Back to Journals » Infection and Drug Resistance » Volume 14
rpoB Mutations and Effects on Rifampin Resistance in Mycobacterium tuberculosis
Authors Li M, Lu J , Lu Y, Xiao T , Liu H , Lin S , Xu D, Li G, Zhao X, Liu Z, Zhao L, Wan K
Received 9 August 2021
Accepted for publication 21 September 2021
Published 5 October 2021 Volume 2021:14 Pages 4119—4128
DOI https://doi.org/10.2147/IDR.S333433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Ma-chao Li,1,* Jie Lu,2,* Yao Lu,1,3,* Tong-yang Xiao,4,* Hai-can Liu,1 Shi-qiang Lin,5 Da Xu,1 Gui-lian Li,1 Xiu-qin Zhao,1 Zhi-guang Liu,1 Li-li Zhao,1 Kang-lin Wan1
1State Key Laboratory of Infectious Disease Prevention and Control, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, People’s Republic of China; 2Beijing Key Laboratory for Pediatric Diseases of Otolaryngology, Head and Neck Surgery, MOE Key Laboratory of Major Diseases in Children, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 3School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, People’s Republic of China; 4Guangdong Key Laboratory for Diagnosis & Treatment of Emerging Infectious Diseases, Shenzhen Third People’s Hospital, Southern University of Science and Technology, Shenzhen, People’s Republic of China; 5Department of Bioinformatics, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li-li Zhao; Kang-lin Wan
National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, P.O. Box 5, Changping, Beijing, 102206, People’s Republic of China
Tel/Fax +86 10 58900779
Email [email protected]; [email protected]
Objective: To investigate the mutations within the whole rpoB gene of Mycobacterium tuberculosis and analyze their effects on rifampin (RIF) resistance based on crystal structure.
Methods: We sequenced the entire rpoB gene in 175 tuberculosis isolates and quantified their minimum inhibitory concentrations using microplate-based assays. Additionally, the structural interactions between wild-type/mutant RpoB and RIF were also analyzed.
Results: Results revealed that a total of 34 mutations distributed across 17 different sites within the whole rpoB gene were identified. Of the 34 mutations, 25 could alter the structural interaction between RpoB and RIF and contribute to RIF resistance. Statistical analysis showed that S450L, H445D, H445Y and H445R mutations were associated with high-level RIF resistance, while D435V was associated with moderate-level RIF resistance.
Conclusion: Some mutations within the rpoB gene could affect the interaction between RpoB and RIF and thus are associated with RIF resistance. These findings could be helpful to design new antibiotics and develop novel diagnostic tools for drug resistance in TB.
Keywords: Mycobacterium tuberculosis, rifampin resistance, structure, mutation
Introduction
Although the incidence and mortality rates of tuberculosis (TB) are slowly declining, TB is still a major global public health threat with reports of ~10 million new cases and 1.2 million deaths annually.1 In recent years, there has been an alarming increase in drug-resistant TB, which poses a serious challenge for TB control. Rapid and accurate detection of drug resistance in TB patients is essential for the successful control of the disease.
Rifampin (RIF) is one of the most important anti-TB drugs. Its highly effective bactericidal activity against Mycobacterium tuberculosis (M. tuberculosis) has made it a principal drug in TB treatment. RIF targets the DNA-dependent RNA polymerase β-subunit, encoded by the rpoB gene.2,3 Mutated rpoB brings about a conformational change relevant to the binding affinity of RIF at β-subunit of the RNA polymerase (RNAP) and consequently the drug became inactive without proper binding to the target site.2,3 Approximately 90–95% of RIF-resistant isolates have been shown to harbor mutations within the rpoB gene,4–6 and RIF resistance is largely associated with mutations in an 81-bp fragment of the rpoB gene,6–9 which located between rpoB codons 426 and 452 in M. tuberculosis.7,9–11 However, the mechanism of resistance concerning the rest 5% RIF-resistant isolates is still unknown, indicating there might be other mechanisms, such as lowered cell wall permeability12 or enhanced efflux pump.13,14
Prior reports suggest that particular mutations within rpoB RRDR are more likely to confer higher levels of RIF resistance.5,15,16 Intriguingly, several rpoB mutations were also observed in RIF-susceptible phenotypes.5,17,18 These contradictory results suggest that the association between RIF resistance and rpoB mutations should be explored in more detail for understanding the RIF resistance mechanism. Furthermore, the information on RIF resistance levels in M. tuberculosis strains without rpoB mutations is limited. In the present study, we systematically performed quantitative RIF resistance phenotyping with MIC measurements for 175 M. tuberculosis clinical isolates, and the mutations in the entire rpoB gene and their effects on RIF resistance were also analyzed.
