Back to Journals » OncoTargets and Therapy » Volume 12
S100 Calcium Binding Protein A11 (S100A11) Promotes The Proliferation, Migration And Invasion Of Cervical Cancer Cells, And Activates Wnt/β-Catenin Signaling
Authors Meng M, Sang L, Wang X
Received 29 July 2019
Accepted for publication 20 September 2019
Published 22 October 2019 Volume 2019:12 Pages 8675—8685
DOI https://doi.org/10.2147/OTT.S225248
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Man Meng,1 Lin Sang,2 Xiangyu Wang3
1Department of Oncology, The Second People’s Hospital of Hefei City Affiliated to Anhui Medical University, Hefei City, Anhui Province, 230000, People’s Republic of China; 2Department of Obstetrics and Gynecology, The Second People’s Hospital of Hefei City Affiliated to Anhui Medical University, Hefei City, Anhui Province, 230000, People’s Republic of China; 3Department of Obstetrics and Gynecology, The Affiliated Hospital of Qingdao University, Qingdao City, Shandong Province, 266003, People’s Republic of China
Correspondence: Xiangyu Wang
Department of Obstetrics and Gynecology, The Affiliated Hospital of Qingdao University, No. 16, Jiangsu Road, Qingdao City, Shandong Province 266003, People’s Republic of China
Tel +86-15863061517
Email [email protected]
Purpose: This study is aimed to investigate the specific regulatory role of S100 calcium binding protein A11 (S100A11) on cervical cancer (CC), and reveal the potential mechanisms relating to Wnt/β-catenin signaling.
Patients and methods: The expression of S100A11 in cervical squamous cell carcinoma (CSCC), adjacent non-cancerous, cervical intraepithelial neoplasia (CIN), and normal cervical tissues was detected by quantitative real-time PCR and/or immunohistochemistry. After transfection of pENTER-S100A11 or sh-S100A11-1/sh-S100A11-2, the viability, cell cycle, migration and invasion of C33A or SiHa cells were detected. The tumor volume and tumor weight were measured after injection of transfected C33A cells into mice. The expression of E-caherin (CDH2), N-caherin (CDH1), β-catenin (CTNNB1), and c-Myc (MYC) in C33A and SiHa cells was detected by Western blot.
Results: The expression of S100A11 was significantly higher in CSCC tissues than in adjacent non-cancerous, CIN, and normal cervical tissues (P < 0.05). S100A11 expression was positively correlated with the FIGO stage and lymph node metastasis of CSCC patients (P < 0.05). The transfection of pENTER-S100A11 into C33A cells significantly increased the cell viability, the percentage of cells in G2/M phase, the numbers of migratory and invasive cells, as well as the tumor volume and weight in mice (P < 0.05). Overexpression of S100A11 also significantly downregulated E-caherin, and upregulated N-caherin, β-catenin, and c-Myc in C33A cells (P < 0.05). The transfection of sh-S100A11-1/sh-S100A11-2 exhibited the opposite results to that of pENTER-S100A11 on SiHa cells.
Conclusion: Overexpression of S100A11 promotes the proliferation, migration, invasion, and epithelial-mesenchymal transition of CC cells, and activates Wnt/β-catenin signaling.
Keywords: S100A11, cervical cancer, Wnt/β-catenin signaling, proliferation, migration, invasion
Introduction
Cervical cancer (CC) is the fourth most common cancer and the fourth leading cause of cancer-related death in women in the world.1 CRC is a severe cancer originating from the cervix, and about 90% of CC cases are cervical squamous cell carcinoma (CSCC), and 10% cases are adenocarcinoma.2 Human papillomavirus (HPV) infection, especially HPV16 and HPV18 infection, is a necessary cause of CC. HPV16 and HPV18 infection can be observed in more than 90% of CC patients.3 Up to now, surgery, chemotherapy and radiotherapy are still the main therapeutic strategies for CC in clinical practice.4 However, metastasis of CC cells to lymph nodes or adjacent tissues greatly limits the prognosis of CC patients.5 Since molecular targeted therapy is a promising therapeutic strategy for CC, researching of novel therapeutic targets for CC is urgently needed.
