Back to Journals » Clinical Ophthalmology » Volume 17
Safe Removal of Sticky Silicone Oil Using Perfluorocarbon Liquid Injection and Emulsification with a Fragmatome
Authors Takashina H , Watanabe A, Nakano T
Received 14 March 2023
Accepted for publication 23 May 2023
Published 29 May 2023 Volume 2023:17 Pages 1481—1488
DOI https://doi.org/10.2147/OPTH.S412379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hirotsugu Takashina,1 Akira Watanabe,2 Tadashi Nakano2
1Department of Ophthalmology, Tokyo Rosai Hospital, Tokyo, Japan; 2Department of Ophthalmology, The Jikei University School of Medicine, Tokyo, Japan
Correspondence: Hirotsugu Takashina, Department of Ophthalmology, Tokyo Rosai Hospital, 4-13-21 Omori-minami Ota-ku, Tokyo, 143-0013, Japan, Tel +81-3-3742-7301 (ext. 8116), Fax +81-3-3744-9310, Email [email protected]
Purpose: We examine the safe removal of a sticky silicone oil (SO) with an inconspicuous perfluorocarbon liquid (PFCL) layer.
Methods: In the first experiment, we injected PFCL into the SO bubble on the bottom of a transparent container or into the chili oil layer of an immiscible droplet composed of PFCL and chili oil on the posterior retina of a vitrectomized pig eye, in order to release the SO bubble or the chili oil layer from the downward adhesion. In the second experiment, we sucked out the SO layer of an immiscible droplet composed of PFCL and SO on the bottom of a transparent container or on the posterior retina of a vitrectomized pig eye using a fragmatome.
Results: In the first experiment, the PFCL injection caused the SO bubble to move from the bottom of the transparent container and the chili oil to move from the posterior retina of the pig eye. In the second experiment, when the suction pressure was high, this made it easy and possible to remove the SO layer in the transparent container or the vitrectomized pig eye when emulsification was done by a fragmatome.
Conclusion: Moving the SO layer of a sticky SO from the posterior retina after a PFCL injection followed by sucking out of the relocated SO layer using emulsification and high suction pressure through the utilization of a fragmatome can safely remove sticky SO with an inconspicuous PFCL layer.
Keywords: silicone oil, perfluorocarbon, fragmatome, emulsification, vitrectomy
Introduction
Sticky silicone oil (SO), which is defined as the unexpected adhesion of an SO bubble to the posterior retina, is one of the refractory complications of SO removal. Not only has sticky SO removal been causing issues over a long period of time, but it has also been reported that there are cases of unexpected occurrences of iatrogenic retinal tears during attempts to forcibly remove the sticky SO.1,2 It is known that the use of perfluorocarbon liquid (PFCL) during vitrectomy with SO tamponade can cause sticky SO1–5 and that the prior removal of the PFCL layer between the sticky SO and the retinal surface can make it easier to remove the sticky SO.3,4 Thus, the identity of a sticky SO is thought to be an immiscible droplet composed of PFCL and SO on the retinal surface. However, only a few previous studies have reported finding a visible PFCL layer between the SO layer of the sticky SO and the retinal surface.3,4 Moreover, optical coherence tomography performed in another study was not able to detect a PFCL layer between the sticky SO bubble and the retinal surface.1 Therefore, overall these results suggest that a clearly visible PFCL layer might not actually be present in the majority of sticky SO cases. In our present study, we examined a possible methodology that can potentially be used for the safe removal of a sticky SO with an inconspicuous PFCL layer. The Ethics Committee has ruled that approval was not required for this study, and we adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Materials and Methods
Two procedures are required in order to perform a safe removal of the sticky SO with an inconspicuous PFCL layer. In the first part, the SO layer of a sticky SO needs to be moved from the retinal surface in order to avoid any histological invasion when sucking out the SO layer. The second part involves the sucking out of the SO layer that was moved of a sticky SO without causing any histological invasion, such as iatrogenic retinal tear.