Materials and Methods
M. tuberculosis Isolates
A total of 175 stored M. tuberculosis clinical isolates (collected from 2005 to 2012) were included in this study. These isolates were obtained from 175 epidemiologically unrelated adult patients with pulmonary tuberculosis in China, of which 48.6% (85/175) were newly diagnosed cases. All isolates were cultured on Lowenstein-Jensen (L-J) medium and freshly subcultured before being used for MIC testing.
MIC Testing
MICs were determined by in vitro 96-well microplate-based assay. Briefly, a 100 µL volume of Middlebrook 7H9 broth was dispensed in each well of the plate. The drug concentrations tested for RIF were 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64 and 128. The inoculum was prepared from fresh L-J medium in 7H9 broth, then adjusted to a McFarland no. 1 turbidity standard and diluted 1:50. A 100 µL volume was used to inoculate each well of the plate. Furthermore, drug-free (inoculum-only) controls were used to determine the time to read results. Plates were sealed and incubated at 37°C for approximately 7–10 days. After incubation, results were read using a Sensititre VIZION® system (Trek Diagnostic Systems, East Grinstead, WS, UK) Growth was evident as turbidity or as a deposit of cells at the bottom of the well. The MIC was recorded as the lowest concentration of antibiotics that inhibited visible growth. Control wells were evaluated first, and results were considered invalid when growth was not observed. The critical concentration was taken as 1 µg/mL for RIF.19,20 H37Rv was used as a control with each batch of microplate-based assays.
DNA Extraction, Amplification and Sequencing
Genomic DNAs were extracted using the cetyltrimethylammonium bromide (CTAB) method21 and stored at −20°C for further use.
The entire rpoB gene was amplified using primers: rpoB-F1 (5ʹ-GCGGCTCAGCGGTTTAGTTG-3ʹ), rpoB-R1 (5ʹ-ACAGCGGGTTGTTCTGGTCC-3ʹ), rpoB-F2 (5ʹ-GACGACATCGACCACTTC-3ʹ), rpoB-R2 (5ʹ-GATCACCTTGCCGGATTC-3ʹ), rpoB-F3 (5ʹ-CACATCGAGGAGCATGA-3ʹ), and rpoB-R3 (5ʹ-CTCGCCATAGGACCATTG-3ʹ). The cycling conditions were 94°C for 10 min, followed by 35 cycles of 94°C for 1 min, 57°C for 40 sec, and 72°C for 50 sec, and a final extension at 72°C for 10 min. All amplicons were purified, dried, and loaded onto an ABI 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA). The sequences generated were aligned to the H37Rv reference genome (GenBank accession number NC_000962) using BioEdit v7.05.3 (Tom A. Hall, Department of Microbiology, North Carolina State University, North Carolina, USA). All nucleotide sequences of rpoB presented in the study were deposited in the NCBI SRA BioProject under the accession number: PRJNA722811.
Quality Control
Mixed peaks within a chromatogram and novel mutations were treated as true if they were reproducible. Furthermore, repeat testing was also performed on any isolate with a discrepancy between phenotypic susceptibility results and genotypic data.
