Back to Journals » Cancer Management and Research » Volume 12
S1PR2 Knockdown Promotes Migration and Invasion in Multiple Myeloma Cells via NF-κB Activation
Authors Pang M, Li C , Zheng D, Wang Y, Wang J, Zhang W, Li F, Jing H
Received 5 November 2019
Accepted for publication 4 August 2020
Published 26 August 2020 Volume 2020:12 Pages 7857—7865
DOI https://doi.org/10.2147/CMAR.S237330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Meng Pang,1 Chunyuan Li,1 Dong Zheng,1 Ying Wang,2 Jing Wang,1 Weilong Zhang,1 Fang Li,1 Hongmei Jing1
1Department of Hematology, Third Hospital of Peking University, Beijing, People’s Republic of China; 2Department of Immunology, Key Laboratory of Medical Immunology of Ministry of Health, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, People’s Republic of China
Correspondence: Fang Li; Hongmei Jing Email [email protected]; [email protected]
Background: The presence of circulating plasma cells (cPCs) was associated with a worse prognosis in multiple myeloma patients. However, the underlying mechanisms involved in the migration and invasion of bone marrow myeloma cells (BMMCs) to cPCs remains unclear. Here, we investigate the possible factors related to hematogenous myeloma cell dissemination and potential regulatory mechanisms.
Methods: BMMCs and cPCs of five extramedullary plasmacytoma (EMP) patients were selected for single cell RNA sequencing, We found that the expression level of sphingosine-1-phosphate receptor 2 (S1RP2) was lower in cPCs compared with that in BMMCs. Then, we investigated the effect of S1PR2 in cell migration and invasion through pharmacologic inhibition with a S1PR2-selective antagonist JTE-013 or knockdown of S1PR2 expression in MM cell line U266.
Results: The results showed that S1PR2 inhibition with JTE-013 or S1PR2-shRNA significantly promoted cell migration and invasion in U266 cells. We measured the expression of invasion-related proteins by Western blot and found that knockdown of S1PR2 could reduce MMP-9 expression in U266 cells. Furthermore, we found NF-κB pathway may mediate the inhibition effects of S1PR2 on cell migration and invasion in MM cells.
Conclusion: Our findings demonstrated that S1PR2 downregulation may contribute to the initial extramedullary translocation by promoting cell migration and invasion through NF-κB pathway activation.
Keywords: multiple myeloma, extramedullary plasmacytoma, circulating plasma cells, NF-κB, S1PR2
Introduction
Multiple myeloma (MM) is an incurable hematologic cancer and accounts for about 13% of hematologic malignancies.1 MM is characterized by infiltration of clonal plasma cells (PCs) in bone marrow.2 While, in some cases, PCs can develop autocrine supportive loops and become stromal independent, which could form soft-tissue extramedullary plasmacytomas (EMPs).3 MM patients with extramedullary invasion were associated with poorer prognosis and were difficult to treat.4 However, the mechanisms of extramedullary spread of PCs in MM have not been defined. There is a hypothesis that metastatic PCs initially leave the bone marrow, enter into blood in the form of clonal circulating plasma cells (cPCs), and finally locate in peripheral tissues and develop into EMP.5 Possible mechanisms of extramedullary myeloma cells spread include decreased expression of adhesion molecules (VLA-4, CD44, CD56), low expression of chemokine receptors (CCR1, CCR2 and CXCR4), upregulation of genes involved in angiogenesis and adhesion (angiopoietin-1, Notch3, and fibronectin-1) in EMPs compared with MM.3 However, the exact mechanisms involved in hematogenous myeloma cell dissemination and cell growth at extramedullary sites are still not clear.
