Back to Journals » Journal of Blood Medicine » Volume 14
Safe Blood Donation from Donors Using Antihypertensive Medication. A Multi-Center Retrospective Quality Study from South-East Norway
Authors Johnsen KMN, Magnussen K, Erstad C , Bhatti SN, Nissen-Meyer LSH
Received 28 December 2022
Accepted for publication 21 April 2023
Published 3 May 2023 Volume 2023:14 Pages 337—343
DOI https://doi.org/10.2147/JBM.S390609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
Kathrine M Neuman Johnsen,1,2 Karin Magnussen,3 Christian Erstad,3 Sadaf Nabi Bhatti,4 Lise Sofie H Nissen-Meyer2
1Center for Laboratory Medicine and Blood Bank, Østfold Hospital Trust, Grålum, Norway; 2Department of Immunology, Oslo University Hospital, Oslo, Norway; 3Department of Immunology and Transfusion Medicine, Innlandet Hospital Trust, Lillehammer, Norway; 4Department of Immunology and Transfusion Medicine, Akershus University Hospital, Lørenskog, Norway
Correspondence: Kathrine M Neuman Johnsen, Østfold Hospital Trust, P.O. Box 300, Grålum, 1714, Norway, Tel +47 669868410 ; +4796090859, Email [email protected]
Purpose: In Norway, blood donors using antihypertensive medication were deferred until 2015. Following revision of the national directive, these donors could be allowed, providing stable dose for at least 3 months, adequate blood pressure control and no adverse effects caused by the therapy. The new practice was evaluated by a quality study where the major aim was to establish whether donations from blood donors on antihypertensive medication pose a risk to the donor. The risk was assessed by counting the number and categorizing the adverse events related to blood donation. In addition, the quantitative effect of including these donors was calculated.
Subjects and Methods: In this retrospective quality study, blood donors on antihypertensive therapy were recruited from four different blood centers to fill out a questionnaire. A total of 265 donors answered questions regarding their health status, type of medication used, and adverse events connected to blood donation both before and after starting the therapy.
Results: No severe adverse events were observed in donors on antihypertensive medications. The amount of mild adverse events, as exhibited by only 7 persons (0.46%) in this donor population, was the same as for donors without hypertensive treatment.
Conclusion: Blood donation from persons on antihypertensive therapy poses no extra risk of severe adverse events, given the use of screening criteria to identify and bleed only low-risk donors.
Keywords: donor safety, hemovigilance, hypertension, deferral
Introduction
Donors with moderately elevated blood pressure are allowed to donate blood as long as blood pressure measurements performed in the blood center are below the limit (180/100 mmHg). Despite it being counter-intuitive to defer them from blood donation following the start of relevant medical therapy, Norwegian blood banks generally deferred blood donors on antihypertensive treatment until 2015. The national guidelines allowed only donors using diuretics (thiazides) to donate blood without further assessment, and all other antihypertensive medications depended upon the doctors’ evaluation and approval. Without guidelines to instruct the physicians about the safety of antihypertensive medications in the context of blood donation, each doctor was left to make her own decision.1 This led to different practices in different blood donation centers. The donors on antihypertensive medication were generally deferred due to the principle that blood donors should be completely healthy. In addition, since the physiological mechanisms which compensate for donation-induced hypotension were perceived to be inhibited by the therapy, there was a concern for donor safety. The assumption was made that rapid blood loss due to donation could increase the risk of hypotensive reactions, ie, dizziness and syncope. As a result, a significant number of otherwise healthy and highly motivated donors were deferred. Although the evidence in the literature is poor,2 a few reports have indicated that blood donation from donors using antihypertensive therapy is unproblematic.3–5
Therefore, the responsible medical consultants in four different blood centers of southeast Norway decided to adjust the local guidelines, to allow donors using antihypertensive drugs to donate blood from 2015. To help us choose which donors to accept for donation, we established a set of guidelines (Box 1). In addition, the following safety rules were generally applied: Donors using angiotensin II receptor antagonists (AIIAs) and angiotensin converting enzyme inhibitors (ACEi) were deferred from donation by apheresis, due to possible risk of anaphylaxis and hypotension mediated by the bradykinin axis.6 These donors were, however, allowed to donate whole blood. Donors using calcium channel inhibitors were checked for edemas and pulse irregularities before and after donation, these being the most common side effects of antihypertensive drugs. Donors treated with alpha and/or beta receptor blockers have been deferred both before and after the 2015 revision, mostly because these medications are not first-line therapeutics for primary hypertension.