Structure Modelling and Analysis
The complex structure of each RpoB mutant with RIF was modelled by SWISS-MODEL (https://swissmodel.expasy.org/) using Protein Data Bank (PDB) (http://www.rcsb.org/pdb/) file 5UHB as template.22 Structure analysis of protein-ligand complex was carried out by Discovery Studio Visualizer v.4.5 software (BIOVIA, Dassault Systèmes, San Diego, CA, USA). The interactions between wild-type/mutant RpoB and RIF were analyzed using the “Structure Monitor” and the ‘Receptor-Ligand Interactions’ modules.
Data Analysis
The association of the rpoB mutation with the MICs was assessed using a regression multivariate model. P value less than 0.01 was considered to be statistically significant.23,24 All data were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Mutations Within rpoB
Among the 175 tested isolates, 125 (71.4%) were resistant to RIF, and 50 (28.6%) were susceptible to RIF (Table 1). In order to facilitate interpretation, MIC ranges were classified into three categories according to RIF resistance: low group (MIC 1–8 µg/mL), moderate group (MIC 16–64 µg/mL) and high group (MIC ≥128 µg/mL). Among 125 RIF-resistant isolates, 19 (15.2%), 18 (14.4%) and 88 (70.4%) had low, moderate and high levels of RIF resistance, respectively.
 |
Table 1 Distribution of rpoB Mutations and MICs of Rifampin |
DNA sequencing showed that 118 isolates, including one susceptible isolate, 13 low-level resistant isolates, 18 moderate-level resistant isolates and 86 high-level resistant isolates, carried at least one non-synonymous mutation within the sequenced rpoB gene (Table 1). Among them, 96 isolates (81.4%) had a single mutation, while 22 isolates (18.6%) had two mutations. For all mutations, 34 genotype patterns distributed across 17 different sites were detected, including 25 polymorphisms in the RRDR and nine polymorphisms outside the RRDR.
The most prevalent mutation was observed at codons 450, 445 and 435, which had mutated frequencies of 47.5% (56/118 isolates), 28.0% (33/118 isolates) and 16.1% (19/118 isolates), respectively (Table 1). Ser450 was replaced by Leu (54 isolates), Phe (1 isolate) and Gly (1 isolate); His445 was replaced by Asp (11 isolates), Tyr (9 isolates), Arg (4 isolates), Asn (4 isolates), Leu (2 isolates), Cys (1 isolate), Gln (1 isolate) and Pro (1 isolate); Asp435 was replaced by Val (10 isolates), Gly (5 isolates), Tyr (3 isolates) and Glu (1 isolate). Other mutations were also observed at codons 45, 170, 400, 429, 430, 431, 432, 441, 446, 452, 488, 491, 759 and 1056, which had a total mutated frequency of only 27.1% (32/118 isolates). Notably, of these 32 isolates, 21 (65.6%, 21/32 isolates) were combined with additional mutations at 435, 445 and 450. Moreover, two novel mutations (G759S and Q1056K) were identified in rpoB (Table 1).
Association of rpoB Mutations with RIF Resistance
As 18.6% of isolates with rpoB mutations harbored more than one mutation, the association between mutations and drug resistance was estimated by multivariate regression (Table 2). In the multivariate model, the rpoB mutations S450L, H445D, H445Y, and H445R were significantly associated with RIF resistance (P<0.01), and isolates with these four mutations had a median MIC belonging to the high MIC category (128 µg/mL). Mutation D435V was also significantly correlated with RIF resistance (P<0.01) according to multivariate analysis. Notably, D435V mutant had a median MIC of 64 µg/mL, which was within the range of moderate MIC category.
 |
Table 2 Logistic Regression Multivariate Model Results Between rpoB Mutations and RIF Resistance |
Besides S450L, H445D, H445Y, H445R and D435V, there were some other mutations that were not identified by our multivariate analysis possibly due to small sample sizes. These mutations might also confer RIF resistance since our data showed that some isolates did not have the above-mentioned five mutations but were resistant to RIF. As shown in Tables 1 and 2, isolates within the moderate and high MIC category, harbored single mutation V170F, L430P, S441L, H445C, H445L, and Q432K, Q432L, Q432P, and S450F, respectively, as well as double mutations V170F/H445N, L430P/S431G, L430R/D435G, L430P/D435G, D435G/L452P, D435G/I491N, and L430P/H445N, D435E/S441L, H445N/L452P, H445P/K446Q, respectively. It was notable that one RIF-susceptible isolate (MIC=0.5 µg/mL) harbored single D435Y mutation. Another isolate that harbored the single D435Y mutation had a MIC of 1 µg/mL. Finally, six low-level RIF-resistant isolates and two high-level RIF-resistant isolates harbored no mutation in the whole rpoB gene.