A member of the G protein receptor family (GPCR), sphingosine-1-phosphate receptor 2 (S1PR2), could be activated by sphingosine-1-phosphate (S1P) and couple with different Gα proteins (such as Gi, Gq, G12/13), which activates a variety of signaling pathways and exerts different biological effects.6 Of note, S1PR2 involves in promoting the maturing B cells to the follicle center and inhibiting their growth through Gα13/p115RhoGEF/Rho pathway activation that subdues the ability of B cells to respond to chemokines providing chemoattractant and prosurvival signals.7,8 In the B cell tumors, the research related to S1PR2 is mainly focused on B-cell lymphoma and recent studies have confirmed that S1PR2 was an important tumor suppressor in diffuse large B-cell lymphoma (DLBCL).9 In germinal center B-cell-like (GCB) DLBCL, S1PR2/GNA13/ARHGEF1 pathway exerted dual actions in inhibiting growth and restricting dissemination of germinal center B cells and was frequently deficient.7 While in activated B-cell-like (ABC) DLBCL, S1PR2 promoted cell apoptosis through Gα13-dependent pathway and the expression of S1PR2 was directly repressed by FOXP1.9 Furthermore, S1PR2 was regarded as a negative prognosticator of survival in patients with DLBCL.10 As for the role S1PR2 played in multiple myeloma, few studies have been performed.
In this study, we applied single-cell RNA-seq to find transcriptomic differences between bone marrow myeloma cells (BMMCs) and peripheral cPCs in five EMP-positive patients, and found downregulated expression of S1PR2 in cPCs. Then we demonstrated both JTE-013, S1PR2 specific inhibitor, and S1PR2 knockdown significantly promoted cell migration and invasion ability in MM cells. We found that nuclear factor kappa-B (NF-κB) signaling pathway may account for the tumor suppression function of S1PR2 in MM cells. Overall, we demonstrated S1PR2 activation suppressed cell migration and invasion ability of MM cell through NF-κB pathway interruption, which may partially account for the hematogenous myeloma cell dissemination at extramedullary sites.
Materials and Methods
Agents and Antibodies
Primary antibodies directed against S1PR2 (sc-365589), p-p65 (Ser536) (Sc-136548) were obtained from Santa Cruz Biotechnology (California, USA). Antibodies for detecting p65 (8242) were purchased from Cell Signaling Biotechnology (Massachusetts, USA). The anti-MMP9 antibody (10375-2-AP) was obtained from Proteintech (Shanghai, China). JTE-013 was purchased from Selleck (Houston, USA). The cell counting kit-8 (CCK8) (CK04) was purchased from DOJINDO (Kyushu, Japan). Transwell chamber and Matrigel were obtained from CORNING (California, USA).
Samples and Single-Cell RNA Sequencing
Five patients with EMP were involved in this study and they were a subset of patients from the study we previously published.5 They were diagnosed according to the 2009 International Myeloma Working Group (IMWG) consensus criteria.12 This project was approved by the Ethics Committee of Peking University Third Hospital (IRB00006761-2016120) and written informed consent was given by each patient according to the Declaration of Helsinki protocol. Bone marrow and peripheral blood samples from each patient were simultaneously collected in a sterile manner. BMMCs and cPCs were isolated by Ficoll-Paque PLUS (GE Healthcare Bio-Sciences, Pittsburgh, USA) and CD138+ MACS microbeads separation (Miltenyi Biotec, Bergisch Gladbach, Germany). Then the myeloma cells were further sorted and enriched by flow cytometry. Single-cell RNA sequencing was performed as previously reported.5
Cell Cultures and Lentiviral Vector Transfection
The multiple myeloma cell line U266 was provided by Central Laboratory of the Peking University Third Hospital. Cell STR authentication was made by Beijing MDHS Testing technology Co., Ltd before we conducted the following experiments as below. Cells were cultured at 37°C/5% CO2 in RPMI1640 containing 10% fetal bovine serum, supplemented with 1% penicillin/streptomycin. The lentiviral shRNAs were constructed by Generay Biotech Co., Ltd (Shanghai, China). The siRNA sequences for S1RP2 were: 5′-CAACAAGGTCCAGGAACACTATAAT-3′ and the siRNA sequences for NC were: 5′-TTCTCCGAACGTGTCACGTAA-3′. The plasmids including negative control (NC) short-hairpin RNA (shRNA) and S1PR2 gene shRNA were constructed by Hanbio (Shanghai, China). The U266 cells were inoculated to 24 well-plates (2.5⨰10^5/mL) and cultured for 24 h. When the cells reached 50–70% confluence they were infected with S1PR2-shRNA lentivirus or NC-shRNA lentivirus using transfection agents. After 48 h, quantitative polymerase chain reaction (qPCR) and Western blot assays were performed to estimate the transfection efficiency.