S100 is a large subfamily of calcium binding proteins comprising at least 25 members.6 S100 calcium binding protein A11 (S100A11), also known as calgizzarin or S100C, is an important member of S100 protein that is involved in the initiation and progression of cancer.7 S100A11 is upregulated in a variety of cancers, such as lung,8 ovarian,9 renal,10 pancreatic11 and gastric cancer.12 S100A11 exerts key regulatory role in diverse cellular processes of cancer, such as the proliferation, apoptosis, cell cycle, migration, invasion, and epithelial-mesenchymal transformation (EMT). For example, overexpression of S100A11 promotes the proliferation of PANC-1 cells (pancreatic cancer), decreases the percentage of early apoptotic cells, and increases the percentage of cells in the S phase.13 Knockdown of S100A11 inhibits the migration and invasion of 786-O cells (renal cancer) via upregulating E-cadherin.14 Knockdown of S100A11 inhibits transforming growth factor-β1 (TGF-β1)-induced migration and invasion of RBE cells (cholangiocarcinoma) and upregulates the expression of N-cadherin (CHD2), β-catenin (CTNNB1), Vimentin (VIM), Slug (SLUG) and Snail (SNAL1) and downregulates E-cadherin (CDH1).15 In addition, previous studies have proved that the overexpression of both, S100A14 and S100A7, can promote the migration, invasion, and EMT of cervical cancer C33A cells.16,17 However, the specific regulatory role of S100A11 on CC remains unclear.
Wnt/β-catenin signaling plays an important role in regulating the proliferation, apoptosis, invasion, and migration of cancer cells.18 Since Wnt/β-catenin signaling is usually activated in cancer, inhibition of Wnt/β-catenin signaling has become a promising therapeutic target for CC.19 However, the regulatory relationship between S100A11 and Wnt/β-catenin signaling on CC remains unclear. In this study, the expression of S100A11 was detected in CSCC, adjacent non-cancerous, cervical intraepithelial neoplasia (CIN), and normal cervical tissues. Based on the overexpression and silencing of S100A11, the specific regulatory role of S100A11 on the proliferation, invasion, migration, and EMT of CC cells were evaluated. The potential regulatory mechanism of S100A11 related to Wnt/β-catenin signaling was further analyzed. Our findings may reveal a promising therapeutic target for CC, and the underlying mechanisms responsible for CC treatment.
Materials And Methods
Patients And Tissue Samples
A total of 127 CSCC and 86 CIN patients were enrolled in the Affiliated Hospital of Qingdao University between July 2006 and August 2012. CSCC was staged in accordance with the International Federation of Gynecology and Obstetrics (FIGO) criteria, and graded in accordance with the World Health Organization criteria. Histopathologically confirmed CSCC and CIN tissues were collected from patients receiving biopsy or primary surgery. A total of 30 normal cervical tissues were collected as controls. In addition, CSCC tissues and adjacent non-cancerous tissues were collected from 27 representative CSCC patients. This study was approved by the Institutional Review Board of Qingdao University (Examination and Approval Number of Ethics Committee: 2016-Gynaecology-021103), and written informed consents were obtained from all patients.
Cell Culture
Human CC cell lines, including SiHa (HTB-35, CSCC), HeLa (CCL-2, adenocarcinoma), C33A (HTB-31, CSCC), and CaSki (CRL-7915, CSCC) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). SiHa, HeLa, and C33A cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma, St Louis, MO, USA) containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), 100 U/mL streptomycin, and 100 U/mL penicillin. CaSKi cells were maintained in McCoy’s 5A medium (Sigma) containing 10% FBS, 100U/mL streptomycin, and 100 U/mL penicillin. All cells were cultured in an incubator at 37°C with 5% CO2. Cells were passaged until 90% confluence, and logarithmic growth phase cells were used for further assays.