Changes of Intermolecular Interactions Between RpoB Mutants and RIF
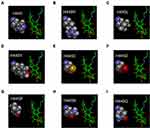
The effects of mutations on the interactions between RpoB mutants and RIF were explored using the crystal structure of RIF-RNAP complex. Figure 1A shows the detailed intermolecular interactions between wild-type RpoB and RIF. Overall, 17 residues of RpoB had mutations possible for RIF resistance, and the receptor–ligand interaction changes of mutations are summarized in Table 3.
 |
Table 3 Summary for Interaction Changes of Mutations Based on Structural Analysis |
Seven residues with mutations in this study do not directly interact with RIF in wild-type RpoB-RIF complex. Six residues, namely P45, T400, K446, I488, G759 and Q1056 are not involved in RIF binding, and the mutations of these residues do not have the interaction with RIF (Table 3). The other residue, S441, does not have any direct interaction with RIF in wild-type RpoB-RIF complex, but S441L mutation introduces steric hindrance between L441 and H445, leading to the loss of hydrogen bond between H445 and RIF (Figure 1B). Notably, the double mutant of D435E/S441L not only loses the hydrogen bond between H445 and RIF but also introduces steric hindrance between E435 and RIF (Figure 1C).
The other 10 residues directly interact with RIF, namely V170, Q429, L430, S431, Q432, D435, H445, S450, L452 and I491 (Figure 1A). Residues Q432, H445 and S450 have intermolecular hydrogen bonds with RIF. Residues L430, L452 and I491 participate in hydrophobic interactions between RpoB and RIF. Residues V170, Q429, D435 and S431 involve in van der Waals interactions.
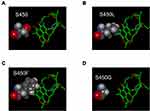
Structural studies reveal that intermolecular hydrogen bonds are essential for the binding of RIF,2,25 and H445 and S450 are the crucial residues for the intermolecular hydrogen bonds. As shown in Figure 2, the eight mutations of H445 all lead to loss of hydrogen bond, and H445Y mutation additionally introduces steric hindrance; thus, all the mutations of H445 contribute to RIF resistance in TB. For residue 450, S450G only loses the intermolecular hydrogen bond, while S450L and S450F result in both loss of hydrogen bond and introduction of steric hindrance (Figure 3). Moreover, Q432 also participates in the formation of intermolecular hydrogen bonds, and Q432P and Q432L lead to loss of hydrogen bond and confer RIF resistance in TB. Mutations of hydrophobic residues L430, L452 and I491 result in loss of hydrophobic interaction between RpoB and RIF, leading to RIF resistance as well. In addition, some mutations introduce steric hindrance and electrostatic repulsion in RpoB-RIF complex, which are unfavorable for RIF binding. However, mutations Q429H, S431G and D435G do not change the intermolecular interactions between wild-type RpoB and RIF (Table 3).
Discussion
Rapid and accurate detection of drug resistance in M. tuberculosis is essential for improving treatment success and decreasing the spread of disease. In recent years, several molecular diagnostics techniques, such as GeneXpert MTB/RIF and Geno Type MTBDRplus, have been developed to allow the rapid identification of M. tuberculosis drug resistance directly from specimens.26–28 However, these molecular methods can miss isolates with mutations outside the target region, and cannot determine the level of resistance. In addition, several reports suggest that some isolates with rpoB mutations are RIF-susceptible according to culture-based phenotypic drug susceptibility testing.4,5,18,29 Previous studies indicate that RIF resistance is a surrogate marker of MDR-TB,4,5 hence rapid and accurate detection of RIF resistance is important.
