Back to Journals » Patient Preference and Adherence » Volume 17
Safe and Effective Subcutaneous Self-Injection of Bimekizumab with Safety Syringe and Auto-Injector Devices: Results from a Multicenter, Randomized, Open-Label Study in Patients with Psoriatic Arthritis
Authors Kivitz A, Ellis AM, Shende V, Lambert J , Tatla D
Received 22 July 2023
Accepted for publication 8 September 2023
Published 3 October 2023 Volume 2023:17 Pages 2451—2461
DOI https://doi.org/10.2147/PPA.S427809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Alan Kivitz,1 Alicia M Ellis,2 Vishvesh Shende,3 Jérémy Lambert,4 Daljit Tatla2
1Altoona Center for Clinical Research, Duncansville, PA, USA; 2UCB Pharma, Raleigh, NC, USA; 3UCB Pharma, Slough, UK; 4UCB Pharma, Colombes, France
Correspondence: Daljit Tatla, UCB Pharma, 4000 Paramount Parkway, Morrisville, NC, USA, Tel +1 919 767-2518, Email [email protected]
Purpose: Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A, key drivers of chronic inflammation. Bimekizumab must be injected subcutaneously and so patients require self-injection options that meet their preferences. This study evaluated safe and effective self-injection of bimekizumab by patients with psoriatic arthritis using the 1 mL safety syringe (SSy) or the 1 mL auto-injector (AI).
Patients and Methods: The DV0004 devices study (NCT04109976) was a sub-study of BE VITAL, a multicenter, open-label extension of BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) in patients with active psoriatic arthritis. After receiving training, patients subcutaneously self-injected bimekizumab 160 mg at Baseline and Week 4. The primary and secondary endpoints were the proportion of patients self-injecting bimekizumab safely and effectively at Week 4 and Baseline, respectively. Patient self-injection experience was evaluated using the pain visual analog scale (VAS) and the Self-Injection Assessment Questionnaire (SIAQ).
Results: Overall, 214 patients were randomized 1:1 at Baseline. All evaluable patients safely and effectively self-injected bimekizumab at Week 4 (SSy: n=105; AI: n=104) and Baseline (SSy: n=106; AI: n=106). Mean pain VAS scores were generally low at Week 4 (SSy: 11.0; AI: 11.4) and Baseline (SSy: 9.5; AI: 14.9). High mean pre- and post-injection SIAQ scores (≥ 6.7) were observed for both devices indicating a positive overall patient experience with self-injection. Self-injection was well tolerated with no reports of treatment-emergent adverse device effects (TEADEs), serious TEADEs or discontinuations due to TEADEs. Four non-device-related injection site reactions during the sub-study were reported in the parent study; all were mild, did not lead to discontinuation and resolved without treatment. All devices maintained their structural and functional integrity post-use.
Conclusion: All patients self-injected subcutaneous bimekizumab safely and effectively using either device at Baseline and Week 4. Overall, patients reported a positive self-injection experience.
Keywords: bimekizumab, clinical trial, patient experience, psoriatic arthritis, self-injection devices
Introduction
Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits the pro inflammatory cytokine interleukin (IL)-17F, in addition to IL-17A, which are key independent drivers of chronic inflammation.1,2 The safety and efficacy of bimekizumab treatment has been evaluated in Phase 2 and 3 trials of patients with active psoriatic arthritis (PsA), psoriasis and axial spondyloarthritis, with clinically meaningful improvements observed across all patient populations.3–9 The drug is administered subcutaneously, either by a healthcare professional or via self-injection.2
Self-injection offers patients control and independence over the injection, the setting, and the injection schedule.2 Previous studies have also reported that availability of, and education on, a self-injection device that meets patients’ preferences is associated with increased adherence to treatment, a greater proportion of patients self-injecting medication and a smaller proportion of patients attending primary care for administration, translating into a reduction in healthcare costs.10,11 Despite these benefits, some patients face barriers to self-injection such as needle anxiety and lack of confidence.2 While some patients prefer greater control over the self-injection process, others prefer less control and a more automatic self-injection.2,12 Given these considerations, it is important that patients who require bimekizumab treatment have safe and effective self-injection options that meet their needs and preferences.