RNA Isolation and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Whole RNA of control and S1PR2 shRNA U266 cells were extracted and reversely transcribed into cDNA using FastKing First Strand cDNA Synthesis Kit (TIANGEN, Shanghai, China) according to the instructions. Then the real-time quantitative PCR was performed in triplicate with β-actin as endogenous control. The Livak method was used to calculate the level of the S1PR2 mRNA. Specific primer sequences for S1PR2 and β-actin were indicated in Table 1.
 |
Table 1 Primer Sequences |
Protein Extraction and Western Blot Analysis
After transfection, cells were collected and washed with phosphate-buffered saline three times. Total protein of the cells was extracted using RIPA lysis buffer and the protein was quantitated with BCA protein assay. The samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis with 30 µg per hole and then transferred onto nitrocellulose membranes. After being blocked with 5% BSA for one hour, the membranes were incubated with primary antibodies overnight at 4°C. After washing three times with TBST, the membranes were incubated with secondary fluorescence antibodies for one hour at room temperature. The images were collected and analyzed by Odyssey two-color infrared laser imaging system.
Cell Proliferation Assay
Cell Counting Kit-8 (CCK-8) assay was applied to determine the proliferation ability. After transfection, cells were diluted to 1⨰10^5/mL and plated in 96 well plates with 100 µL per well and grown for 24, 48, and 72 h. Then 10 μL CCK-8 solution was added to each well and the cells were incubated at 37°C for one hour. Finally, the absorbance at 450 nm was measured by enzyme-linked immunosorbent assay. All the experiments were repeated three times independently.
Transwell Assay for the Examination of Cell Migration and Invasion
Transwell chamber with an 8.0-μm pore membranes was used for cell migration and invasion test. For the invasion assay, the transwell chamber was firstly coated with 60 µL Matrigel (800 μg/mL) for one hour. After transfection, U266 cells were serum-free starved for 12 h and then resuspended to 5⨰10^6 cells/mL in RPMI 1640 containing 0.1% BSA. Then, 600 µL of the 10% FBS culture medium was added to the lower chamber and 100 µL of the cell suspension was added to the upper chamber. After culturing at 37°C for 24 h, the cells transmigrated into the lower chamber were counted and photographed by microscope (Nikon, Tokyo, Japan).
Statistics
All graphs represent mean plus standard deviation (SD) of at least three independent assays. Two-tailed Student's t-test was used to accomplish statistical analysis p<0.05, p<0.01, p<0.001.
