Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
RUNX2 Reverses p53-Induced Chemotherapy Resistance in Gastric Cancer
Authors Huang Y, Liang L, Zhao YX, Yao BH, Zhang RM, Song L, Zhang ZT
Received 21 October 2022
Accepted for publication 25 February 2023
Published 27 March 2023 Volume 2023:16 Pages 253—261
DOI https://doi.org/10.2147/PGPM.S394393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Yuan Huang,1 Lu Liang,2 Yong-Xiang Zhao,3 Bi-Hui Yao,2 Rui-Min Zhang,3 Lei Song,2 Zhong-Tao Zhang1
1Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, People’s Republic of China; 2Department of General Surgery, Baotou Central Hospital, Baotou, 014000, People’s Republic of Chin; 3Department of Pediatrics and Urology Surgery, Baotou No.4 Hospital, Baotou, 014000, People’s Republic of China
Correspondence: Zhong-Tao Zhang, Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, No. 95 Yongan Road, Xicheng District, Beijing, 100050, People’s Republic of China, Tel +8613801060364, Email [email protected]
Objective: Gastric cancer is one of the most common malignancies worldwide; however, its overall mortality has not improved significantly over the last decade. Chemoresistance plays a critical role in this issue. This study aimed to clarify the role and mechanism of runt-related transcription factor 2 (RUNX2) in platinum-based chemotherapy resistance.
Methods: First, a drug-resistant model of gastric cancer cells was established to evaluate the relative expression level of the RUNX2 as a potential biomarker of chemotherapy resistance. Next, exogenous silencing was conducted to study whether RUNX2 could reverse drug resistance and understand the underlying mechanisms. Simultaneously, the correlation between the clinical outcomes of 40 patients after chemotherapy and the RUNX2 expression levels in tumor samples was analyzed.
Results: We discovered that RUNX2 was significantly expressed in drug-resistant gastric cancer cells and tissues; it was also reversibly resistant to transformation treatment by exogenous RUNX2 silencing. It is confirmed that RUNX2 negatively regulates the apoptosis pathway of the p53 to reduce the chemotherapeutic effects of gastric cancer.
Conclusion: RUNX2 is a possible target for platinum-based chemotherapy resistance.
Keywords: gastric cancer, RUNX2, platinum, chemotherapy resistance
Introduction
Gastric cancer is one of the most common malignancies worldwide, with the third-highest global morbidity and mortality.1,2 Although continuous advancement has been achieved in diagnosis and treatment technologies, both the number of newly diagnosed patients and the overall mortality rate have risen over the past decade. The cure rate of early-stage gastric cancer is reported to be as high as 90%,3 however, most patients are diagnosed with advanced gastric cancer when the disease is confirmed.4
Chemotherapy is one of the critical treatment strategies for gastric cancer. However, more than 50% of patients have intrinsic or acquired drug resistance, with a 5-year survival rate of approximately 20%.5 Platinum-based or fluorouracil-based chemotherapy is the primary treatment modality for advanced gastric cancer. Platinum resistance can be modulated by multiple factors, including the reduction of drug influx, the improvement of drug flux or metabolism, the enhancement of DNA repair, the activation of pro-survival signaling pathways, and the inhibition of pro-apoptotic signaling pathways.6 The molecular mechanisms underlying these phenomena remain to be investigated to develop novel treatment strategies for gastric cancer.
Runt-related transcription factor 2 (RUNX2) (also known as Osf2/Cbfa1, AML-3, or Pebp2ÁA) is a member of the RUNX family,7 which is one of the core determinants of osteoblast differentiation and osteogenesis. It was reported that RUNX2-deficient mice died shortly after birth due to a complete lack of osteogenesis and osteoblast differentiation.8,9
It is worth noting that the overexpression of RUNX2 causes chemotherapy insensitivity in some types of cancer cells. For instance, the RUNX2 gene is occasionally amplified and overexpressed in osteosarcoma, acting as a reliable indicator for predicting osteosarcoma chemotherapy resistance.10,11 Roos et al found that the lack of RUNX2 in osteosarcoma cells increased the adverse drug reaction (ADR) sensitivity of cells.12 However, a correlation between high levels of RUNX2 in gastric cancer and chemotherapy prognosis has not been reported to date.