Safe and effective subcutaneous self-injection and a positive patient self-injection experience with the 1 mL bimekizumab safety syringe (SSy) and the 1 mL bimekizumab auto-injector (AI) device has been demonstrated in patients with psoriasis in two sub-studies (DV0002 and DV0006) of the BE BRIGHT (NCT03766685) open-label extension study in the bimekizumab clinical program.2 However, self-injection using these devices in patients with active PsA has not been evaluated. PsA is a chronic, heterogenous, inflammatory disease with broad clinical manifestations, including musculoskeletal (stiffness, pain, and swelling of the small joints of the hand) and dermatological (psoriatic skin lesions) symptoms of varying severity.13–15 As these symptoms may impact hand grip and function, it is important to evaluate safe and effective self-injection of bimekizumab in patients with active PsA.16,17
Here, the results from DV0004 (NCT04109976), a sub-study of the Phase 3 study BE VITAL (NCT04009499) are presented. The study evaluated safe and effective self-injection of bimekizumab by patients with PsA using the 1 mL bimekizumab SSy or the 1mL AI.18
Materials and Methods
Study Endpoints
The primary endpoint was the safe and effective self-injection of bimekizumab at 4 weeks, which is the inter-dose interval for bimekizumab treatment of patients with PsA, using the SSy or the AI. The secondary objective was the safe and effective self-injection of bimekizumab immediately after training (at Baseline), using the SSy or the AI and is representative of a patient starting treatment. Other study objectives included: (i) evaluation of the patient experience of self-injection using the pain visual analog scale (VAS) for injection site pain and the pre- and post-injection versions of the Self-Injection Assessment Questionnaire (SIAQ) and (ii) evaluation of the structural and functional integrity of each self-injecting device after completion of self-injection.
Patient Population
The DV0004 study (NCT04109976) was a Phase 3 sub-study of BE VITAL (NCT04009499), a multicenter, open-label extension of the Phase 3 studies BE OPTIMAL (NCT03895203)7 and BE COMPLETE (NCT03896581)6 conducted in North America and Europe between August 13th, 2019 and November 19th, 2020 to evaluate the safety and efficacy of bimekizumab in patients with active PsA. The study included patients ≥18 years old diagnosed with active PsA. All patients were willing to self-inject bimekizumab (full study inclusion and exclusion criteria are listed in Table S1). This study was designed to be representative of real-life clinical practice where the ability of the patient to self-inject safely and effectively is of primary importance. As consistent with real-life clinical practice and to ensure patient safety, the study did not measure degree of hand involvement or hand function and only included patients who, in the opinion of the investigator, were capable of safe and effective self-injection. This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidance for Good Clinical Practice. Ethical approvals were obtained from the relevant Institutional Review Boards at participating sites (listed in Table S2) and all patients provided written informed consent in accordance with local requirements.
Self-Injecting Devices
Two self-injecting devices were assessed in this study: the SSy and the AI. The SSy is a modified syringe with a large smooth grip area for optimal needle control, extended finger flange, and plunger rod for enhanced device usability, depicted in Bagel et al, 2022.2 To use the SSy, patients removed the over-cap, safely positioned the visible needle at the injection site (either the lateral abdominal wall or outer thigh), fully depressed the plunger rod which emptied the syringe contents (bimekizumab) through the needle into the subcutaneous space, and upon completion of the injection, automatically withdrew the needle into the body of the SSy. The AI was designed with a large smooth grip area, hidden needle to reduce needle phobia, and a window to ensure all syringe contents are emptied. To use the AI, patients removed the ring-pull cap and positioned the AI against the skin at the injection site (either the lateral abdominal wall or outer thigh). The AI was then depressed against the skin surface which automatically activated the device to push the hidden needle tip into the subcutaneous space to administer bimekizumab.
Study Design
At Baseline, patients were randomly assigned to either the SSy group or AI group in a 1:1 ratio to self-inject bimekizumab 160 mg every 4 weeks (Q4W) (Figure 1). Patients were randomized using a unique 5-digit identification number to maintain confidentiality. An Interactive Response Technology (IRT) was used to generate individual assignments to the two devices. Patients received training in subcutaneous self-injection technique for the allocated device at Baseline. Prior to self-injection at Baseline, patients performed the pre-injection SIAQ. Patients self-injected bimekizumab immediately after training at Baseline and at Week 4 (without training). Following self-injection at Baseline and Week 4, patients completed the pain VAS (to evaluate injection site pain) and the pre-injection SIAQ (to assess their self-injection experience). The study was designed to represent real life in that the primary endpoint evaluated safe and effective self-injection after 4 weeks which is the inter-dose interval. After completing the DV0004 sub-study, patients continued participation in the parent study (BE VITAL [NCT04009499]).