Results
Downregulated Expression of S1PR2 May Contribute to cPCs Extramedullary Translocation and Survival in Peripheral Blood
In Table 2, clinical characteristic analysis is performed for the five EMP-positive patients. The average age of the five patients was 66.2 years old with a standard deviation of 4.92 years. There was an even distribution of gender and ISS stages. All five patients have anemia and higher β2-MG detection. Extramedullary translocation is the primary step that enables cPCs to migrate from the bone marrow. To explore the possible factors associated with the translocation and cell survival of cPCs, bone marrow and peripheral blood samples from each patient were simultaneously collected. Consistent with myeloma diagnosis, BMMCs were detected in all five bone marrow samples, but cPCs were positive only in two peripheral blood samples (P02 and P04) (Table 3). Then we performed single-cell RNA seq using the BMMCs and cPCs we enriched and the results showed the expression level of S1RP2 in cPCs was lower than that in BMMCs (P<0.05) (Figure 1). Recent studies have confirmed that S1PR2 was an important tumor suppressor in DLBCL and the loss of S1PR2 allele significantly accelerated tumor formation in an MYC-driven B-cell lymphoma model.7,9
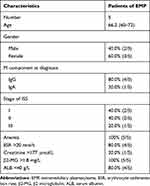 |
Table 2 The Clinical Characteristics of Five Patients with Extramedullary Plasmacytoma |
 |
Table 3 Sample Collection and Cell Detection |
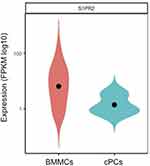 |
Figure 1 The S1PR2 mRNA expression levels in bone marrow myeloma cells (BMMCs) and circulating plasma cells (cPCs). Fragments per kilobase million (FPKM) was chosen as standardized value. |
JTE-013 Promotes Cell Migration and Invasion in U266 Cells
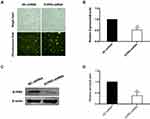
JTE-013, a selective antagonist of S1PR2, was used to explore the role of S1PR2 on cell migration and invasion in MM cell line U266. A transwell chamber assay was conducted to detect the effect of JTE-013 treatment on U266 cell migration and invasion. As shown in Figure 2A and B, compared with the control group, there was an 8.83-, 20.90-, and 28.86-fold increase in the number of U266 cells transmigrated after 1 μM, 2.5 μM and 5 μM exposures to JTE-013 respectively (P<0.01). As shown in Figure 2C and D, 2.5 μM and 5 μM JTE-013 significantly induced U266 cell invasion with a 9.59- and 13.72-fold increase in the cell counts respectively (P<0.0001). Taken together, the data showed that JTE-013 promoted u266 cell migration and invasion in a concentration-dependent manner.
Construction and Verification of S1PR2 Knockdown U266 Cells
To further evaluate the function of S1PR2 in MM, U266 was transfected with a lentivirus carrying S1PR2-shRNA or NC-shRNA. Lentiviral transfection efficiency for shRNAs was monitored by fluorescent microscopy (Figure 3A) and we observed about 90% transfection efficiency for both S1PR2-shRNA and NC-shRNA. Further, qPCR and Western blot analysis was performed to verify the expression levels of S1PR2 mRNA and protein in S1PR2-shRNA and NC-shRNA U266 cells. The results showed the mRNA level of S1PR2 decreased to 52% in the shS1PR2-transfected cells compared with the cells infected with NC-shRNA (P<0.01) (Figure 3B). Further, Western blot assay proved that the expression level of S1PR2 protein was also significantly decreased in the S1PR2-shRNA group compared with that in the negative control group (P<0.01) (Figure 3C and D).
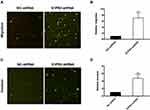
Downregulation of S1PR2 Promotes Cell Migration and Invasion in U266 Cells
We examined whether the downregulation of S1PR2 had the same impact on cell migration and invasion that S1PR2 specific inhibitor JTE-013 had by transwell chamber assay. As shown in Figure 4A and B, shS1PR2-transfection resulted in a 6.07-fold increase at the migration of U266 cells compared with the negative control group (P<0.01) (Figure 4A and B). Besides, S1PR2 knockdown enhanced cell invasion ability in U266 cells (3.74-fold) compared with the negative control group (P<0.001) (Figure 4C and D). The results showed the stimulating effect on cell migration and invasion of S1PR2 knockdown was similar to that of JTE-013.
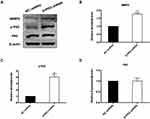
Knockdown of S1RP2 Regulates the Phosphorylation of NF-κB Pathway
Since we found that S1PR2 knockdown participated in the regulation of cell migration and invasion, we further investigated the molecular mechanism involved. We found that the expression level of phosphorylation-P65 (p-P65) was upregulated in S1PR2-shRNA U266 cells compared with the negative control group, which indicated thatS1PR2 might be involved in the NF-κB pathway regulation (Figure 5A and C). While the expression level of P65 showed no difference in the two groups (Figure 5A and D). Besides, the results showed that the expression level of matrix metallopeptidase 9 (MMP9) in S1PR2-shRNA U266 cells was higher than that in NC group, which further confirmed the role of S1PR2 in MM cell migration and invasion (Figure 5A and B).