Cell Treatments
The plasmids of pENTER-S100A11 and pENTER-Control (pENTER-Con) (vector, pENTER; promoter, CMV; Tag, FLAG and His) were purchased from the ViGene Biosciences (Rockville, MD, USA). shRNAs of shRNA-S100A11-1 (sh-S100A11-1), sh-S100A11-2, and shRNA Control (sh-Con) (vector, pAV-U6-GFP; promoter, U6; Tag, GFP) were purchased from the GenePharma (Shanghai, China). At 80% confluence, C33A cells were transfected with pENTER-S100A11 and pENTER-Con, and SiHa cells were transfected with shRNA-S100A11-1/shRNA-S100A11-2 and sh-Con by using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) for 72 h. Stable transfection was selected by using 2 µg/mL puromycin (Sigma). After the transfection for 72 h, C33A cells were treated with 200 ng/mL dickkopf-1 (DKK-1) (a Wnt inhibitor) for another 48 h.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from specific tissues and cells using TRIzol reagent (Invitrogen), and reverse-transcribed using a cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) in accordance with manufacturers’ instructions. qRT-PCR was performed using SYBRs Green PCR Master Mix (Applied Biosystems)
on the StepOne PlusTM Real-Time PCR System (Applied Biosystems). Relative expression of target genes was calculated according to the 2−ΔΔCt method.20 Specific primers used in qRT-PCR were shown in Table 1.
 |
Table 1 Primers Used In Quantitative Real-Time PCR |
Immunohistochemistry (ICH)
Paraffin-embedded tissue sections were dewaxed in xylene, dehydrated in graded ethanol, incubated in 3% H2O2 for 30 min, and heated in citrate buffer (pH 6.0) at 95°C for 25 min. The sections were then blocked with 10% normal goat nonimmune serum at 37°C for 30 min, and incubated with primary antibody, anti-S100A11 (1:200, ProteinTech, Rosemont, IL, USA) overnight at 4°C. IHC was performed by using immunohistological staining kit (Golden Bridge, Beijing, China) in accordance with the manufacturer’s instructions. The sections were stained with diaminobenzidine (DAB) and Hematoxylin and observed under Aperio scanning system (Aperio, San Diego, USA). The expression of S100A11 was quantitatively analyzed by Aperio Image Scope software (Aperio) in 5 randomly selected fields.
Western Blot
Total proteins were isolated from specific tissues and cells using RIPA Lysis buffer (Invitrogen), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to polyvinylidenefluoride membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% skim milk for 1 h, and incubated with specific primary antibodies, including anti-S100A11 (1:500, ProteinTech), -E-cadherin, -N-cadherin (1:500, Santa Cruz Biotechnology, CA, USA), -β-catenin, -c-Myc (1:500, Abcam, Cambridge, England), and -β-actin (1:500, Sigma) overnight at 4°C. Then the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000, Abcam) for 1 h at 25°C. The protein bands were visualized using the enhanced chemiluminescence kit (Pierce, Rockford, IL, USA) in accordance with manufacturer’s instructions. The relative expression level of protein was calculated by comparing with β-actin and normalized to the control (set as 1).
MTS Assay
Cell viability was detected by using MTS assay kit (Promega, Madison, WI, USA) in accordance with the manufacturer’s instructions. Briefly, cells were seeded in 96-well plates at a density of 3 × 103/well and incubated with MTS for 4 h. After 10 min of incubation with DMSO for 10 min, optical density (OD) at 490nm was detected by a spectrophotometer (Invitrogen).
Migration And Invasion Assays
Migration and invasion assays were performed using Boyden chambers (polycarbonate membrane with 8 μm pores) (Costar, Cambridge, MA, USA). Briefly, cells were seeded in chambers for migration assay, and in chambers coated with Matrigel (BD Biosciences, San Jose, USA) for invasion assay. The lower chamber was filled with 600 µl RPMI 1640 medium containing 10% FBS. After 48 h of incubation at 37°C, cells on the top surface of the insert were removed with cotton swab. Cells on the bottom surface of the insert were stained with 0.3% crystal violet for 30 min. The migratory and invasive cells were observed under microscope (Olympus, Tokyo, Japan), and counted in 5 randomly selected fields.
