syndrome; UK, United Kingdom; USA, United States.
Ethics Approval and Informed Consent
The PATRO Children study protocol was approved by the ethics review committee of participating centers in accordance with national laws and regulations. The study was conducted according to the ethical principles of the Declaration of Helsinki (1964) and its later amendments. All patients (and/or their parents/guardians) provided written informed consent. Patients are permitted to withdraw their informed consent at any time or discontinue treatment for any reason.
Acknowledgments
The authors would like to thank all patients and investigators who participated in the PATRO Children study. Medical writing support was provided by Eve Blumson Ph.D. and Caroline McGown Ph.D. of Apollo, OPEN Health Communications, and funded by Sandoz/Hexal AG, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022). Statistical input was provided by Masiur Rahman of Novartis Pharmaceuticals, Wallingford, CT, USA.
Funding
The PATRO Children study was funded by HEXAL AG (a Sandoz company). HEXAL AG (a Sandoz company) was involved in all stages of the study, from design to submission of the paper for publication.
Disclosure
Sandro Loche is a member of the PATRO Children Advisory Board and the PATRO Children Global Steering Committee, and has received advisory board and lecture fees from Sandoz, Merck Serono, Ipsen, and Pfizer. Shankar Kanumakala has received support to attend scientific meetings from Sandoz in previous years, and is a member of the PATRO Children Global Steering Committee. Philippe Backeljauw is a member of the PATRO Children Advisory Board, and has received advisory board, consultancy, and lecture fees from Novartis/Sandoz, BioMarin, Ascendis, Tolmar, Cavalry Biosciences, Novo Nordisk, and Ipsen. Karl Otfried Schwab is a member of the German PATRO Children Advisory Board and the PATRO Children Advisory Board, and has received advisory board and lecture fees from Akcea, Alexion, Merck Healthcare, Novartis, Pfizer, and Sanofi-Aventis. Alfonso M Lechuga-Sancho is a member of an external advisory board for Sandoz and Ipsen, and has received research grants from Abbott, Medtronic, Menarini, Merck Serono, Sanofi, and Novo Nordisk. Altaher Esmael and Markus Zabransky are employees of HEXAL AG (a Sandoz company). Dragan Urosevic is an employee of Novartis Sandoz Biopharmaceutical AG. Anca Boldea was an employee of HEXAL AG (a Sandoz company) when the study and manuscript development were undertaken. The authors report no other conflicts of interest in this work.
References
1. European Medicines Agency [Internet]. Omnitrope® summary of product characteristics, updated 2023; 2008. Available from: https://www.ema.europa.eu/en/documents/product-information/omnitrope-epar-product-information_en.pdf.
2. Tidblad A. The history, physiology and treatment safety of growth hormone. Acta Paediatr. 2022;111(2):215–224. doi:10.1111/apa.15948
3. Ranke MB, Wit JM. Growth hormone – past, present and future. Nat Rev Endocrinol. 2018;14(5):285–300. doi:10.1038/nrendo.2018.22
4. Dahlgren J, Noordam C. Growth, endocrine features, and growth hormone treatment in Noonan syndrome. J Clin Med. 2022;11(7):2034. doi:10.3390/jcm11072034
5. European Medicines Agency [Internet]. Omnitrope® European public assessment report (EPAR), updated 2018; 2008. Available from: https://www.ema.europa.eu/en/documents/overview/omnitrope-epar-summary-public_en.pdf.
6. López-Siguero J, Borrás Pérez MV, Balser S, Khan-Boluki J. Long-term safety and efficacy of the recombinant human growth hormone Omnitrope® in the treatment of Spanish growth hormone deficient children: results of a phase III study. Adv Ther. 2011;28(10):879–893. doi:10.1007/s12325-011-0063-8
7. Romer T, Saenger P, Peter F, et al. Seven years of safety and efficacy of the recombinant human growth hormone Omnitrope in the treatment of growth hormone deficient children: results of a phase III study. Horm Res. 2009;72(6):359–369. doi:10.1159/000249164
8. Pfäffle R, Schwab KO, Marginean O, et al. Design of, and first data from, PATRO Children, a multicentre, noninterventional study of the long-term efficacy and safety of Omnitrope® in children requiring growth hormone treatment. Ther Adv Endocrinol Metab. 2013;4(1):3–11. doi:10.1177/2042018813479644
9. Iughetti L, Tornese G, Street ME, et al. Long-term safety and efficacy of Omnitrope®, a somatropin biosimilar, in children requiring growth hormone treatment: Italian interim analysis of the PATRO Children study. Ital J Pediatr. 2016;42(1):93. doi:10.1186/s13052-016-0302-3
10. Pfäffle R, Bidlingmaier M, Kreitschmann-Andermahr I, et al. Safety and effectiveness of Omnitrope®, a biosimilar recombinant human growth hormone: more than 10 years’ experience from the PATRO Children study. Horm Res Paediatr. 2020;93(3):154–163. doi:10.1159/000508190