 |
Box 1 Local Guidelines for Blood Donation from Donors Using Antihypertensive Medication |
Two years after the guideline changes, we conducted a follow-up quality study with the following aims: To investigate whether it is safe for the donors who use antihypertensive medication to donate blood and to see if these types of donors experience adverse events more frequently compared to other donors. We also wanted to validate the selection criteria for donors on antihypertensive medication and estimate the number of extra donors and donations gained by acceptance of these donors.
Materials and Methods
Information from 265 donors was collected from four blood centers in the southeast part of Norway. The centers were located in Oslo, Akershus, Innlandet og Østfold counties. Blood donation data for the participating blood centers are shown in Table 1. Data collection was performed for 3–4 months at each site, whereafter de-identified data was combined and analyzed using Microsoft Excel.
 |
Table 1 Blood Donation Data from the Participating Blood Centres |
All donors reporting relevant medication were given the opportunity to participate in the study. Established donors on antihypertensive medication who were allowed to donate following the guidelines (Box 1) had been given a special code in the blood bank laboratory system that enabled us to locate and address them for inclusion. In addition, new donors using this type of medication who were admitted during the inclusion period were also invited to participate. Eleven donors did not wish to attend. One donor was deferred and excluded from further analysis due to treatment with the beta receptor blocker bisoprolol.
Each donor was asked to complete a questionnaire we had developed for this study.
We made queries regarding health status including additional diagnoses, type of medications used, and efficacy of and side effects to antihypertensive treatment. We also requested information about any adverse reactions related to blood donation while on medication and asked the donors to estimate their extra fluid intake in connection with donations. Signed informed consent from each donor was obtained and stored along with the above mentioned questionnaire. The study complies with the Declaration of Helsinki and was approved as a quality study by the data protection officer at Oslo University Hospital (2017-6600) and by equivalent bodies in the other hospitals.
Results
A total of 264 donors who responded to the survey and gave their consent to data collection used one of the approved medications. The demographic characteristics of the participating donors are shown in Figure 1. The average age of the participants was 55 years (170 men and 94 women). Most of the donors were prescribed antihypertensive medication due to a diagnosis of essential hypertension. Of these donors, 70% were treated in primary health care. Other causes for hypertension, eg, stress and obesity were noted in 13% of the participants. Analysis showed that two of the donors included were taking antihypertensive medication for migraines. While these persons were not within the initial scope of the study, the novelty of acceptance of antihypertensive medication in this instance had overshadowed the indication. Since they had consented to participation, we decided to include the information from these donors in the analysis.
 |
Figure 1 Demographics of the donors included in the study (n=264). Histogram showing the distribution of male (blue columns) and female (red columns) donors in groups based on age (horizontal axis). |
None of the donors had secondary hypertension or serious underlying conditions. Before starting the antihypertensive medication, 85% of the donors had donated a median of 27 times (variation: 0–319; Table 2), with a total of 9661 donations.
 |
Table 2 Summary of Donor Characteristics |
Figure 2 shows the distribution of different antihypertensive medication used by the donors in the study. Angiotensin II (AII) receptor antagonists in monotherapy were found in 54% of donors. More men than women were using angiotensin-converting enzyme (ACE)-inhibitors, whereas more women than men were using Ca2+- channel inhibitors. Of the 80 (30%) using combination therapies, as many as 67 (5 out of 6) were using combinations containing angiotensin II receptor antagonists. The median treatment length was 14 months (range 1 to 414).
A total of 68% of the donors had no other illnesses and used no other therapies, whereas 84 donors (32%) reported using additional medications. In this group, 30% used cholesterol-reducing prescriptions. Additional medications in the form of anti-allergic or anti-asthmatic prescriptions were present in 25% of donors, 10% with thyroid substitution and 10% used proton pump inhibitors. Only 5% reported other hormonal substitution therapies and 10% used more than one medication (of which one is cholesterol-reducing).