Relocation of the SO Layer of a Sticky SO with PFCL Injection
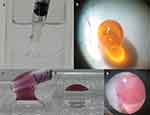
The first part of the procedure involves the moving of the SO layer of a sticky SO from the retinal surface via the use of a PFCL injection. We planned to perform this challenging injection of PFCL into the SO layer of an immiscible droplet composed of PFCL and SO, which is the identity of sticky SO, as creating an enlargement of the immiscible droplet due to the PFCL injection should result in moving of the SO layer from the retinal surface. We applied SO (SILIKONTM 1000; Alcon Laboratories, Inc., Fort Worth, TX) to the dry bottom of a transparent container and then filled the container with water. Subsequently, we then carefully injected additional SO on the original SO application area. The additionally injected SO easily adhered to the original SO application area (Figure 1a), with this viewed as being almost the same situation as that observed for sticky SO with an inconspicuous PFCL layer. Based on this supposition, we carefully injected PFCL (Perfluoron; Alcon Laboratories, Inc.) into the adhered SO bubble, in order to loosen and move the adhered SO bubble from the bottom. In a subsequent step, we then injected PFCL into the chili oil layer of the immiscible droplet composed of PFCL and chili oil on the posterior retina of the vitrectomized pig eye in an attempt to move the chili oil layer from the posterior retina (Figure 1b). This step, which was without utilization of SO, was performed because it is impossible to create a small immiscible droplet composed of PFCL and SO on the posterior retina of a vitrectomized pig eye via the pars plana. That is, this cannot be done due to the high viscosity of SO, which will disable an injection of a small amount of SO on the posterior retina. Therefore, chili oil, which is one of the soybean oils, was utilized as a substitute of SO, as the interfacial tension and the gravity of soybean oil are very close to that found for SO as compared to water.6 Moreover, due to the low viscosity of chili oil, this makes it possible to perform an injection of a small amount of chili oil on a posterior retina via a 27-gauge needle.
Use of a Fragmatome to Suck Out the SO Layer of a Sticky SO
The second part of the procedure involved sucking out the moved SO layer. Due to the length of each tube and the high viscosity of SO, it is impossible to suck out a SO bubble using a vitreous cutter or a backflush needle. Thus, the Viscous Fluid Control Pak® (VFC), which is the specialized tube for the utilization of SO during a surgery, was developed (Alcon Laboratories, Inc.). However, there is less intraocular operability due to the shortness of the VFC tube, which is thought to disturb the contact between the relocated SO layer and the tip of a VFC tube. Therefore, we attempted to remove the SO layer by utilizing emulsification using a fragmatome (Alcon Laboratories, Inc.) to overcome these issues. In this procedure, we injected PFCL on the bottom of a transparent container filled with water. Subsequently, we carefully placed nearly the same amount of a purple colored SO on the surface of the PFCL bubble so that it adhered and formed an immiscible droplet composed of PFCL and SO (Figure 1c and d), which was an intentionally created sticky SO. Afterward, we then attempted to suck out the SO layer of this immiscible droplet with/without emulsification using a fragmatome. Finally, we then attempted to suck out the SO layer of the created immiscible droplet composed of PFCL and SO on the posterior retina of a vitrectomized pig eye by performing emulsification using a fragmatome. This was done through insertion of the device via a temporary 20-gauge sclerotomy (the pore size of a fragmatome is 20-gauge), which was similar to an actual sticky SO in a human eyeball (Figure 1e).
Results
Movement of the SO Layer of a Sticky SO Using a PFCL Injection
The PFCL bubbles that were injected into the adhered SO bubble on the bottom of the transparent container were found to sink through the SO bubble (Figure 2a), after which they then moved underneath the SO bubble (Figure 2b). Subsequently, there was formation of an immiscible droplet composed of PFCL and SO (Figure 2c), with the SO bubble then released from the bottom of the transparent container. After the release of the SO bubble from the bottom, a further PFCL injection into the PFCL layer of the immiscible droplet resulted in movement of the SO layer from the bottom (Figure 2d). After these changes, the bottom of the transparent container was uniformly filled with only PFCL, without any boundaries observed between the different liquids, such as remnant SO. As a result, this made it possible for the entire SO bubble to be released from downward adhesion. Similarly, the PFCL injection into the chili oil layer of the created immiscible droplet on the posterior retina of the vitrectomized pig eye also caused movement of the chili oil layer from the posterior retina (Figure 3a and b).
