Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Rosacea: Practical Guidance and Challenges for Clinical Management
Authors Nguyen C , Kuceki G, Birdsall M , Sahni DR , Sahni VN, Hull CM
Received 22 June 2023
Accepted for publication 2 December 2023
Published 23 January 2024 Volume 2024:17 Pages 175—190
DOI https://doi.org/10.2147/CCID.S391705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Cassidy Nguyen,1,* Guilherme Kuceki,1,* Michael Birdsall,1 Dev Ram Sahni,2,3 Vikram Nath Sahni,2 Christopher M Hull2
1School of Medicine, University of Utah, Salt Lake City, UT, USA; 2Department of Dermatology, University of Utah Health, Salt Lake City, UT, USA; 3Department of Dermatology, Brigham and Women’s Hospital, Boston, MA, USA
*These authors contributed equally to this work
Correspondence: Dev Ram Sahni, Brigham and Women’s Hospital, 221 Longwood Ave, Boston, MA, 02115, USA, Tel +16177324918, Email [email protected]
Abstract: Rosacea is a common chronic dermatosis. Clinically, rosacea can present with flushing, erythema, papules, pustules, telangiectasias, phymatous changes, pruritus, burning, and stinging. In 2017, the National Rosacea Society Expert Committee recommended a phenotype-based classification for therapy. In this review, we identify monotherapies and multimodal treatment approaches for the clinical management of rosacea including topical, systemic, laser and light, alternative, and combination therapies.
Keywords: combination therapy, management, monotherapy, rosacea, therapy, treatment
Introduction and Epidemiology
Rosacea is a common chronic dermatosis with a prevalence of approximately 5.46% of the global population.1 However, prevalence is theoretically higher as these percentages do not account for patients with milder, unrecognized disease. The first reported account of rosacea was in the 14th century by Dr Guy de Chauliac, a French surgeon, who described “red lesions on the face” and termed the condition “goutterose”—French for “pink droplet”—or “couperose”, which remains as a term still used in French medical language.1 Onset of illness commonly emerges in middle to late adulthood and affects males and females equally, with a predominance of disease occurring in Northern European populations.2
Rosacea clinically presents with centrofacial erythema, prominent blood vessels, papules, and pustules.3 In 2002, the National Rosacea Society Expert Committee developed a subtype-based standardized classification system.4 The proportions of those affected by rosacea were found to be 56.7% (95% CI, 51.4–62.0%) for erythematotelangiectatic rosacea (ETR), 43.2% (95% CI, 38.8–47.6%) for the papulopustular subtype (PPR), 7.4% (95% CI, 6.1–8.9%) the phymatous subtype, and 11.1% (95% CI, 6.7–16.3%) the ocular subtype.5 These subjective subtype designations made it difficult to categorize patients considering the overlap of symptoms between categories. Over the next 15 years, further insights into the pathogenesis and pathophysiology of rosacea led to the 2017 updated classification guidelines that provided a phenotype-based approach that aimed to describe more explicit parameters based on clinical presentation and their respective, diagnostic positive predictive values.6 Instead of subtyping, the new classification system groups pathognomonic centrofacial erythema with major phenotypic criteria—papules, pustules, flushing, telangiectasia, and ocular involvement. Secondary phenotypes include burning, stinging, edema, and dry appearance.6 These new guidelines facilitate the individualization and customization of treatment for each patient.