It is known that the β-subunit of DNA-dependent RNA polymerase encoded by rpoB is a target for rifamycin drugs, and that amino acid changes in this protein can confer rifamycin resistance.9,10,30 Our current results revealed that 93.6% (117/125 isolates) of RIF-resistant isolates carried at least one mutation in rpoB. Overall, 17 distinct codons in rpoB had mutations contributing to amino acid replacements, of which 10 codons are located in the RRDR and seven are located outside the RRDR. Interestingly, almost all mutations outside the RRDR were accompanied by mutations within the RRDR. These results indicate the potential for using DNA sequencing of RRDR to rapidly predict RIF.4,7
As evidenced in a number of studies, the most frequently mutated codons associated with RIF in the rpoB gene are codons 435, 445 and 450.4,5,16,31,32 The reported frequencies of these three mutations from world geographic regions range from 1.1% to 20.4% for codon 435, from 6.8% to 32% for codon 445 and from 31% to 80.9% for codon 450, respectively.4,6,7,33,34 In this study, the majority of mutations in RIF-resistant isolates were at codons 435, 445 and 450, with the mutation frequencies of 16.1%, 28.0% and 47.5%, respectively. The levels of RIF resistance in isolates with rpoB mutations at these three codons were observed to be dependent on the amino acid change. In multivariate analysis, S450L, H445D, H445Y and H445R were strongly associated with high-level RIF resistance; D435V was strongly associated with moderate-level RIF resistance.5,15,16,32 In contrast, isolates with mutations S450G, H445C, H445L and D435Y, belong to low-level or susceptible categories. In addition, several mutations, such as Q432K, Q432L, Q432P and S450F occurred exclusively in high-level RIF resistant isolates. For some mutations, however, the quantities of the isolates in this study are very small, which could affect the statistical power of our model. It is possible that the mutations are actually causing resistance, without being recognized by the statistical model.
RIF resistance is usually caused by mutations within RIF resistance-determining regions (RRDRs) of RpoB that are involved in the formation of the RIF-binding pocket.35,36 Accordingly, all 11 mutated sites contributing to RIF-resistance in our study, including V170, Q429, L430, S431, Q432, D435, S441, H445, S450, L452 and I491, are located in the RRDRs of RpoB,35 while four residues outside RRDRs, namely P45, T400, G759 and Q1056, are not involved in RIF binding. Though in this study, mutations P45S, T400I, G759S and Q1056K occurred in isolates belonging to high-level RIF resistant group, these mutations were accompanied by additional mutations associated with high-level RIF resistance, S450L or H445Y. In addition, mutations on codons V170 and I491 were considered to lead to RIF resistance, which necessitated investigation beyond the RRDR, especially when patient treatment effects are not as expected.
Our analysis also showed that in the RRDRs, K446 and I488, do not directly interact with RIF and then mutations of these residues do not have the interaction with RIF. In addition, mutations Q429H, S431G and D435G do not have effect on interactions between wild-type RpoB and RIF. These contradictory results probably owe to using the different crystal structure models and different algorithms.35,36 Only by combining the results of structural prediction with phenotypic data could we determine whether a mutation contributes to drug resistance. However, in this study, these five mutations occurred in RIF-resistant isolates, and these mutations were accompanied by an additional RIF resistance-associated mutation. Interestingly, single mutation, for example, Q429H, was observed in both RIF-susceptible isolates and RIF-resistant isolates.6 Thus, several intra-RRDR mutations are considered not to involve in the development of RIF resistance. Instead, they may perform as compensatory mutations to alleviate fitness impairment incurred by other mutations directly associated with drug resistance.6 Further research might be required to confirm their functions.