Cell Cycle Assay
Cells were washed with phosphate buffered saline (PBS), and fixed in methyl alcohol overnight. Then cells were incubated with PBS containing 50 μg/mL propidium iodide, 100 μg/mL RNase, and 0.1% Nonidet P-40 for 30 min at 37°C. The number of cells in particular cell cycle phases was determined by the nuclear DNA content on FACS Calibur cell flow cytometer (BD Biosciences).
Establishment Of Tumor Model In Mice
Female BALB/c-nude mice (4–6 weeks old) were purchased from Vital River Laboratory Animal Technology (Beijing, China). Mice were fed in specific pathogen free animal room at 22°C-25°C and 40–50% humidity with free access to water and food. The transfected C33A cells (5×105) were subcutaneously injected into mice. The tumor volume was measured using vernier caliper every 5 days. After the injection for 35 days, the tumor weight was measured on an analytical balance. Animal experiment was approved by the Animal Care and Use Committee of the Affiliated Hospital of Qingdao University (Examination and Approval Number of Ethics Committee: 2016-Gynaecology-021103).
Statistical Analyses
All experiments were performed at least in triplicate, and data were expressed as mean ± standard deviation. Statistical analysis was performed by SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). The clinical parameters were compared by Mann–Whitney U (two groups) and Kruskal-Wallis test (more than two groups). Other quantitative parameters were compared by Student’s t-test (two groups). A P-value less than 0.05 was considered to be significantly different.
Results
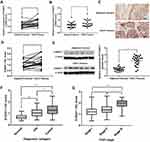
S100A11 Was Upregulated In CSCC Tissues
The expression of S100A11 was detected in 27 cases of CSCC tissues and adjacent non-cancerous tissues. qRT-PCR showed that the expression of S100A11 at the mRNA level in 21 out of 27 CSCC patients was significantly higher in CSCC tissues than that in adjacent non-cancerous tissues (P < 0.05) (Figure 1A). Among 27 CSCC patients, the mean expression of S100A11 was significantly higher in CSCC tissues than in adjacent non-cancerous tissues (P < 0.05) (Figure 1B). In addition, IHC showed that S100A11 was mainly located in the cytoplasm and cytomembrane of CSCC cells (Figure 1C). The S100A11 IHC score in 21 out of 27 CSCC patients was significantly higher in CSCC tissues than that in adjacent non-cancerous tissues (P < 0.05) (Figure 1D). Significantly higher expression of S100A11 at the protein level was also identified in CSCC tissues than in adjacent non-cancerous tissues from 6 representative CSCC patients by Western blot (Figure 1E).
The Expression Of S100A11 Was Positively Correlated With The FIGO Stage And LN Metastasis Of CSCC Patients
The expression of S100A11 was further analyzed in 127 cases of CSCC tissues (74 cases at stage I, 38 cases at stage II and 15 cases at stage III), 86 cases of CIN tissues, and 30 normal cervical tissues by IHC. As shown in Figure 1F, the S100A11 IHC score was significantly higher in CIN tissues than in normal cervical tissues (P < 0.01), and significantly higher in CSCC tissues than in CIN tissues (P < 0.05) (Figure 1F). CSCC tissues at stage III exhibited significantly higher S100A11 IHC score than those at stage I and II (P < 0.05). There was no significantly difference in S100A11 IHC score between stage I and II CSCC tissues (Figure 1G). The correlation between S100A11 expression (IHC score) and clinical characteristics of CSCC patients was further analyzed. As shown in Table 2, the expression of S100A11 was positively correlated with the FIGO stage and lymph node (LN) metastasis in CSCC patients (P < 0.05) (Table 2).
 |
Table 2 The Correlation Between S100A11 Expression (IHC Score) And Clinical Characteristics Of Cervical Squamous Cell Carcinoma (CSCC) Patients |
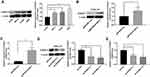
S100A11 Overexpression Promoted The Proliferation, Migration, And Invasion Of CC Cells
The expression of S100A11 was detected in four CC cell lines, including SiHa, HeLa, C33A, and CaSki cells. Western blot showed that the expression of S100A11 was the highest in SiHa cells and lowest in C33A cells (Figure 2A). The transfection of pENTER-S100A11 significantly upregulated S100A11 in C33A cells (P < 0.01) (Figure 2B and C). The transfection of sh-S100A11-1/sh-S100A11-2 significantly downregulated S100A11 in SiHa cells (P < 0.05) (Figure 2D and E).