Therefore, the authors of the present study constructed a gastric cancer platinum-based chemotherapy-resistant cell model and confirmed its drug resistance using flow cytometry and the cell counting kit-8 (CCK-8) method. The increased expression of RUNX2 in drug-resistant cells was measured via quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot (WB). Simultaneously, immunohistochemistry confirmed that RUNX2 was highly expressed in patients with chemotherapy resistance. Flow cytometry and WB detection were performed after further knocking down RUNX2 in drug-resistant cells, with the results demonstrating that high RUNX2 expression was negatively correlated with platinum-mediated apoptosis.
Finally, it was stated that RUNX2 inhibits the p53 tumor protein and hinders the downstream apoptotic process from enhancing platinum-based chemotherapy resistance. Therefore, the RUNX2/ p53 axis is a prognostic indicator of chemotherapy for human gastric cancer as well as a potential therapeutic target.
Materials and Methods
Tissue Samples
A total of 40 patients admitted to Beijing Friendship Hospital were enrolled in the study. The patients were pathologically confirmed with primary gastric cancer (stage II or III) and underwent radical D2 resection and postoperative platinum-based chemotherapy using oxaliplatin and capecitabine between 2013 and 2016. All patients were followed up after operation. The baseline data of their clinical characteristics are presented in Table 1. The tissue specimens of each patient were stored in liquid nitrogen until further analysis. The recurrence within 5 years after surgery was defined as the recurrence group. 15 patients died in the recurrence group (n=17), no recurrence within 5 years after surgery is defined as the sensitive group, and 3 patients died in the sensitive group (n=23).
 |
Table 1 The Correlation Between RUNX2 Expression in Tumors and Clinicopathological Features of GC Patients |
Cell Culture
The MGC803 human gastric cancer cell line was purchased from the China National Experimental Cell Resource Sharing Platform (Beijing Headquarters). The MGC803/cisplatin (DDP) drug-resistant cell line was successfully established in our laboratory after 8 months of using a gradient drug induction method. Cells were cultured in Dulbecco’s modified minimal essential medium (Gibco, USA) supplemented with 10% fetal bovine serum (Livning, China) and incubated at 37°C. A 1% mixture of penicillin and streptomycin (Livning, China) was added to the cell culture media of the MGC803 and MGC803/DDP cell lines, respectively. Additionally, to maintain their resistant phenotype, 0.5-µg/mL DDP (Selleck, USA) was added to the culture medium of MGC803/DDP.
Cell Counting Kit-8 Assay
The MGC803 and MGC803/DDP cells were seeded into a 96-well plate (3 × 103 cells/well) in triplicate for each group before being incubated for 24 h. The DDP cell line was freshly prepared before the experiment. The cells were subjected to chemotherapy at a series of concentration gradients for 24 h. Subsequently, the CCK-8 (Dojindo, Japan) solution was added to each well. Then, the cells were incubated for 1h. Next, the cells were processed on a 549-nm microplate reader (A549) to determine the absorbance of the samples. The half-maximal inhibitory concentration (IC50) of the two groups of cells was estimated from the relative survival curve.
Cell Transfection
The MGC803/DDP cells were transfected with RUNX2 small inhibitory RNA (siRNA) (#sc-37145 -SH; SANTA, USA) or the corresponding control siRNA (#sc-37007; SANTA, USA). The cells were added to 6-well plates (3 × 103 cells/well). When the cells reached 70–80% confluence, Lipofectamine 3000 (Invitrogen USA) was utilized for transfection into cells. The transfected cells were collected 24 h after transfection and harvested for the function experiments.