11. Hermanussen M, Assmann C, Wöhling H, Zabransky M. Harmonizing national growth references for multi-centre surveys, drug monitoring and international postmarketing surveillance. Acta Paediatr. 2012;101(1):78–84. doi:10.1111/j.1651-2227.2011.02415.x
12. Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95(1):167–177. doi:10.1210/jc.2009-0178
13. Child CJ, Zimmermann AG, Chrousos GP, et al. Safety outcomes during pediatric GH therapy: final results from the prospective GeNeSIS observational program. J Clin Endocrinol Metab. 2019;104(2):379–389. doi:10.1210/jc.2018-01189
14. Cutfield WS, Lindberg A, Rapaport R, Wajnrajch MP, Saenger P. Safety of growth hormone treatment in children born small for gestational age: the US trial and KIGS analysis. Horm Res. 2006;65(Suppl 3):153–159. doi:10.1159/000091719
15. Sävendahl L, Pournara E, Pedersen BT, Blankenstein O. Is safety of childhood growth hormone therapy related to dose? Data from a large observational study. Eur J Endocrinol. 2016;174(5):681–691. doi:10.1530/eje-15-1017
16. Sävendahl L, Maes M, Albertsson-Wikland K, et al. Long-term mortality and causes of death in isolated GHD, ISS, and SGA patients treated with recombinant growth hormone during childhood in Belgium, The Netherlands, and Sweden: preliminary report of 3 countries participating in the EU SAGhE study. J Clin Endocrinol Metab. 2012;97(2):E213–E217. doi:10.1210/jc.2011-2882
17. Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database). J Pediatr. 2010;157(2):265–270. doi:10.1016/j.jpeds.2010.02.028
18. Poidvin A, Weill A, Ecosse E, Coste J, Carel JC. Risk of diabetes treated in early adulthood after growth hormone treatment of short stature in childhood. J Clin Endocrinol Metab. 2017;102(4):1291–1298. doi:10.1210/jc.2016-3145
19. Swerdlow AJ, Cooke R, Beckers D, et al. Cancer risks in patients treated with growth hormone in childhood: the SAGhE European cohort study. J Clin Endocrinol Metab. 2017;102(5):1661–1672. doi:10.1210/jc.2016-2046
20. Maghnie M, Ranke MB, Geffner ME, et al. Safety and efficacy of pediatric growth hormone therapy: results from the full KIGS cohort. J Clin Endocrinol Metab. 2022;107(12):3287–3301. doi:10.1210/clinem/dgac517
21. Coutant R, Bosch Muñoz J, Dumitrescu CP, et al. Effectiveness and overall safety of NutropinAq® for growth hormone deficiency and other paediatric growth hormone disorders: completion of the International Cooperative Growth Study, NutropinAq® European Registry (iNCGS). Front Endocrinol. 2021;12:676083. doi:10.3389/fendo.2021.676083
22. Sävendahl L, Polak M, Backeljauw P, et al. Long-term safety of growth hormone treatment in childhood: two large observational studies: NordiNet IOS and ANSWER. J Clin Endocrinol Metab. 2021;106(6):1728–1741. doi:10.1210/clinem/dgab080
23. Poidvin A, Touzé E, Ecosse E, et al. Growth hormone treatment for childhood short stature and risk of stroke in early adulthood. Neurology. 2014;83(9):780–786. doi:10.1212/wnl.0000000000000737
24. Ogle GD, James S, Dabelea D, et al. Global estimates of incidence of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res Clin Pract. 2022;183:109083. doi:10.1016/j.diabres.2021.109083
25. Wu H, Patterson CC, Zhang X, et al. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. 2022;185:109785. doi:10.1016/j.diabres.2022.109785
26. Craig ME, Cowell CT, Larsson P, et al. Growth hormone treatment and adverse events in Prader-Willi syndrome: data from KIGS (the Pfizer International Growth Database). Clin Endocrinol. 2006;65(2):178–185. doi:10.1111/j.1365-2265.2006.02570.x
27. Damen L, Donze SH, Kuppens RJ, et al. Three years of growth hormone treatment in young adults with Prader-Willi syndrome: sustained positive effects on body composition. Orphanet J Rare Dis. 2020;15(1):163. doi:10.1186/s13023-020-01440-6
28. Lindgren AC, Lindberg A. Growth hormone treatment completely normalizes adult height and improves body composition in Prader-Willi syndrome: experience from KIGS (Pfizer International Growth Database). Horm Res. 2008;70(3):182–187. doi:10.1159/000145019
29. Bonfig W, Lindberg A, Carlsson M, et al. Efficacy of growth hormone treatment in children with type 1 diabetes mellitus and growth hormone deficiency—an analysis of KIGS data. J Pediatr. 2018;198:260–264. doi:10.1016/j.jpeds.2018.02.035
30. Polak M, Blair J, Kotnik P, Pournara E, Pedersen BT, Rohrer TR. Early growth hormone treatment start in childhood growth hormone deficiency improves near adult height: analysis from NordiNet® International Outcome Study. Eur J Endocrinol. 2017;177(5):421–429. doi:10.1530/eje-16-1024
31. Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB. Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: analysis of a large international database. J Clin Endocrinol Metab. 2006;91(6):2047–2054. doi:10.1210/jc.2005-2284
32. Sandoz. Data on file: Omnitrope periodic safety update report (PSUR); 2022.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.