Study Outcomes
Safe and Effective Self-Injection
Safe and effective self-injection was evaluated by study personnel and was defined as complete bimekizumab dose delivery, assessed by visual inspection of the device and confirmation that the dose was delivered completely, as well as the absence of treatment-emergent adverse device effects (TEADEs) leading to sub-study withdrawal. A TEADE was defined as an adverse event resulting from insufficiencies in the instructions for use, deployment, operation or malfunction of the investigational medical device and/or misuse of the device by the operator. All other adverse events were reported in a common database for BE VITAL; injection site reactions that were not device related and were reported in the parent study are reported here for completeness. The structural and functional integrity of the SSy and AI were evaluated following self-injection by appropriately trained site personnel.
VAS for Injection Site Pain
The pain VAS was completed post-injection to evaluate injection site pain after self-injection, at Baseline and Week 4. Patients indicated their pain level from 0 (no pain) to 100 (worst possible pain) by placing a mark on a 100 mm line. Patients completed the VAS prior to the post-injection SIAQ.
Pre- and Post-Injection SIAQ
Patients completed the pre-injection SIAQ at Baseline prior to self-injection and the post-injection SIAQ 30 minutes after self-injection at Baseline and Week 4. The pre-injection SIAQ comprised seven items grouped into three domains: i) feelings about injection, ii) self-confidence and iii) satisfaction with the mode of administration. The post-injection SIAQ comprised 21 items grouped into six domains i) feelings about injections, ii) self-image, iii) self-confidence, iv) injection site reactions, v) ease of use and vi) satisfaction with self-injection. Patients assigned to the SSy and the AI completed versions 2.0 and 2.1 of the SIAQ, respectively. The versions are identical except for the eleventh question which discusses the use of a plunger (version 2.0; SSy) or depression of the device (version 2.1; AI).
The individual item scores of the pre- and post-injection SIAQ items were transformed from Likert-type scale ratings between 1–5 (1–6 for the post-injection SIAQ domain “ease of use”) to a 0–10 scale (0 being worst experience and 10 being best experience), enabling comparison between the pre- and post-injection questionnaires.17 Domain scores were calculated as a mean of the item scores if ≥50% of the items in the domain had been answered. Domain scores ranged from 0 to 10, higher scores reflect more confidence, higher satisfaction and less concern about self-injection.
Statistical Analysis
The analysis of the primary and secondary objectives used all evaluable patients who self-injected and injections performed by site personnel were not included. Missing data were not imputed.
The analysis of the pain VAS included data collected from the subgroup of patients that completed all available pain VAS questionnaires as per protocol. The analysis of the SIAQ also included data collected from the subgroup of patients that completed all available SIAQ questionnaires per protocol. Questionnaires were considered impacted if they were performed out of the predefined order (e.g., the pre-injection SIAQ was administered after self-injection, the post-injection SIAQ was completed before self-injection, or the pain VAS was completed prior to self-injection) or because patients were administered the incorrect version of the SIAQ for their assigned device group. If patients had only completed one of either the pre-injection SIAQ or post-injection SIAQ, and this was completed correctly, it was included in this subgroup analysis. To assess the generalizability of these results, data from all completed questionnaires (including those not completed as per protocol) for all evaluable patients were also analyzed; these results are available in the Supplementary Materials.
Summary statistics were used to describe all scores. Mean, median, standard deviation, and minimum and maximum values of the pre- and post-injection pain VAS and SIAQ scores are presented in this study. The distribution of pain VAS scores are also presented by pain intensity category, in accordance with published literature: “No to minimal pain” (0–≤4mm), “mild” (>4–≤44), “moderate” (>44–≤74) and “severe” (>74–≤100).19 The proportion (%) of patients reporting individual scores for SIAQ questions is also reported.