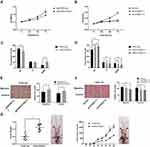
The regulatory role of S100A11 on the proliferation, migration, and invasion of C33A and SiHa cells were further evaluated. MTS showed that the cell viability (OD490) was significantly higher in pENTER-S100A11-transfected C33A cells than in pENTER-Con-transfected cells at 48 and 72 h post-transfection (P < 0.05). The cell viability was significantly lower in sh-S100A11-1/sh-S100A11-2-transfected SiHa cells than in sh-Con-transfected cells at 48 and 72 h post-transfection (P < 0.05) (Figure 3A and B). After transfection of pENTER-S100A11 into C33A cells, the percentage of cells in G1 phase decreased significantly and the percentage of cells in G2/M phase increased significantly (P < 0.001) (Figure 3C). The transfection of sh-S100A11-1/sh-S100A11-2 into SiHa cells significantly increased the percentage of cells in G1 phase, and decreased the percentage of cells in S and G2/M phases (P < 0.05) (Figure 3D). In addition, the transfection of pENTER-S100A11 into C33A cells, and sh-S100A11-1/sh-S100A11-2 into SiHa cells significantly increased, and decreased the numbers of migratory and invasive cells, respectively (P < 0.05) (Figure 3E and F). Furthermore, the transfected C33A cells were injected into mice. Mice injected with pENTER-S100A11-transfected C33A cells exhibited significantly higher tumor volume than those injected with pENTER-Con-transfected cells beginning from the 15th day (P < 0.05). After 30 days of breeding, the tumor weight was significantly higher in mice injected with pENTER-S100A11-transfected C33A cells than those injected with pENTER-Con-transfected cells (P < 0.01) (Figure 3G).
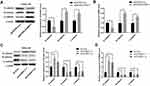
S100A11 Overexpression Enhanced The EMT Of CC Cells
Because S100A11 overexpression promoted the migration and invasion of C33A and SiHa cells, the EMT of C33A and SiHa cells was further analyzed. As shown in Figure 4A and B, overexpression of S100A11 significantly downregulated E-caherin, and upregulated N-caherin and β-catenin in C33A cells at both the mRNA and protein level (P < 0.05) (Figure 4A and B). In contrast, silencing of S100A11 significantly upregulated E-caherin, and downregulated N-caherin and β-catenin in SiHa cells at both the mRNA and protein level (P < 0.05) (Figure 4C and D).
S100A11 Overexpression Activated Wnt/β-Catenin Signaling In CC Cells
The regulatory relationship between S100A11 and Wnt/β-catenin signaling was evaluated. As shown in Figure 5A and B, overexpression of S100A11 significantly upregulated β-catenin and c-Myc in C33A cells at both the mRNA and protein level (P < 0.05) (Figure 5A and B). In contrast, silencing of S100A11 significantly downregulated β-catenin and c-Myc in SiHa cells at both the mRNA and protein level (P < 0.05) (Figure 5C and D). In addition, the intervention of DKK-1 (a Wnt inhibitor) significantly downregulated S100A11, β-catenin and c-Myc in both pENTER-S100A11- and pENTER-Con-transfected C33A cells (P < 0.05) (Figure 5E and F).