Correspondingly, 63 donors (24%) reported to have other conditions: of those, 40% had allergy/asthma, 11% were treated for hypercholesterolemia, 16% had thyroid disorders, 11% suffered from gastric reflux, 5% had migraine, and 5% reported having uric acid arthritis.
This study revealed that, following the changed guidelines, the included donors had contributed a total of 1527 units of blood (ranging from 1 to 21), yielding median 5 units per donor, as shown in Table 2.
Following 7 of these donations, the donors reported adverse events (0.46%), none of which was considered severe (Table 2). Two events of dizziness occurred before the donor had left the blood center, whereas two episodes of dyspnea upon activity and three episodes of dizziness were reported from outside the blood establishment following donation. In the same period, the frequency of mild adverse events in 5645 donations from donors without antihypertensive medication was 0.44% in comparison.
Donors showed excellent compliance following advice given by the blood center staff. More than 90% drank 0.5 liters or more of liquids upon donation, and mean liquid intake was 0.62 liters. Three of the donors who experienced adverse effects had been drinking 0.25–0.3 liters, and all three were female.
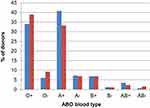
The distribution of blood types among the participating donors is shown in Figure 3.
Discussion
Large studies of whole blood donations have determined predictors of adverse reactions following blood donation to be having a donor blood volume of less than 3500 mL, young age and first-time donation.7 In more than 400 000 allogeneic donations, the reaction prevalence was 1.43%. Blood pressure or pulse frequency was not associated with increased risk for reactions.
Our findings showed a similar number of mild adverse events (AE) in donors on antihypertensive medication (0.46%), compared to the general donor population in this period (0.44%). In line with data discussed above, three of the AEs in this study occurred in females who used to drink less than the average volume. This frequency is, however, low compared to previous reports of donor reactions following whole blood donation, where an overall reaction prevalence ranged from 1.4 to 5%.7,8 The Norwegian Hemovigilance program has no relevant data for comparison locally since only data on severe adverse events is reported (0.05% in 2016 and 2017). However, 0.65% of whole blood donors in Saudi-Arabia experienced mild adverse events including dizziness.9 Our medicated donors reported no severe adverse events. We postulate that this may be attributed to previous donation experience, good compliance with regard to drinking fluids during donation, and extra attention from the staff.
In our study population of blood donors treated for hypertension, the number of male donors was almost twice the number of female donors. This contrasts with the general Norwegian blood donor population. In Oslo Blood Center, females constitute 57% of all donors.10 However, the numbers reflect the age and gender distribution of hypertension treatment in the general population, being a condition of increased age and more common in men.11 Norwegian primary care practitioners diagnose and treat hypertension in line with national recommendations.12 ACE-inhibitors, angiotensin II receptor antagonists, diuretics and calcium channel blockers are used for primary prophylactic treatment, whereas alpha- and beta blockers are considered for secondary prophylaxis following the first incidents of heart disease. Therefore, the distribution of medication in our blood donors reflects current prescription practices. In addition, there is a selection bias since alpha and beta blockers are still not generally accepted in Norwegian blood donors.
In our study, 32% of the donors using antihypertensive medication also used other medication, and 24% reported having other medical conditions. Thus, the idea of the blood donor being “completely healthy” is nowadays less valid than before. In recent years, several eligibility criteria have been revised to increase the availability of blood donors, both due to lack of new donors but also to prevent unnecessary loss of established donors. It has been suggested that several of the traditional causes for blood donor deferrals are not evidence-based but on consensus and “better safe than sorry”-arguments.13
As shown in Figure 3, the blood-type distribution of our medicated population largely reflects the distribution in the general Norwegian population. Although the sample size is small, this indicates that the acceptance of blood donors treated with antihypertensive medication is general and not restricted to the most-needed blood types in the blood center. Thus, the changed rule represents an improved donation policy. Furthermore, the donors on antihypertensive medication provided a much-welcomed addition of blood products (Table 2), from earlier unexploited resources, which will increase in the future. The donors in our study are reliable and highly motivated, and they have reached an age which is associated with low numbers of deferrals for the most common reasons seen in younger donors.