Discussion
In patients with MM, the presence of CPCs is considered a marker for a worse prognosis.11 Extramedullary translocation is the primary step that cPCs migrate from the bone marrow. To further find the possible factors involved in cell migration and invasion, we analyzed the single cell transcriptome of cPCs and BMMCs in five EMP+ patients, and found S1PR2 was downregulated in cPCs compared with BMMCs (Figure 1). As far as we know, S1PR2 could promote the confinement of B cells within the follicle center and limit their growth. In S1PR2−/- mice, loss of germinal center confinement and abnormal growth of B cells accounted for the development of B-cell lymphomas.9 Recent studies have confirmed that S1PR2 was an important tumor suppressor in DLBCL. However, the role of S1PR2 in myeloma, another B cell tumor, has not been clear. In this study, we investigate the function of S1PR2 and the underlying mechanisms in myeloma cells.
In this research, we found S1PR2 has an important role in suppressing MM cell migration and invasion. JTE-013 has been widely used in many preclinical studies as a S1PR2-selective antagonist. In this study, pharmacologic inhibition of S1PR2 with JTE-013 evidently promoted U266 cell migration and invasion in a concentration-dependent manner (Figure 2). However, recent studies have raised a question about the specificity of JTE-013 to S1PR2. It has been proved that JTE013 showed antagonism on S1PR4 in MDA-MB-453 breast cancer cells.6 To further validate the role of S1PR2 in cell migration and invasion, we constructed S1PR2 knockdown U266 cells and found downregulation of S1PR2 significantly promoted cell migration and invasion in MM cells, which was consistent with the results of JTE-013 exposure as described above. Matrix metalloproteinases (MMP) are able to degrade many constituents of extracellular matrix, which leads to invasion, metastasis, and angiogenesis in a number of malignancies including MM.15 In this study, we found the expression level of MMP9 was higher in the S1PR2 knockdown group, which further validated the anti-invasion role of S1PR2 in MM. However, S1PR2 inhibition with JTE-013 or S1PR2-shRNA had no detectable effect on U266 cell proliferation through CCK-8 test (Supplementary Figure 1).
In fact, several lines of evidence have demonstrated that S1PR2 negatively regulated migration and invasion in DLBCL, melanoma, glioblastoma, and Wilms' tumor cells through different mechanisms, such as Rho/Rho kinase/JNK pathway activation, RhoA/ROCK activation, PI3K-AKT inhibition, Rac inhibition.7,16-18 Besides, some reports have determined that NF-κB negatively correlated with S1PR2 expression and might be mediated the pro-tumor effect through suppressing S1P/S1RP2 axis.13,14 In this study, we proved that S1PR2 might inhibit cell migration and invasion in MM cells through regulating phosphorylation of NF-κB signaling pathway. The results indicated that p-P65 was obviously upregulated in S1PR2-shRNA U266 cells compared with the NC group, which indicated S1PR2 suppressed MM cell migration and invasion partially through downregulating NF-κB signaling pathway (Figure 5).
In conclusion, we provide evidence that the downregulated expression of S1PR2 in myeloma cells partially accounts for the translocation and survival of cPCs in peripheral blood. S1PR2 knockdown could promote cell migration and invasion in MM cells, and this effect is associated with NF-κB signaling pathway inhibition. However, the antimetastasis role of S1PR2 and the downstream signals of S1PR2 in MM need to be further investigated.
Abbreviations
MM, multiple myeloma; EMP, extramedullary plasmacytoma; BMMCs, bone marrow myeloma cells; cPCs, circulating plasma cells; S1PR2, sphingosine-1-phosphate receptor 2; S1P, sphingosine-1-phosphate; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B-cell-like; ABC, activated B-cell-like; NC, negative control; shRNA, short-hairpin RNA; NF-κB, nuclear factor kappa-B; p-p65, phosphorylation-p65; MMP9, matrix metallopeptidase 9.