Results
Patient Disposition and Baseline Characteristics
A total of 214 patients started the DV0004 study, with 107 randomized to the SSy group and 107 randomized to the AI group (Figure S1). In the SSy group, one patient discontinued before the Week 4 visit due to withdrawn consent; 99.1% (106/107) of patients completed the study, with 105 and 106 evaluable patients at Week 4 and Baseline, respectively (Figure S1). In the AI group, 100% (107/107) of patients completed the study, with 104 and 106 evaluable patients at Week 4 and Baseline, respectively (Figure S1). One participant in each of the SSy and AI groups was not evaluable at Baseline as they did not self-inject the bimekizumab dose and this was instead administered by site personnel. One participant in the SSy group discontinued before Week 4. Two participants in the AI group had no drug administered at Week 4. All other non-evaluable patients were due to missing data. Patient baseline demographics were similar between groups (Table 1). Patients in the SSy and AI groups had a mean age of 48.3 (SD 13.2) years and 51.5 (SD 11.9) years, respectively; approximately half of the patients in both groups were male (49.5% in each group). Patients had a disease duration of 8.6 (SD 8.9) and 8.4 (SD 7.4) years in the SSy and AI groups, and a body mass index of 31.2 (SD 7.3) and 30.5 (SD 6.2), respectively.
 |
Table 1 Baseline Demographics and Disease Duration |
Safe and Effective Self-Injection
Self-Injection
The study met both primary and secondary objectives, with all evaluable patients in the SSy and AI groups able to safely and effectively self-inject bimekizumab: SSy group at Week 4 (n=105/105) and Baseline (n=106/106); AI group at Week 4 (n=104/104) and Baseline (n=106/106). There were no reports of TEADEs, serious TEADEs or discontinuation due to TEADEs for the SSy or AI groups. There were a total of four non-device-related injection site reactions reported in the parent study; all were mild, none led to discontinuation, and all resolved without treatment.
Device Structural Integrity
Upon post-injection inspection, 100% of the SSy (212/212) and AI (211/211) devices used maintained their post-use structural and functional integrity.
Pain VAS Scores
Pain VAS scores were recorded on a scale from 0 (no pain) to 100 (worst possible pain). In the subgroup of SSy participants that completed all of their available VAS questionnaires as per protocol, 57 and 56 had results for Week 4 and Baseline, respectively, and the mean pain VAS score was 11.0 (SD: 14.1) at Week 4 and 9.5 (SD: 13.7) at Baseline (Table 2). In the subgroup of AI participants that completed all of their available VAS questionnaires as per protocol, 54 and 51 had results for Week 4 and Baseline, respectively, and the mean pain VAS score was 11.4 (SD: 17.4) and 14.9 (SD: 20.1) at Week 4 and Baseline, respectively. Assessment of pain VAS scores by pain intensity category showed that 98.3% and 94.6% of SSy and 92.6% and 86.3% of the AI subgroup participants reported having “no to minimal” or “mild” pain at Week 4 and Baseline, respectively (Table S3).
 |
Table 2 Patient Pain VAS Scores for SSy and AI Groups in Subgroup of Patients Who Completed All Available VAS Questionnaires per Protocol |
The results from all evaluable patients who completed all questionnaires (including those not completed as per protocol) for the SSy and AI groups were analyzed separately to assess the generalizability of the subgroup results. This analysis showed that Week 4 and Baseline pain VAS scores whether reported as mean, median, or pain intensity category, were similar to the subgroup analysis of patients who completed the questionnaires per protocol (Tables S4 and S5).
SIAQ Scores
SIAQ scores range from 0 to 10 for each domain, with higher scores indicating a more positive self-injection experience. Patients who did not complete all available pre- and post-injection SIAQ questionnaires as per protocol at any visit were excluded from analysis. Hence, for the pre-injection SIAQ, 46 out of 107 and 63 out of 107 randomized patients were included in the SSy and AI group analyses, respectively. The mean pre-injection SIAQ score for the subgroup of SSy participants that completed all of their available questionnaires as per protocol was 8.4 (SD: 1.8) in the “feelings about injections” subscore, 6.7 (SD: 2.8) for “self-confidence” subscore and 8.5 (SD: 2.2) for “satisfaction with current mode of administration” subscore (Table 3). Overall, 17.4–50.0% of subjects were “a little” afraid or anxious, “very confident”, or “satisfied” with self-injection whilst 19.6–60.9% of subjects were “not at all” afraid or anxious, “extremely confident”, or “very satisfied” with self-injection (data on file).