Conclusion
Our study supports the continuous acceptance of blood donors despite anti-hypertensive drug therapy, as they do not experience more frequent AEs than other donors. In part, this can be explained by this group having higher baseline blood pressures than other donors, their experience from previous donations, and good compliance with advice offered by the blood bank personnel.
The inclusion of this group of blood donors has led to a welcome extra supply of whole blood units to our blood centers.
Acknowledgments
The authors thank all the blood donors who contributed, as well as the personnel in the blood centers for assistance in recruiting and handling the donors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Helsedirektoratet [Norwegian DIrectorate of Health]. Veileder for transfusjonstjenesten i Norge, utgave 7.3 [Guidelines for the Blood Transfusion Services in Norway, edition 7.3]. 75. Available from: https://www.helsedirektoratet.no/veiledere/transfusjonstjenesten-i-norge-utgave-73.
2. Stainsby D, Brunskill S, Chapman CE, Dorée C, Stanworth S. Safety of blood donation from individuals with treated hypertension or non-insulin dependent type 2 diabetes – a systematic review. Vox Sang. 2010;98:431–440. doi:10.1111/j.1423-0410.2009.01275.x
3. Thomas SHL, Weston Smith S, Slater NGP, Pearson TC, Treacher DF. The haemodynamic responses to venesection and the effects of cardiovascular disease. Clin lab Haematol. 1992;14:201–208. doi:10.1111/j.1365-2257.1992.tb00366.x
4. Weisbach V, Schnabel L, Zimmermann R, Zingsem J, Eckstein R. A pilot study of continuous ambulatory monitoring of blood pressure in repeated preoperative autologous blood donation. Transfusion. 2006;46:934–941. doi:10.1111/j.1537-2995.2006.00825.x
5. Pisciotto P, Sataro P, Blumberg N. Incidence of adverse reactions in blood donors taking antihypertensive medications. Transfusion. 1982;22(6):530–531. doi:10.1046/j.1537-2995.1982.22683068620.x
6. Jurakić Tončić R, Marinović B, Lipozenčić J. Nonallergic hypersensitivity to nonsteroidal antiinflammatory drugs, angiotensin-converting enzyme inhibitors, radiocontrast media, local anesthetics, volume substitutes and medications used in general anesthesia. Acta Dermatovenerol Croat. 2009;17(1):54–69.
7. Wiltbank TB, Giordano GF, Kamel H, Tomasulo P, Custer B. Faint and prefaint reactions in whole-blood donors: an analysis of predonation measurements and their predictive value. Transfusion. 2008;48:1799–1808. doi:10.1111/j.1537-2995.2008.01745.x
8. Newman BH. Donor reactions and injuries from whole blood donation. Transfus Med Rev. 1997;11:64–75. doi:10.1016/S0887-7963(97)80011-9
9. Almutairi H, Salam M, Alajlan A, Wani F, Al-Shammari B, Al-Surimi K. Incidence, predictors and severity of adverse events among whole blood donors. PLoS One. 2017;12(7):e0179831. doi:10.1371/journal.pone.0179831
10. Nissen-Meyer LSH, Heier HE. Probability to be a blood donor in Oslo depending on geographical factors. Abstract, 35th International Congress of the ISBT, Toronto, Canada, June 2–6, 2018. Vox Sang. 2018;113(Suppl 1):5–347. doi:10.1111/vox.12658
11. Klouman M, Åsberg A, Widerøe T-E. Blood pressure level in a Norwegian population – the significance of heritage and lifestyle. Tidsskr nor Laegeforen. 2011;131:1185–1189. doi:10.4045/tidsskr.10.0486
12. Helsedirektoratet [Norwegian DIrectorate of Health]. Legemiddelbehandling av høyt blodtrykk [Pharmacologic antihypertensive therapy]. Available from: https://www.helsedirektoratet.no/retningslinjer/forebygging-av-hjerte-og-karsykdom/legemidler-ved-primaerforebygging-av-hjerte-og-karsykdom/legemiddelbehandling-av-hoyt-blodtrykk.
13. Eder A, Goldman M, Rossmann S, Waxman D, Bianco C. Selection criteria to protect the blood donor in North America and Europe: past (dogma), present (evidence), and future (hemovigilance). Transfus Med Rev. 2009;23(3):205–220. doi:10.1016/j.tmrv.2009.03.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.