We also tested some commonly “disputed” mutations, such as L430P, D435Y, D435N, D435L and L452P, which have been reported to confer highly discordant RIF results by culture-based drug susceptibility testing.31,34,37–39 Crystal structure analysis indicated that these mutations could disrupt the interaction between RpoB and RIF, and then lead to RIF resistance. MIC testing results also indicated that almost all these mutations occurred in RIF-resistant isolates. It is notable that single D435Y mutation was observed in only two isolates. One was a RIF-resistant isolate with a critical concentration MIC value (1 µg/mL). Another was RIF-susceptible isolate but with a MIC of 0.5 µg/mL, which was suggested as the breakpoint for RIF in microplate-based assay to replace the existing one by a number of studies.38–41
There are a few RIF-resistant isolates without rpoB mutations reported in the prior studies.1,5,6,42 We also observed eight RIF-resistant isolates, including two high-level RIF resistance isolates and six low-level RIF resistance isolates (three isolates had a MIC of 1 µg/mL and the other three isolates had MICs of 2, 4 and 8 µg/mL, respectively), in the absence of mutations over the whole rpoB gene. Phenotypic and genotype characteristics of these eight isolates were confirmed by retesting proportion method based on L-J medium and DNA sequencing of rpoB. Whole-genome sequencing and genotype analysis of these eight isolates also showed that they derived from different origins (data not shown in this study). Resistance in these isolates might be attributed to other mechanisms, such as the upregulation of efflux pump,13,16 or the overexpression of transporter.43
However, there are limitations in this study. On the one hand, although 175 isolates represented a quite challenging panel of isolates in the studies on RIF-resistance mechanism so far, the number of isolates harboring certain types of rpoB mutations was still limited. Also, some of the RIF resistant isolates could not be explained in the present study and clarifying them will further help us to reveal RIF resistance in M. tuberculosis.
In sum, we revealed a comprehensive profiles of mutations within the whole rpoB gene and their associations with RIF resistance levels in a variety of M. tuberculosis clinical isolates. The effects of mutations on the interaction between RpoB and RIF were analyzed based on crystal structure. The information of these mutations and interactions will advance our mechanistic understanding of M. tuberculosis RIF-resistance and aid molecular testing and new drug design.
Ethical Approval
This study was approved by the Ethics Committee of National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. All patients participating in the study had written consent from themselves.
Funding
This study was supported by the projects from National Key Program of Mega Infectious Diseases (Grant No. 2018ZX10302302-001) and National Basic Research Program of China (973 Program, Grant No. 2015CB554202). The funder had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Disclosure
All authors have no competing interests.
References
1. World Health Organization. Global tuberculosis report 2020. Geneva, Switzerland: World Health Organization; 2020.
2. Campbell EA, Korzheva N, Mustaev A, et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell. 2001;104:901–912.
3. Swain SS, Sharma D, Hussain T, Pati S. Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerg Microbes Infect. 2020;9:1651–1663. doi:10.1080/22221751.2020.1785334
4. Campbell PJ, Morlock GP, Sikes RD, et al. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55:2032–2041. doi:10.1128/AAC.01550-10
5. Jamieson FB, Guthrie JL, Neemuchwala A, Lastovetska O, Melano RG, Mehaffy C. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol. 2014;52:2157–2162. doi:10.1128/JCM.00691-14