Uses of a Fragmatome to Suck Out the SO Layer of a Sticky SO
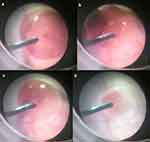
Prior to the experiment that we sucked out the SO layer in a pig eye, we conducted the initial experiment using a transparent container. Although it was impossible to suck out the SO layer of an immiscible droplet composed of PFCL and SO without emulsification using a fragmatome, even if the suction pressure was high (230 mmHg), this was possible when using emulsification with a fragmatome. By using emulsification with a fragmatome, this made it especially simple to suck out the SO layer when the suction pressure was high (230 mmHg) and the tip of the fragmatome was placed near the surface of the SO layer (Figure 4a–c). Conversely, when the suction pressure was low (30 mmHg) and/or the tip of the fragmatome was at the core of the SO layer, it became very difficult to suck out the SO layer. Subsequently, we then conducted a further experiment using a pig eye. When using emulsification and high suction pressure (230 mmHg) in conjunction with the fragmatome, this enabled the easy removal of the SO layer of the created immiscible droplet that was composed of PFCL and SO in the vitrectomized pig eye (Figure 5a–5d). However, when we repeated the procedure several times in a pig eye using the single suction tube, there were multiple SO clogging all over the suction tube (Figure 6), thereby leading to a worsening of the efficiency for the sucking out of the SO.
 |
Figure 6 Multiple clogging of the purple colored silicone oil in the suction tube. |
Discussion
The present experiments evaluated two procedures that used either a transparent container or a pig eye to resolve the hard removal of a sticky SO with an inconspicuous PFCL layer. Although removal of a sticky SO with internal limiting membrane peeling to avoid complications has been previously reported,7 the existence of more than a small histological invasion of the retina cannot be denied. Therefore, in this study, we evaluated procedures that did not cause any histological invasion of the retina during the removal of a sticky SO with an inconspicuous PFCL layer. By utilizing the formation of an immiscible droplet composed of PFCL and SO due to its low interfacial tension,3 this made it possible to attempt to inject PFCL to the SO bubble or the SO layer in order to enlarge the immiscible droplet that consisted of PFCL and SO. As a result, the enlargement of the immiscible droplet easily led to movement of the SO layer from the bottom of the transparent container or the posterior retina of the pig eye to the enough height without histological invasion during the following SO removal. Of course, surgeons should avoid the increase in intraocular pressure during PFCL injection by low perfusion pressure from the vitrectomy machine and adequate drainage of fluid from the sclerotomy. We performed the PFCL injection into the SO droplet instead of onto the SO droplet. This is because even if PFCL bubbles are dropped onto the SO droplet, the PFCL bubbles will only cover the surroundings of the SO droplet without getting into the SO droplet.
These results further suggested that an additional PFCL injection into the enlarged immiscible droplet should be able to lift up the SO layer to just beneath the crystalline lens or intraocular lens, if this procedure is performed during an actual surgery. However, it should be noted that due to the shortness of the VFC tube, the tip of the VFC tube was not able to reach the center of the relocated SO layer, which is in the vicinity of the orbital axis. Therefore, we attempted to suck out the SO layer using a fragmatome, as the emulsified SO during the late postoperative period can, in general, be easily sucked out when using a vitreous cutter. As we had expected, it was possible to suck out the SO layer with emulsification using a fragmatome, while this was not possible without emulsification. Even so, the sucking out of the SO layer with emulsification did prove to be troublesome when emulsifying the SO at the core of the SO layer and/or when there was low suction pressure (30 mmHg).