 |
Table 3 Baseline Pre-Injection SIAQ Scores for SSy and AI Groups in Subgroup of Patients Who Completed All Available SIAQ Questionnaires per Protocol |
The mean pre-injection SIAQ scores in the subgroup of AI participants that completed all of their available questionnaires per protocol were similar to the SSy group. The mean pre-injection SIAQ score was 8.5 (SD: 1.9) in the “feelings about injections” subscore, 7.3 (SD: 2.4) in the “self-confidence” subscore, and 8.8 (SD: 1.5) in the “satisfaction with current mode of administration” subscore. Overall, 23.8–47.6% of subjects were “a little” afraid or anxious, “very confident”, or “satisfied” with self-injection whilst 28.6–65.1% of subjects were “not at all” afraid or anxious, “extremely confident”, or “very satisfied” with self-injection (data on file).
For the post-injection SIAQ, 46 out of 107 and 45 of 107 patients were randomized to the SSy group and were included for analyses at Week 4 and Baseline, respectively, and 63 patients out of 107 randomized to the AI group were included at both timepoints for AI group analyses.
Mean post-injection SIAQ domain scores for the SSy group ranged from 7.1 (SD: 3.0) at Week 4 for the “self-confidence” subscore to 9.3 for the “self-image” at Baseline (SD: 1.3) and for “injection site reaction” subscore at Week 4 (SD: 1.0) (Table 4). Overall, 24.4–37.8% of subjects were “a little” whilst 55.6–62.2% of subjects were “not at all” afraid or anxious about self-injection; 17.4–17.8% of subjects were “a little” whilst 76.1–77.8% of subjects were “not at all” embarrassed by self-injection; 41.3–57.8% of subjects were “very” whilst 26.7–32.6% of subjects were “extremely” confident about self-injection; 6.5–34.8% of subjects were “a little” whilst 50.0–91.1% of subjects were “not at all” bothered by injection site reactions; 28.3–50.0% and 33.3–54.3% of subjects found the SSy “easy” or “very easy” to use, respectively; 28.3–60.9% of subjects were “satisfied” whilst 23.9–63.0% were “very satisfied” with the SSy; (data on file).
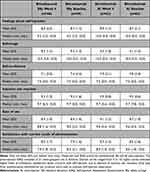 |
Table 4 Week 4 and Baseline Post-Injection SIAQ Scores for SSy and AI Groups in Subgroup of Patients Who Completed All Available SIAQ Questionnaires per Protocol |
Mean post-injection SIAQ domain scores for the AI group ranged from 7.8 (SD: 2.4) at Baseline for the “self-confidence” domain to 9.5 for the “injection site reaction” subscore at Week 4 (SD: 0.8) and at Baseline (SD: 0.6) (Table 4). Overall, 14.3–19.0% of subjects were “a little” whilst 65.1–73.0% of subjects were “not at all” afraid or anxious about self-injection; 7.9–23.8% of subjects were “a little” whilst 69.8–84.1% of subjects were “not at all” embarrassed by self-injection; 36.5–52.4% of subjects were “very” whilst 31.7–42.9% of subjects were “extremely” confident about self-injection; 3.2–41.3% of subjects were “a little” whilst 46.0–95.2% of subjects were “not at all” bothered by injection site reactions; 20.6–33.3% and 50.8–73.0% of subjects found the AI “easy” or “very easy” to use, respectively; 17.5–55.6% of subjects were “satisfied” whilst 38.1–76.2% were “very satisfied” with the AI; (data on file).
The results from all evaluable who completed all questionnaires for the SSy and AI groups were considered separately to assess the generalizability of the subgroup results. The results were similar to the subgroup analysis of patients who completed the SIAQ questionnaires per protocol (Tables S6 and S7).
Discussion
Patients with active PsA face many barriers to successful self-injection,2,12 and previous studies have shown important benefits when patients self-inject using devices that meet their needs and preferences.10,11,20 Therefore, the DV0004 study evaluated safe and effective self-injection of bimekizumab by patients with PsA using the 1mL SSy or AI.
The primary and secondary objectives of this sub-study were met, demonstrating that after training in self-injection at Baseline, all evaluable patients with active PsA were able to safely and effectively self-inject bimekizumab using either the SSy or AI at Baseline and Week 4. These endpoints reflect real life as 4 weeks is the inter-dose interval and training for self-injection at baseline is typical for patients starting a new injectable treatment. There were no TEADEs reported during this sub study and only 4 non-device-related injection site reactions were reported in the parent study, all of which were mild and did not lead to discontinuation or require treatment. Additionally, all devices evaluated in this study maintained their post-use functional and structural integrity.