Acknowledgments
We are grateful to DF, and WJ for their participation in collection of patient samples at Peking University Third Hospital; We appreciate HS, LC and ZD for their support in flow cytometry experiments, and Dr Zhang Weilong for technical support in next-generation sequencing at the Peking University High Throughput Sequencing Center.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Palumbo A, Mina R. Management of older adults with multiple myeloma. Blood Rev. 2013;27:133–142. doi:10.1016/j.blre.2013.04.001
2. Eslick R, Talaulikar D. Multiple myeloma: from diagnosis to treatment. Aust Fam Physician. 2013;42:684–688.
3. Bladé J, Fernández de Larrea C, Rosiñol L, et al. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805–3812. doi:10.1200/JCO.2011.34.9290
4. Wu P, Davies FE, Boyd K, et al. The impact of extramedullary disease at presentation in the outcome of myeloma. Leuk Lymphoma. 2009;50:230–235. doi:10.1080/10428190802657751
5. Geng S, Wang J, Xiannian Z, et al. Single-cell RNA sequencing reveals chemokine self-feeding of myeloma cells promotes extramedullary metastasis. FEBS Lett. 2019. doi:10.1002/1873-3468.13623
6. Adada M, Canals D, Hannun YA, Obeid LM. Sphingosine-1-phosphate receptor 2. FEBS J. 2013;280:6354–6366. doi:10.1111/febs.12446
7. Muppidi JR, Schmitz R, Green JA, et al. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516:254–258. doi:10.1038/nature13765
8. Green JA, Suzuki K, Cho B, et al. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol. 2011;12:672–680. doi:10.1038/ni.2047
9. Flori M, Schmid CA, Sumrall ET, et al. The hematopoietic oncoprotein FOXP1 promotes tumor cell survival in diffuse large B-cell lymphoma by repressing S1PR2 signaling. Blood. 2016;127:1438–1448. doi:10.1182/blood-2015-08-662635
10. Stelling A, Hashwah H, Bertram K, et al. The tumor suppressive TGF-β/SMAD1/S1PR2 signaling axis is recurrently inactivated in diffuse large B-cell lymphoma. Blood. 2018;131:2235–2246. doi:10.1182/blood-2017-10-810630
11. Granell M, Calvo X, Garcia-Guiñón A, et al. Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition. Haematologica. 2017;102:1099–1104. doi:10.3324/haematol.2016.158303
12. Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high‐dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009; 23: 1716–1730. doi: 10.1038/leu.2009.122
13. Tong S, Chen SC, Xu KY, et al. 14-3-3ζ promotes esophageal squamous cell carcinoma invasion by repressing S1PR2 protein expression through NF-κB signaling. Arch Biochem Biophys. 2018;643:7–13. doi:10.1016/j.abb.2018.02.009
14. Hendley AM, Wang YJ, Polireddy K, et al. p120 catenin suppresses basal epithelial cell extrusion in invasive pancreatic neoplasia. Cancer Res. 2016;76:3351–3363. doi:10.1158/0008-5472.CAN-15-2268
15. Valckenborgh EV, Croucher PI, Raeve HD, et al. Multifunctional role of matrix metalloproteinases in multiple myeloma. Am J Pathol. 2004;165(3):869–878. doi:10.1016/S0002-9440(10)63349-4
16. Yamaguchi H, Kitayama J, Takuwa N, et al. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J. 2003;374(Pt 3):715–722. doi:10.1042/bj20030381
17. Arikawa K, Takuwa N, Yamaguchi H, et al. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J Biol Chem. 2003;278:32841–32851. doi:10.1074/jbc.M305024200
18. Lepley D, Paik JH, Hla T, et al. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–3795. doi:10.1158/0008-5472.CAN-04-2311
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.