Discussion
S100 protein is a large subgroup of EF-hand protein family, which plays a key regulatory role in diverse cellular processes, such as calcium homeostasis, endo- and exocytosis, cell cycle, proliferation, apoptosis, migration, inflammation, cytoskeleton dynamics, and DNA repair.21,22 Growing evidence have shown that S100A11 protein is also implicated in tumorigenesis and progression of cancer.23,24 In this study, the expression of S100A11 in CSCC tissues was significantly higher than that in adjacent non-cancerous tissues by qRT-PCR and IHC in 27 cases of CSCC. Our findings are consistent with previous studies on lung cancer,8 ovarian cancer,9 renal cancer,10 pancreatic cancer,11 and gastric cancer.12 We suspect that S100A11 may function as a tumor promoter in CC. In addition, the expression of S100A11 is also closely associated with cancer progression. It has been reported that S100A11 is positively correlated with the regional LN metastasis, and the stage of patients with colorectal cancer.25,26 S100A11 is positively correlated with FIGO stage, ascitic fluid volume, residual disease, as well as poor disease-free and overall survival in patients with high-grade ovarian cancer.27 In this study, we found that the expression of S100A11 was significantly higher in CSCC tissues than in CIN tissues, and significantly higher in stage III CSCC tissues than in stage I and II CSCC tissues. In addition, S100A11 was positively correlated with the FIGO stage and LN metastasis of CSCC patients. Our findings are consistent with previous studies, suggesting that S100A11 may be a promising diagnostic and prognostic factor for CC.
S100A11 plays a key role in regulating the proliferation, migration, and invasion of cancer cells. It has been reported that the overexpression of S100A11 in PANC-1 cells increases the proliferation rate and cell percentage in S phase, and decreases the early apoptotic rate and cell percentage in G0/G1 phase.13 S100A11 knockdown significantly decreases the numbers of migratory and invasive cells in RBE and 786-O cells.14,15 However, the specific regulatory role of S100A11 in CC cells remains unclear. In this study, S100A11 was overexpressed in C33A cells by the transfection of pENTER-S100A11 and silenced in SiHa cells by the transfection of sh-S100A11-1/sh-S100A11-2. We found that overexpression of S100A11 in C33A cells significantly increased the cell viability, the percentage of cells in G2/M phase, as well as the numbers of migratory and invasive cells. The opposite results in SiHa cells were found after S100A11 silencing. These findings are consistent with previous studies on pancreatic cancer, renal cancer, and cholangiocarcinoma mentioned above.13–15 Silencing of S100A11 may inhibit the proliferation of CC cells via blocking cells in G2/M phase, as well as the migration and invasion abilities.
During EMT, polarized epithelial cells are converted into motile mesenchymal cells.28 EMT influences cell-cell and cell-matrix interactions, and enhances cell invasiveness, thereby facilitating the initiation of caner metastasis.29 In this study, overexpression of S100A11 significantly downregulated E-caherin, and upregulated N-caherin and β-catenin in C33A cells. S100A11 silencing had the opposite effect on SiHa cells. Our findings are consistent with a pervious study that knockdown of S100A11 upregulates the expression of N-cadherin, β-catenin, vimentin, Slug and Snail and downregulates E-cadherin in RBE cells.15 We suspect that silencing of S100A11 may suppress the migration and invasion of CC cells through inhibiting EMT.
Wnt/β-catenin signaling is an important regulatory signaling pathway involved in tumorigenesis.30 The regulatory relationship between S100A11 and Wnt/β-catenin signaling is still unclear in CC. In this study, we found that overexpression of S100A11 significantly upregulated β-catenin and c-Myc in C33A cells, and silencing of S100A11 significantly downregulated β-catenin and c-Myc in SiHa cells. These findings indicate that S100A11 has a positive regulation on the activation of Wnt/β-catenin signaling in CC cells. In addition, our further assay showed that the intervention of DKK-1 (a Wnt inhibitor) significantly downregulated S100A11, β-catenin and c-Myc in C33A cells. These results indicate that Wnt/β-catenin signaling can also feedback regulate S100A11 in CC cells. It has been reported that Wnt/β-catenin pathway inhibitor calcimycin inhibits the S100A4-induced migration and invasion of HCT116 cells (colon cancer).31 Knockdown of β-catenin revises the promoting effects of recombinant S100A8 and S100A9 on the viability and migration of HCT116 and SW480 cells.32 We suspect that silencing of S100A11 may inhibit the proliferation, migration, invasion, and EMT of CC cells through blocking Wnt/β-catenin signaling. However, the detailed action mechanism of S100A11 on Wnt/β-catenin signaling in CC remains unclear, and further research is still needed.