Reverse Transcription and Quantitative Real-Time PCR
The RNA was extracted using the reagent TRIzol® (Invitrogen), and a total of 1 µg of RNA was reverse transcribed into complementary DNA (cDNA). To detect the expression of RUNX2, the authors purchased the primers from RiboBio China. In addition, the expression level of RUNX2 was detected using an iScript™ cDNA synthesis kit (#1708890; BIO-RAD, USA) and PowerUp™ SYBR™ Green Master Mix (#A25742; Thermo Fisher Scientific, USA). All primers used are listed in Table 2.
 |
Table 2 Primers Sequences Used for Quantitative Real-Time PCR |
Flow Cytometry Analysis
After 24 h, the cells were treated with chemotherapeutic medication (DDP). Subsequently, the cells were collected and processed for apoptosis measurement using an annexin V–fluorescein isothiocyanate (V-FITC)/propidium iodide (PI) apoptosis detection kit (Pujing Kangli China). The cells were washed twice with chilled phosphate-buffered saline and trypsinized. A 500-μL binding buffer was added to each well, according to the manufacturer’s instructions, followed by 5-μL annexin V-FITC and 5-µL PI. The samples were incubated for 30 min at 4°C in the dark and placed on ice for counting; then, the cells were measured using flow cytometry. A data analysis was performed using ModFit (BD FACSDiva™ 7.0) software.
Western Blot
The cells were collected and homogenized in a ristocetin-induced platelet aggregation lysis buffer mixed with protease inhibitors (Beyotime China). The protein samples were segregated in 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred to a polyvinylidene fluoride membrane, which was incubated separately with a series of primary antibodies against the primary antibody (1:1000, sc-126, SANTA, USA), Bcl-2-associated X protein (BAX) (1:1000, ab32503, Abcam Britain), RUNX2 (1:1000, sc-101145, SANTA, USA), and glyceraldehyde-3-phosphate dehydrogenase (1:1000, G9545, Sigma–Aldrich USA). Then, it was probed for incubation with the secondary antibody (1:3000, Sigma–Aldrich). The chemiluminescent signal of the bands was detected using elc Luminescent Solution (Applygen Gene Biotechnology China).
Immunohistochemistry
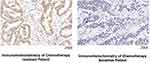
Slides of the tumors were deparaffinized, blocked, and incubated at 4°C overnight with primary antibodies, followed by secondary antibodies at 24 ° C for 1 h. RUNX2 (sc-390715, Santa Cruz USA) antibodies were used. The evaluation criteria for immunohistochemical staining were as follows (Figure 1): Patients with a RUNX2 expression level of >80% were assigned to a high expression group, while those with a RUNX2 expression level of <80% were assigned to a low expression group. The expression level is mainly based on two aspects: the amount of expression and the intensity of staining. The staining intensity is determined by using the CaseViewer 2.4 scanning software to select the target area of the tissue for 400 times imaging. After imaging, use the Image Pro Plus 6.0 analysis software to calculate the average optical density (AOD) value for evaluation.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7.0 and SPSS 26.0 software. The data were presented as mean ± SEM, and a value of P < 0.05 was considered statistically significant. The general data of patients (Table 1) were analyzed by paired t-test and chi square test, and the survival analysis of patients (Table 3) was analyzed by chi square test and logrank regression model.
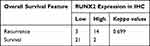 |
Table 3 RUNX2 Expression in IHC and Overall Survival Features of GC Patients |
Results
Establishment of the Cisplatin-Resistant Cell Line (MGC803/DDP)
The CCK-8 method was employed to measure the MGC803 and MGC803/DDP cell lines. The results revealed that the IC50 value of MGC803/DDP was significantly higher (Figure 2a) than that of MGC803. These two cell lines were examined using a flow cytometer, which revealed that the MGC803/DDP cell line had a considerably higher drug resistance (Figures 2b and c) than MGC803.
Elevated Expression of RUNX2 in MGC803/DDP Cells and Tissues
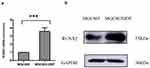
A qRT-PCR was performed to detect the RUNX2 expression level in the MGC803 and MGC803/DDP cells. It was found that the mRNA level of RUNX2 was significantly elevated in the MGC803/DDP cells (Figure 3a). Furthermore, WB detected a high protein level of RUNX2 in the MGC803/DDP cells (Figure 3b). Finally, the results from the immunohistochemical staining of RUNX2 (Figure 1) and the clinical efficiency of chemotherapy were combined to analyze correlation (Figure 4).