6. Jagielski T, Bakula Z, Brzostek A, et al. Characterization of mutations conferring resistance to rifampin in Mycobacterium tuberculosis clinical strains. Antimicrob Agents Chemother. 2018;62:e01093–18. doi:10.1128/AAC.01093-18
7. Zaw MT, Emran NA, Lin Z. Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis. J Infect Public Health. 2018;11:605–610. doi:10.1016/j.jiph.2018.04.005
8. Williams DL, Spring L, Collins L, et al. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:1853–1857. doi:10.1128/AAC.42.7.1853
9. Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis. 1998;79:3–29. doi:10.1054/tuld.1998.0002
10. Rothman-Denes LB. Structure of Escherichia coli RNA polymerase holoenzyme at last. Proc Natl Acad Sci U S A. 2013;110:19662–19663. doi:10.1073/pnas.1320604110
11. Andre E, Goeminne L, Cabibbe A, et al. Consensus numbering system for the rifampicin resistance-associated rpoB gene mutations in pathogenic mycobacteria. Clin Microbiol Infect. 2017;23:167–172. doi:10.1016/j.cmi.2016.09.006
12. Danilchanka O, Pires D, Anes E, Niederweis M. The Mycobacterium tuberculosis outer membrane channel protein CpnT confers susceptibility to toxic molecules. Antimicrob Agents Chemother. 2015;59:2328–2336. doi:10.1128/AAC.04222-14
13. Louw GE, Warren RM, Gey van Pittius NC, McEvoy CR, Van Helden PD, Victor TC. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother. 2009;53:3181–3189. doi:10.1128/AAC.01577-08
14. Xu G, Liu H, Jia X, Wang X, Xu P. Mechanisms and detection methods of Mycobacterium tuberculosis rifampicin resistance: the phenomenon of drug resistance is complex. Tuberculosis. 2021;128:102083. doi:10.1016/j.tube.2021.102083
15. Ohno H, Koga H, Kohno S, Tashiro T, Hara K. Relationship between rifampin MICs for and rpoB mutations of Mycobacterium tuberculosis strains isolated in Japan. Antimicrob Agents Chemother. 1996;40:1053–1056. doi:10.1128/AAC.40.4.1053
16. Pang Y, Lu J, Wang Y, Song Y, Wang S, Zhao Y. Study of the rifampin monoresistance mechanism in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2013;57:893–900. doi:10.1128/AAC.01024-12
17. Berrada ZL, Lin SY, Rodwell TC, et al. Rifabutin and rifampin resistance levels and associated rpoB mutations in clinical isolates of Mycobacterium tuberculosis complex. Diagn Microbiol Infect Dis. 2016;85:177–181. doi:10.1016/j.diagmicrobio.2016.01.019
18. Wi YM, Greenwood-Quaintance KE, Brinkman CL, Lee JYH, Howden BP, Patel R. Rifampicin resistance in Staphylococcus epidermidis: molecular characterisation and fitness cost of rpoB mutations. Int J Antimicrob Agents. 2018;51:670–677. doi:10.1016/j.ijantimicag.2017.12.019
19. World Health Organization. Guidelines for Surveillance of Drug Resistance in Tuberculosis.
20. Hall L, Jude KP, Clark SL, Wengenack NL. Antimicrobial susceptibility testing of Mycobacterium tuberculosis complex for first and second line drugs by broth dilution in a microtiter plate format. J Vis Exp. 2011. doi:10.3791/3094
21. Somerville W, Thibert L, Schwartzman K, Behr MA. Extraction of Mycobacterium tuberculosis DNA: a question of containment. J Clin Microbiol. 2005;43:2996–2997. doi:10.1128/JCM.43.6.2996-2997.2005
22. Lin W, Mandal S, Degen D, et al. Structural basis of Mycobacterium tuberculosis transcription and transcription inhibition. Mol Cell. 2017;66:169–179 e8. doi:10.1016/j.molcel.2017.03.001
23. Farhat MR, Jacobson KR, Franke MF, et al. Gyrase mutations are associated with variable levels of fluoroquinolone resistance in Mycobacterium tuberculosis. J Clin Microbiol. 2016;54:727–733. doi:10.1128/JCM.02775-15
24. Sun Q, Xiao TY, Liu HC, et al. Mutations within embCAB are associated with variable level of ethambutol resistance in Mycobacterium tuberculosis isolates from China. Antimicrob Agents Chemother. 2018;62. doi:10.1128/AAC.01279-17
25. Artsimovitch I, Vassylyeva MN, Svetlov D, et al. Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins. Cell. 2005;122:351–363. doi:10.1016/j.cell.2005.07.014