Conclusions
S100A11 was upregulated in CSCC tissues, and its expression was positively correlated with the FIGO stage and LN metastasis of CSCC patients. Overexpression of S100A11 promoted the proliferation, invasion, migration, and EMT of CC cells in vitro, as well as the tumor growth in vivo (mice). In addition, S100A11 could activate Wnt/β-catenin signaling in CC cells. Silencing of S100A11 may be a promising therapeutic target for CC. Further research on the efficacy and safety of S100A11 silencing in the treatment of CC were still needed.
Ethics Approval And Consent To Participate
This study was conducted after obtaining local ethical committee approval from the Affiliated Hospital of Qingdao University. Written informed consent was obtained from patients over the age of 18 years and parents of patients under the age of 18 years. This was conducted in accordance with the Declaration of Helsinki. All animal experiments were conducted after obtaining approval from the Affiliated Hospital of Qingdao University’s Ethics Committee. The Affiliated Hospital of Qingdao University’s Ethics Committee granted ethical and legal approval for the involvement of animals in this study. All animal experiments were carried out in accordance with the guidelines of Qingdao animal protection society.
Acknowledgements
This research was supported by Projects of the Medical and Health Technology Development Program in the Shandong province (2016WS0279).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Stewart BW, Kleihues P. World cancer report. World Cancer Rep. 2014;45:12–351.
2. Ghazi A, Najla AH, Medhat ES, Ismail AB. HPV prevalence and genetic predisposition to cervical cancer in Saudi Arabia. Infect Agents Cancer. 2013;8(1):15. doi:10.1186/1750-9378-8-15
3. Goodman A. HPV testing as a screen for cervical cancer. BMJ. 2015;350:h2372. doi:10.1136/bmj.h2372
4. Gadducci A, Tana R, Cosio S, Cionini L. Treatment options in recurrent cervical cancer (Review). Oncol Lett. 2010;1(1):3. doi:10.3892/ol_00000001
5. Mountzios G, Soultati A, Pectasides D, Pectasides E, Dimopoulos MA, Papadimitriou CA. Developments in the systemic treatment of metastatic cervical cancer. Cancer Treat Rev. 2013;39(5):430–443. doi:10.1016/j.ctrv.2012.05.009
6. Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. 2004;322(4):1111–1122. doi:10.1016/j.bbrc.2004.07.096
7. He H, Li J, Weng S, Li M, Yu Y. S100A11: diverse function and pathology corresponding to different target proteins. Cell Biochem Biophys. 2009;55(3):117. doi:10.1007/s12013-009-9061-8
8. Woo T, Okudela K, Mitsui H, et al. Up-regulation of S100A11 in lung adenocarcinoma – its potential relationship with cancer progression. PLoS One. 2015;10(11):e0142642. doi:10.1371/journal.pone.0142642
9. Liu Y, Han X, Gao B. Knockdown of S100A11 expression suppresses ovarian cancer cell growth and invasion. Exp Ther Med. 2015;9(4):1460–1464. doi:10.3892/etm.2015.2257
10. Liu L, Miao L, Liu Y, et al. S100A11 regulates renal carcinoma cell proliferation, invasion, and migration via the EGFR/Akt signaling pathway and E-cadherin. Tumour Biol. 2017;39(5):101042831770533. doi:10.1177/1010428317705337
11. Ohuchida K, Mizumoto K, Ohhashi S, et al. S100A11, A putative tumor suppressor gene, is overexpressed in pancreatic carcinogenesis. Clin Cancer Res. 2006;12(18):5417–5422. doi:10.1158/1078-0432.CCR-06-0222
12. Qian YY, Xiao MB, Run-Zhou NI, Digestive DO. Expression and clinical significance of S100 A11 in gastric cancer abstract. Chin J Cancer Prev Treat. 2014;21(13):1014–1017.