Inhibition of RUNX2 in the Drug-Induced P53/BAX Apoptosis Pathway
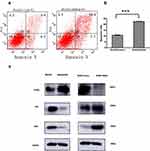
To conduct further experiments, the RUNX2 gene was knocked out in the MGC803/DDP cells. It was found that the drug resistance was significantly reduced after the gene’s knockout (Figures 5a and b), indicating that RUNX2 directly affected drug resistance. The following investigations were performed to confirm the role of RUNX2 inhibition on p53 in initiating and activating the apoptotic process downstream to increase resistance to platinum-based chemotherapy. The WB results revealed a low expression of p53 in the MGC803/DDP cells, which exhibited an enhanced expression of P53 and Bax after transfection with RUNX2-siRNA (Figure 5c), suggesting that RUNX2 could impede the sensitivity of MGC803/DDP cells to drug-induced apoptosis.
Discussion
Multiple studies have reported the high expression of RUNX2 in human osteosarcoma. For example, Bekim Sadikovic et al11 found that among patients with osteosarcoma who received chemotherapy (all received standard treatment regimens for osteosarcoma, including DDP, doxorubicin, and methotrexate), a significantly elevated level of RUNX2 was observed in a subgroup of patients with acquired drug resistance. This discovery suggested that RUNX2 could be used as a biomarker to predict chemotherapy failure in patients with osteosarcoma. However, it was unclear whether it was platinum resistance or the underlying drug-resistance pathways.
Taisuke Ozaki et al revealed for the first time that the loss of RUNX2 in osteosarcoma cells significantly intensified their doxorubicin ADR sensitivity.13,14 They found RUNX2 inhibits DNA damage-induced transcriptional as well as pro-apoptotic activity of p53 through the functional collaboration with HDAC6.Later, it was demonstrated that Silencing of RUNX2 enhances gemcitabine sensitivity of p53-deficient human pancreatic cancer AsPC-1 cells.15,16 The above studies have fully demonstrated that RUNX2 plays an important role in chemotherapy resistance. However, whether RUNX2 can mediate platinum chemotherapy for gastric cancer has not been clarified. We found for the first time that RUNX2 was significantly elevated in both transcription and translation levels by comparing MGC803/DDP cells with their parental MGC803 cells.
Simultaneously, the expression level of RUNX2 in tissue specimens from patients with gastric cancer was also measured. Surprisingly, the RUNX2 level was significantly higher in the samples from the chemotherapy resistance group, which further indicated that RUNX2 was correlated with drug resistance changes in gastric cancer.
Furthermore, the RUNX2 gene in MGC803/DDP cells was knocked out, and flow cytometry measurements were performed. It was discovered that the drug resistance of RUNX2-knockout cells decreased, confirming the dominant role of RUNX2 in the mechanism of drug resistance.
The p53 level is an intranuclear sequence-specific transcription factor that contains an N-terminal transactivation domain, a central sequence-specific DNA binding domain, and a C-terminal oligo domain.17–19 Under normal circumstances, the p53 level remains very low. However, under cellular stress, including DNA damage and oncogene activation, p53 stabilizes at the protein level via post-translational modifications. Sequentially, p53 can trigger a series of molecular events that determine cellular fate, including cell cycle arrest, cell senescence, and/or cell death.20 Therefore, p53 is a determining factor in cell survival and death, with the outcome possibly dependent on the intensity of stress signals and/or the degree of cell damage.
Several studies have reported the p53 status in cancer cells to be related closely to the sensitivity of malignant cells to anti-cancer drugs.21–24 Consequently, it was interesting to investigate whether the knockout of RUNX2 could activate the so-called “gene guardian” p53-dependent pathway.25 It is also worth noting the type of P53, As we know, Wild-type p53 induces tumor cell cycle arrest and apoptosis, while Mutant P53 lost this function and may have the opposite effect. However, there is a genetic feature of Mutant P53 in MGC-803 gastric cancer cells.26,27 Because MGC803DDP gastric cancer cells could not determine the mutation type of p53, we chose total p53 for pathway verification.