26. Friedrich SO, Venter A, Kayigire XA, Dawson R, Donald PR, Diacon AH. Suitability of Xpert MTB/RIF and genotype MTBDRplus for patient selection for a tuberculosis clinical trial. J Clin Microbiol. 2011;49:2827–2831. doi:10.1128/JCM.00138-11
27. Di Tanna GL, Khaki AR, Theron G, et al. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Glob Health. 2019;7:e191–e199. doi:10.1016/S2214-109X(18)30458-3
28. Xie YL, Chakravorty S, Armstrong DT, et al. Evaluation of a rapid molecular drug-susceptibility test for tuberculosis. N Engl J Med. 2017;377:1043–1054. doi:10.1056/NEJMoa1614915
29. Atashi S, Izadi B, Jalilian S, Madani SH, Farahani A, Mohajeri P. Evaluation of GeneXpert MTB/RIF for determination of rifampicin resistance among new tuberculosis cases in west and northwest Iran. New Microbes New Infect. 2017;19:117–120. doi:10.1016/j.nmni.2017.07.002
30. Goldstein BP. Resistance to rifampicin: a review. J Antibiot. 2014;67(9):625–630. doi:10.1038/ja.2014.107
31. Farhat MR, Sixsmith J, Calderon R, Hicks ND, Fortune SM, Murray M. Rifampicin and rifabutin resistance in 1003 Mycobacterium tuberculosis clinical isolates. J Antimicrob Chemother. 2019;74:1477–1483. doi:10.1093/jac/dkz048
32. Shea J, Halse TA, Kohlerschmidt D, et al. Low-level rifampin resistance and rpoB mutations in Mycobacterium tuberculosis: an analysis of whole-genome sequencing and drug susceptibility test data in New York. J Clin Microbiol. 2020;59:e01885–20.
33. Mohajeri P, Sadri H, Farahani A, Norozi B, Atashi S. Frequency of mutations associated with rifampicin resistance in Mycobacterium tuberculosis strains isolated from patients in West of Iran. Microb Drug Resist. 2015;21:315–319. doi:10.1089/mdr.2014.0075
34. Nebenzahl-Guimaraes H, Jacobson KR, Farhat MR, Murray MB. Systematic review of allelic exchange experiments aimed at identifying mutations that confer drug resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2014;69:331–342. doi:10.1093/jac/dkt358
35. Ning Q, Wang D, Cheng F, Zhong Y, Ding Q, You J. Predicting rifampicin resistance mutations in bacterial RNA polymerase subunit beta based on majority consensus. BMC Bioinform. 2021;22:210. doi:10.1186/s12859-021-04137-0
36. Molodtsov V, Scharf NT, Stefan MA, Garcia GA, Murakami KS. Structural basis for rifamycin resistance of bacterial RNA polymerase by the three most clinically important RpoB mutations found in Mycobacterium tuberculosis. Mol Microbiol. 2017;103:1034–1045. doi:10.1111/mmi.13606
37. Van Deun A, Barrera L, Bastian I, et al. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol. 2009;47:3501–3506. doi:10.1128/JCM.01209-09
38. Rigouts L, Gumusboga M, de Rijk WB, et al. Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol. 2013;51:2641–2645. doi:10.1128/JCM.02741-12
39. Miotto P, Cabibbe AM, Borroni E, Degano M, Cirillo DM. Role of disputed mutations in the rpoB gene in interpretation of automated liquid MGIT culture results for rifampin susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol. 2018;56. doi:10.1128/JCM.01599-17
40. Fowler PW. Epidemiological cutoff values for a 96-well broth microdilution plate for high throughput research antibiotic susceptibility testing of M. tuberculosis. medRxiv. 2021. doi:10.1101/2021.02.24.21252386
41. World Health Organization. Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). World Health Organization; 2021. Available from: https://apps.who.int/iris/handle/10665/339275.
42. Comas I, Borrell S, Roetzer A, et al. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat Genet. 2011;44:106–110. doi:10.1038/ng.1038
43. Sharma D, Bisht D, Khan AU. Potential alternative strategy against drug resistant tuberculosis: a proteomics prospect. Proteomes. 2018;6(2):26. doi:10.3390/proteomes6020026
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.