13. Xiao M, Li T, Ji Y, et al. S100A11 promotes human pancreatic cancer PANC-1 cell proliferation and is involved in the PI3K/AKT signaling pathway. Oncol Lett. 2018;15(1):175–182. doi:10.3892/ol.2017.7295
14. Liu L, Miao L, Liu Y, et al. S100A11 regulates renal carcinoma cell proliferation, invasion, and migration via the EGFR/Akt signaling pathway and E-cadherin. Tumour Biol. 2017;39(5):1010428317705337. doi:10.1177/1010428317705337
15. Zhang M, Zheng S, Jing C, et al. S100A11 promotes TGF-beta1-induced epithelial-mesenchymal transition through SMAD2/3 signaling pathway in intrahepatic cholangiocarcinoma. Future Oncol. 2018;14(9):837–847. doi:10.2217/fon-2017-0534
16. Wang X, Yang J, Qian J, Liu Z, Chen H, Cui Z. S100A14, a mediator of epithelial-mesenchymal transition, regulates proliferation, migration and invasion of human cervical cancer cells. Am J Cancer Res. 2015;5(4):1484–1495.
17. Tian T, Li X, Hua Z, et al. S100A7 promotes the migration, invasion and metastasis of human cervical cancer cells through epithelial-mesenchymal transition. Oncotarget. 2017;8(15):24964–24977. doi:10.18632/oncotarget.15329
18. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985. doi:10.1016/j.cell.2017.05.016
19. Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15(7):873. doi:10.1517/14728222.2011.577418
20. Livak KJST. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T))Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
21. Shankar J, Messenberg AJ, Underhill TM, Foster LJ, Nabi IR. Pseudopodial actin dynamics control epithelial-mesenchymal transition in metastatic cancer cells. Cancer Res. 2010;70(9):3780. doi:10.1158/0008-5472.CAN-09-4439
22. Huang Y, Zhao M, Xu H, et al. RASAL2 down-regulation in ovarian cancer promotes epithelial-mesenchymal transition and metastasis. Oncotarget. 2014;5(16):6734–6745. doi:10.18632/oncotarget.2244
23. Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15(2):96–109. doi:10.1038/nrc3893
24. Schönekess BO, Walsh MP. Molecular cloning and expression of avian smooth muscle S100A11 (calgizzarin, S100C). Biochem Cell Biol. 1997;75(6):771.
25. Meding S, Balluff B, Elsner M, et al. Tissue-based proteomics reveals FXYD3, S100A11 and GSTM3 as novel markers for regional lymph node metastasis in colon cancer. J Pathol. 2012;228(4):459–470. doi:10.1002/path.4021
26. Wang G, Wang X, Wang S, et al. Colorectal cancer progression correlates with upregulation of S100A11 expression in tumor tissues. Int J Colorectal Dis. 2008;23(7):675–682. doi:10.1007/s00384-008-0464-6
27. Li Y, Zhang J. Expression of S100A11 is a prognostic factor for disease-free survival and overall survival in patients with high-grade serous ovarian cancer. Appl Immunohistochem Mol Morphol. 2017;25(2):110–116. doi:10.1097/PAI.0000000000000275
28. Li X, Kong X, Huo Q, et al. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci. 2011;102(6):1151–1157. doi:10.1111/j.1349-7006.2011.01919.x
29. Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796(2):75–90. doi:10.1016/j.bbcan.2009.03.002
30. He H, Xi H. Wnt/β-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20(2):119–125. doi:10.1016/j.ceb.2008.01.009
31. Sack U, Walther W, Scudiero D, et al. S100A4-induced cell motility and metastasis is restricted by the Wnt/beta-catenin pathway inhibitor calcimycin in colon cancer cells. Mol Biol Cell. 2011;22(18):3344–3354. doi:10.1091/mbc.E10-09-0739
32. Duan L, Wu R, Ye L, et al. S100A8 and S100A9 are associated with colorectal carcinoma progression and contribute to colorectal carcinoma cell survival and migration via Wnt/beta-catenin pathway. PLoS One. 2013;8(4):e62092. doi:10.1371/journal.pone.0062092
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.