Patients reported a positive self-injection experience using these devices. Reported injection site pain VAS scores were low with >86% of subjects reporting “no to minimal” or “mild” pain for the AI and SSy at both visits, indicating injection site pain was low and self-injection was well tolerated using either device. This is in contrast to previous studies which reported lower injection site pain with the AI compared to the syringe.10,11 The reasons for these different observations are not clear but may relate to differences between the syringes and AIs used, the medications administered (bimekizumab vs adalimumab), or differing study patient populations (patients with PsA vs a mixed population of patients with PsA, rheumatoid arthritis, or ankylosing spondylitis).
Pre-SIAQ domain sub-scores (“feelings about self-injection”, “self-confidence”, and “satisfaction with self-injection”) were high, indicating patients overall had a positive perception of self-injection. Reported domain sub-scores from the post-injection SIAQ (“feelings about self-injection”, “self-image”, “self-confidence”, “injection site reactions”, “ease of use”, and “satisfaction with self-injection”) were also high with mean scores above 7 for both devices at Week 4 and Baseline. The high post-injection SIAQ scores for both devices at both visits indicate that patients had an overall positive self-injection experience.
The outcomes of the DV0004 sub-study reported here are consistent with the findings of Bagel et al, 2022 who reported an overall positive self-injection experience for patients with psoriasis self-injecting bimekizumab using the same 1mL SSy and AI devices.2
Limitations
The analyses of the primary and secondary objectives presented in this study used data from all evaluable patients, but the analysis of the VAS and SIAQ questionnaires used data from a subgroup of patients who completed all available VAS and SIAQ questionaries per protocol. This was necessary as some pain VAS and SIAQ questionnaires were not administered in the correct, pre-defined order, or the incorrect version of the SIAQ was administered. However, the results from these subgroup analyses were consistent with the results observed when VAS and SIAQ questionnaires from all evaluable patients were analyzed, which increases confidence in the subgroup analysis results.
Furthermore, this study assessed the ability of patients to self-inject 4 weeks after receiving self-injection training, and was conducted in a controlled clinical trial context, which may not be fully reflective of the real-world situation for patients. Additionally, this study did not formally assess how the patients’ handgrip strength or dexterity may have been affected by active PsA. Additional studies in a real-world setting would be needed to further investigate whether patients retain the ability to self-inject safely and effectively for longer periods of time after training, and to assess if a positive self-injection experience is maintained. However, in this study all patients were able to safely and effectively self-inject with their assigned device, and these results can be used to provide the patient with reassurance and confidence.
Conclusion
This study demonstrates that self-injection of bimekizumab by patients with active PsA using the 1 mL SSy or AI was safe and effective. Overall, patients reported a positive self-injection experience and generally low injection site pain when using either device. The SSy and AI devices provide patients with PsA two safe and effective options for the self-injection of bimekizumab that will suit their personal preferences.
Abbreviations
AI, auto-injector; BMI, body mass index; GPP, Good Publication Practice; IL, interleukin; IRT, Interactive Response Technology; PsA, psoriatic arthritis; Q4W, every 4 weeks; SD, standard deviation; SIAQ, Self-Injection Assessment Questionnaire; SSy, safety syringe; TEADE, treatment-emergent adverse device effect; VAS, visual analog scale.
Data Sharing Statement
Underlying data from this manuscript may be requested by qualified researchers six months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password protected portal.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidance for Good Clinical Practice. Ethical approvals were obtained from the relevant institutional review boards at participating sites and all patients provided written informed consent in accordance with local requirements.
Consent for Publication
All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study.
Acknowledgments
The authors thank the patients, the investigators, and their teams who took part in these studies. The authors acknowledge the contribution of Deepak Assudani, UCB Pharma, Scarlett Hellot, UCB Pharma and Valerie Ciaravino, UCB Pharma to the study. The authors also acknowledge Heather Edens, PhD, UCB Pharma, Smyrna, GA, USA for publication coordination.