This study explored whether RUNX2 in gastric cancer cells could regulate the p53-mediated apoptosis pathway to inhibit Bax, the p53-upregulated modulator of apoptosis, and phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1) from participating in the apoptosis process, thereby leading to chemotherapy resistance and the negative regulation of the p53/Bax-mediated apoptosis pathway. For this reason, WB was used to measure the expression levels of p53 and Bax in MGC803 and MGC803/DDP cells. The gene knockout of RUNX2 in MGC803/DDP cells reactivated the p53/Bax -mediated apoptosis pathway, which ultimately reversed the drug resistance of the MGC803/DDP cells.
The current findings indicate first, that RUNX2 expression is elevated in gastric cancer cells and tissues resistant to platinum-based chemotherapy and second, that RUNX2 can decrease the sensitivity of gastric cancer cells to platinum-based chemotherapy by impeding cell apoptosis.
This study’s results provide a fresh understanding of the mechanisms involved in p53-mediated drug resistance and may provide the basis for a novel treatment strategy for gastric cancer. The authors believe that patients with gastric cancer and high RUNX2 levels may not benefit from postoperative platinum-based chemotherapy. Conversely, patients at low risk should be advised to undergo platinum-based chemotherapy.
This study’s potential limitations must be considered. For example, it was a small-scale study that used patients from a single center as subjects. Additionally, the drug-resistant cell line was established using DDP, while the chemotherapeutic drug received by patients in this study was oxaliplatin. Finally, the specific isotype of p53 has not been determined. Therefore, the above limitations form the direction of future research and improvement.
Abbreviations
RUNX2, Runt-related transcription factor 2; CCK-8, Cell Counting Kit-8; qRT-PCR, quantitative real-time polymerase chain reaction; WB, Western blot; DDP, Cisplatin; RIPA, Radio Immunoprecipitation Assay; ADR, Adriamycin; V–fluorescein isothiocyanate, (V-FITC); propidium iodide, PI; PMAIP1, phorbol-12-myristate-13-acetate-induced protein 1; IHC, immunohistochemistry.
Acknowledgments
We appreciate all the laboratory members for their valuable suggestions and kind help.
Ethics Approval
This study was approved by the Ethics Committee of Baotou Central Hospital (Date18-Sep-2021/No KYLL2021 005). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants signed written informed consent.
Funding
This work was supported by Baotou Municipal Health Commission Science and Technology Project [Grant no. wsjkwkj032].
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. PMID: 25651787. doi: 10.3322/caac.21262
2. Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20(16):4483–4490. PMID: 24782601; PMCID: PMC4000485. doi:10.3748/wjg.v20.i16.4483
3. Wang J, Yu JC, Kang WM, Ma ZQ. Treatment strategy for early gastric cancer. Surg Oncol. 2012; (2):119–123. PMID: 21256735. doi:10.1016/j.suronc.2010.12.004
4. Mirzaei H, Khataminfar S, Mohammadparast S, et al. Circulating microRNAs as potential diagnostic biomarkers and therapeutic targets in gastric cancer: current status and future perspectives. Curr Med Chem. 2016;23(36):4135–4150. PMID: 27538692. doi: 10.2174/0929867323666160818093854
5. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. PMID: 16682732. doi:10.1200/JCO.2005.05.2308
6. Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. PMID: 21892204. doi:10.1038/onc.2011.384
7. Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. PMID: 19575648. doi:10.1146/annurev.cellbio.042308.113308
8. Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. PMID: 9182763. doi:10.1016/s0092-8674(00)80258-5
9. Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–771. PMID: 9182764. doi:10.1016/s0092-8674(00)80259-7
10. Man TK, Lu XY, Jaeweon K, et al. Genome-wide array comparative genomic hybridization analysis reveals distinct amplifications in osteosarcoma. BMC Cancer. 2004;4:45. PMID: 15298715; PMCID: PMC514550. doi:10.1186/1471-2407-4-45
11. Sadikovic B, Thorner P, Chilton-Macneill S, et al. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer. 2010;10:202. PMID: 20465837; PMCID: PMC2875220. doi:10.1186/1471-2407-10-202
12. Roos A, Satterfield L, Zhao S, et al. Loss of Runx2 sensitises osteosarcoma to chemotherapy-induced apoptosis. Br J Cancer. 2015;113(9):1289–1297. PMID: 26528706; PMCID: PMC4815801. doi:10.1038/bjc.2015.305
13. Ozaki T, Wu D, Sugimoto H, Nagase H, Nakagawara A. Runt-related transcription factor 2 (RUNX2) inhibits p53-dependent apoptosis through the collaboration with HDAC6 in response to DNA damage. Cell Death Dis. 2013;4(4):e610. PMID: 23618908; PMCID: PMC3641350. doi:10.1038/cddis.2013.127
14. Ozaki T, Sugimoto H, Nakamura M, et al. Runt-related transcription factor 2 attenuates the transcriptional activity as well as DNA damage-mediated induction of pro-apoptotic TAp73 to regulate chemosensitivity. FEBS J. 2015;282(1):114–128. PMID: 25331851; PMCID: PMC4368372. doi:10.1111/febs.13108
15. Sugimoto H, Nakamura M, Yoda H, et al. Silencing of RUNX2 enhances gemcitabine sensitivity of p53-deficient human pancreatic cancer AsPC-1 cells through the stimulation of TAp63-mediated cell death. Cell Death Discov. 2015;1:15010. PMID: 27551445; PMCID: PMC4981025. doi:10.1038/cddiscovery.2015.10
16. Nakamura M, Sugimoto H, Ogata T, et al. Improvement of gemcitabine sensitivity of p53-mutated pancreatic cancer MiaPaCa-2 cells by RUNX2 depletion-mediated augmentation of TAp73-dependent cell death. Oncogenesis. 2016;5(6):e233. PMID: 27294865; PMCID: PMC4945741. doi:10.1038/oncsis.2016.40
17. Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007; (9):1306–1316. PMID: 17322916. doi:10.1038/sj.onc.1210263
18. Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137(4):609–622. PMID: 19450511; PMCID: PMC3737742. doi:10.1016/j.cell.2009.04.050
19. Meek DW. Regulation of the p53 response and its relationship to cancer. Biochem J. 2015;469(3):325–346. PMID: 26205489. doi:10.1042/BJ20150517
20. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. PMID: 11099028. doi:10.1038/35042675
21. Fan S, el-Deiry WS, Bae I, et al. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994;54(22):5824–5830. PMID: 7954409.
22. Lai SL, Perng RP, Hwang J. p53 gene status modulates the chemosensitivity of non-small cell lung cancer cells. J Biomed Sci. 2000;7(1):64–70. PMID: 10644891. doi:10.1007/BF02255920
23. Huang Y, Sadée W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006;239(2):168–182. PMID: 16169662. doi:10.1016/j.canlet.2005.07.032
24. Xiao SX, Li SJ, Fang WX, Chen J, Li HJ, Situ YL. Exploring the mechanism of Tripterygium wilfordii against cancer using network pharmacology and molecular docking. World J Tradit Chin Med. 2022;8:417–425.
25. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358(6381):15–16. PMID: 1614522. doi:10.1038/358015a0
26. Zhang GH, Xue WB, An YF, et al. Distinct novel quinazolinone exhibits selective inhibition in MGC-803 cancer cells by dictating mutant p53 function. Eur J Med Chem. 2015;95:377–387. PMID: 25828929. doi:10.1016/j.ejmech.2015.03.053
27. Wei XW, Yuan JM, Huang WY, et al. 2-Styryl-4-aminoquinazoline derivatives as potent DNA-cleavage, p53-activation and in vivo effective anticancer agents. Eur J Med Chem. 2020;186:111851. PMID: 31761381. doi:10.1016/j.ejmech.2019.111851
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.