Furthermore, when the experiment was repeated for the emulsifying of the SO layer in the pig eye, there was a decreasing efficiency for the sucking out of the SO, which was presumed to be caused by the SO clogging the suction tube (Figure 6). Therefore, even after the SO is emulsified using a fragmatome one time, the formation of SO bubbles can clog a fragmatome or a suction tube. Our current findings infer that this is likely due to the innumerable bindings of the emulsified SO particles in the fragmatome and/or suction tube. Similarly, it was also our belief that the easy formation of SO bubbles during the emulsification of the SO at the core of the SO layer would lead to a difficult removal of the SO, as the suction inlet of a fragmatome would be filled with the emulsified SO. Conversely, there is less binding of the innumerable emulsified SO particles due to the mixture of emulsified SO and water, which can enable the easy suctioning of the SO layer during the emulsification of the SO near the surface of the SO layer. Even so, our results did show that suction of the SO layer with low suction pressure (30 mmHg) was troublesome. Therefore, higher suction pressures are needed for suction of SO with emulsification, even if emulsified SO and water are mixed. Based on the above experiments, the validity of the use of the two procedures inside of a vitreous cavity was confirmed, even though an in vivo experiment cannot be performed due to the rare occurrence of sticky SO. Furthermore, the procedures have the remarkable advantage in that they can be performed by instruments that in general are always prepared and ready to be used in operating rooms. Of course, utilizing a fragmatome in actual surgery requires more than enough caution to retinal damage, as with the treatment to nuclear fragments of a cataract fallen onto the posterior retina.
There were some limitations to our present study. First, we could not rule out the possibility of an SO or chili oil remnant at the molecular level beneath the PFCL layer of the immiscible droplet after the PFCL injection. However, because of the lack of any visible boundaries for the different liquids at the bottom of the transparent container, we believe that an SO bubble remnant that can potentially significantly disturb the visual function will not exist during an actual surgery. Second, we could not demonstrate the validity of this procedure for a sticky SO after the use of a long SO tamponade period. However, as it has been reported that there is no significant difference between the SO tamponade period and the occurrence of sticky SO,1,2 it is our belief that these procedures are valid due to the largely similar mechanism underlying the occurrence of sticky SO, even if there is a long SO tamponade period. Third, Veckeneer et al reported finding that the sticky patches had been at or near the posterior pole in all of their cases.2 However, if a sticky SO occurs at the peripheral retina, the injected PFCL into the SO layer will not move to the peripheral retina, and if this in fact does occur, then the procedures in this study may not be valid. In anyway, due to the fact that there is no attempt within humans as of yet, the applicability for this study may be limited. Therefore, when removal of SO layer is troublesome during an actual surgery, the surgeon should finish the surgery once with the sticky SO left in the vitreous cavity.
In conclusion, moving the SO layer of a sticky SO from the posterior retina using a PFCL injection followed by the sucking out of this relocated SO layer with emulsification and high suction pressure using a fragmatome can be safely used to remove a sticky SO with an inconspicuous PFCL layer.
Abbreviations
SO, silicone oil; PFCL, perfluorocarbon liquid; VFC, Viscous Fluid Control Pak®.
Acknowledgment
The authors thank Mai Goto MD, at Tokyo Rosai Hospital for providing the resources to perform the experiment and subsequent helpful discussions regarding the results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ghoraba HH, Zaky AG, Abd A, Fatah HM, Gemai E, Heikal MA. Sticky silicone oil. Retina. 2017;37:1599–1606. doi:10.1097/IAE.0000000000001377
2. Veckeneer MA, Voogd S, Lindstedt EW, Menz DH, van Meurs JC. An epidemic of sticky silicone oil at the Rotterdam Eye Hospital. Patient review and chemical analysis. Graefes Arch Clin Exp Ophthalmol. 2008;246:917–922. doi:10.1007/s00417-008-0768-9
3. Takashina H, Watanabe A, Nakano T. Influence of perfluorooctane liquids in the formation of sticky silicone oil. J Ophthalmol. 2022;2022:8434102. doi:10.1155/2022/8434102
4. Fukumoto M, Nishida Y, Kida T, Sato T, Kobayashi T, Ikeda T. A case of silicone oil adhered to the retinal surface via perfluorocarbon liquid. BMC Ophthalmol. 2018;18:82. doi:10.1186/s12886-018-0745-y
5. Dresp JH, Menz DH. The phenomenon of “sticky” silicone oil. Graefes Arch Clin Exp Ophthalmol. 2007;245:863–868. doi:10.1007/s00417-006-0450-z
6. Sahasrabudhe SN, Rodriguez-Martinez V, O’Meara M, Farkas BE. Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: measurement and modeling. Int J Food Prop. 2017;20:1965–1981.
7. Lin HS, Tang YP, Zhang L, et al. New technique for removal of perfluorocarbon liquid related sticky silicone oil and literature review. Int J Ophthalmol. 2021;14:1903–1908. doi:10.18240/ijo.2021.12.14
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.