Medical writing and editorial assistance was provided by Megan Thomas, MSc, and Ben McNally, PhD, Costello Medical, UK. This study was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by UCB Pharma. Support for third-party writing assistance for this article was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure
Alan Kivitz: Consulting fees from Horizon, Frescenius Kabi, Pfizer, GSK, Janssen, Selecta, Genzyme, Gilead, Prime, Princeton, Synact, Takeda, and Grunenthal. Honoraria from GSK, Eli Lilly, Pfizer, AbbVie, Amgen, and UCB Pharma. Part of a board or advisory board for Horizon, Janssen, Chemocentryx, Princeton Biopartners, UCB Pharma, and Novartis. Stock or stock options in Pfizer, GSK, Gilead, Novartis, and Amgen. Alicia M Ellis: Employee and stockholder of UCB Pharma. Vishvesh Shende: Employee of UCB Pharma. Jérémy Lambert: Employee and stockholder of UCB Pharma. Daljit Tatla: Employee of UCB Pharma. The authors report no other conflicts of interest in this work.
References
1. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390. doi:10.1007/s12016-018-8702-3
2. Bagel J, Tatla D, Hellot S, et al. Bimekizumab self-injection devices: two multicenter, randomized, open-label studies on self-administration by patients with psoriasis. J Drugs Dermatol. 2022;21(2):162–171. doi:10.36849/JDD.6274
3. Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–486. doi:10.1016/S0140-6736(21)00126-4
4. Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157(11):1279–1288. doi:10.1001/jamadermatol.2021.2905
5. Van der Heijde D, Deodhar A, Baraliakos X, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomized controlled trials. Ann Rheum Dis. 2023;82(4):515–526. doi:10.1136/ard-2022-223595
6. Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet. 2023;401(10370):38–48. doi:10.1016/S0140-6736(22)02303-0
7. McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet. 2023;401(10370):25–37. doi:10.1016/S0140-6736(22)02302-9
8. Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498. doi:10.1016/S0140-6736(21)00125-2
9. Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus Adalimumab in Plaque Psoriasis. N Engl J Med. 2021;385(2):130–141. doi:10.1056/NEJMoa2102388
10. Borrás-Blasco J, Gracia-Pérez A, Dolores Rosique-Robles J, et al. Acceptability of switching Adalimumab from a prefilled syringe to an autoinjection pen. Expert Opin Biol Ther. 2010;10(3):301–307. doi:10.1517/14712590903530633
11. Borrás-Blasco J, Gracia-Pérez A, Casterá MD, et al. Educational session as a tool to increase patient satisfaction of switching etanercept from the prefilled syringe to the autoinjection pen. Expert Opin Biol Ther. 2013;13(8):1103–1108. doi:10.1517/14712598.2013.795942
12. Tornero Molina J, López Robledillo JC, Casamira Ruiz N. Potential benefits of the self-administration of subcutaneous methotrexate with autoinjector devices for patients: a review. Drug Healthc Patient Saf. 2021;13:81–94. doi:10.2147/DHPS.S290771
13. Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med. 2017;17(1):65–70. doi:10.7861/clinmedicine.17-1-65
14. Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi:10.1007/s40265-014-0191-y
15. Jevtic V, Lingg G. Differential diagnosis of rheumatoid and psoriatic arthritis at an early stage in the small hand and foot joints using magnetic resonance imaging. Handchir Mikrochir Plast Chir. 2012;44(03):163–170. doi:10.1055/s-0032-1321760
16. Candiri B, Talu B, Demirtas Karaoba D, Ozaltin GE, Yolbas S. Effect of psoriatic arthritis on the strength, proprioception, skill, coordination, and functional condition of the hand. Int J Rheum Dis. 2022;25(1):47–55. doi:10.1111/1756-185X.14241
17. Keininger D, Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the self-injection assessment questionnaire (SIAQ). Health Qual Life Outcomes. 2011;9(1):2. doi:10.1186/1477-7525-9-2
18. UCB Biopharma SRL. A study to assess the long-term safety, tolerability, and efficacy of bimekizumab in the treatment of subjects with active psoriatic arthritis (BE VITAL). NLM identifier: NCT04009499. Available from: https://clinicaltrials.gov/ct2/show/NCT04009499.
19. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. 2011;63(Suppl 11):S240–S252.
20. Gau M, Takasawa K. Initial patient choice of a growth hormone device improves child and adolescent adherence to and therapeutic effects of growth hormone replacement therapy. J Pediatr Endocrinol Metab. 2017;30(9):989–993. doi:10.1515/jpem-2017-0146
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