Pathophysiology
The pathophysiology of rosacea is not entirely understood, but a multifactorial component is suspected with both genetic and environmental factors contributing to its development and progression.7 Mechanisms of disease propagation include both contribution from innate and adaptive immunity. Inflammation may be caused by dysregulation of proinflammatory cytokines (eg, IL-1, IL-3, and IL-8), alteration and dilation of small blood vessels, imbalances in the skin’s microbiome, genetic factors, and environmental triggers.1,7,8
There remains a lack of consensus on whether varying subtypes of rosacea are individualized clinical entities or if they represent a spectrum of disease progression. However, emerging studies have revealed that the diverse features of this disease and its numerous phenotypes may be part of a range of inflammation that is detectable both histologically and biochemically.9–12
UV light induces the production of vitamin D, which promotes cathelicidin expression in keratinocytes. Biochemically, patients with rosacea exhibit aberrant expression of cathelicidin, kallikrein-related peptidase 5 (KLK5), and subsequent cleaved product LL-37. The aberrantly cleaved LL-37 leads to disruption in regulation of leukocyte chemotaxis, angiogenesis (facilitated by CXCR chemokine receptor-ligands), expression of extracellular matrix components, and activation of NF-κB, which clinically correlate with the characteristic facial erythema, telangiectasias, papules, and pustules of rosacea. When mouse skin is injected with aberrant LL-37 a similar skin presentation to rosacea is exhibited, which purports immune dysregulation as a key factor in rosacea.7
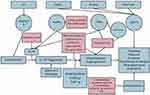
Additionally, Toll-like receptor 2 (TLR2), a member of the TLR family and innate immunity detection system, is highly active in rosacea patients. It typically responds to stimuli including the environment, extreme temperatures, microbial skin colonization, chemical and physical trauma, and ultraviolet (UV) light exposure.1 TLR2 activity also promotes expression of KLK5, further exacerbating this pathway of increased expression of LL-37 (Figure 1). Additional immune players that contribute to rosacea include matrix metalloproteases (MMPs), specifically MMP2 and MMP9, and mast cells, which also contribute to downstream KLK5 and LL-37 activity.7 Sun/heat exposure, alcohol consumption, exercise, and stress represent environmental factors that have also been associated with an increased risk of rosacea, although a recent study failed to confirm the correlation between lifetime UV exposure and prevalence of rosacea.13 Some hypothesize that these trigger factors hyperactivate and hypersensitize the TRPV1 (transient receptor potential (TRPV) vanilloid receptor 1), an ion channel, which may be overexpressed in rosacea patients, and contribute to the characteristic flushing, warmth, and burning pain some patients may experience.
New potential therapeutic target genes have been discovered with gene ontology analysis. Genomic association studies have identified three human leukocyte antigen (HLA) alleles and two single-nucleotide polymorphisms (SNPs) to be associated with rosacea, which have also been associated with autoimmune diseases such as type 1 diabetes and celiac disease.7,14
Rosacea is further associated with Demodex folliculorum, a commensal mite that often causes skin infestations by colonizing the pilosebaceous follicles of the skin.15 The pooled prevalence of Demodex infestation was 70.4% in 1150 patients with rosacea compared to 31.8% in 1057 healthy controls (OR 9.04, 95% CI 4.83–16.93).15,16 D. folliculorum antigens propagate disease activity by sensitizing and activating TLR-2 on keratinocytes.7,17 Other organisms that can cause a similar disease presentation include Helicobacter pylori, Staphylococcus epidermis, Bacillus oleronius, or Chlamydia pneumoniae.15,18,19 Associated comorbidities of rosacea include inflammatory bowel disease, depression, migraine, and cardiovascular conditions.20
Avoidance and Patient Education
The initial stages of rosacea may involve frequent blushing or flushing, or occasional episodes of redness on the face. Individuals with a predilection for blushing, a family history of rosacea, or both may be at risk of developing rosacea and are advised to avoid triggers through lifestyle modifications. Common triggers include alcohol, spicy foods, sun exposure, and stress, but specific triggers may vary between patients.21,22
Histopathological Findings
Rosacea is frequently characterized by dilation of small blood vessels in the skin. Histologically, an increase in the number and caliber of blood vessels is seen. The inflammatory infiltrate is predominantly lymphocytic and is located throughout the upper, mid, and deep dermis.23 Rosacea is often associated with an increase in the size and number of sebaceous glands, which histologically presents as sebaceous gland hyperplasia. Histological findings in rosacea can differ depending on the stage and severity of the condition. In the preliminary stages, there may be minimal to no visible changes on histology. As the condition progresses, dilated blood vessels, inflammation, thickening of the skin, sebaceous gland hyperplasia, and fibrosis can be seen.24 In advanced stages, there may be follicular plugging, telangiectasias, large deposits of dermal mucin, and pronounced thickening and fibrosis of the dermis.24 Pustular lesions often show a superficial accumulation of neutrophils extending beyond the follicle, an important distinguishing factor from acne vulgaris.22,24
Current and Emerging Therapeutic Management: Topical, Systemic, Laser, and Light Therapies
Currently, no single treatment has been fully curative for rosacea, and the quality of evidence for available treatments varies depending on the modality, with topical therapies having the highest level of evidence.25 Rosacea patients can present with a multitude of cutaneous features with different severity levels. Individual composition of clinical features guides treatment, ranging from mild monotherapy to combination therapies that address single or multiple elements through additional mechanisms (Table 1). Successful treatment is often aimed at targeting specific phenotypes.
 |
Table 1 Rosacea Treatment Options by Phenotype |
Phenotype-Based Strategies
Persistent Erythema and Flushing
The initial approach to therapy may include avoidance of flushing triggers along with cosmetic concealment, gentle skin care, and sun protection. These conservative approaches should not be discouraged after failure. A journal can be used to identify possible triggers and additional strategies. If resistant to initial measures, topical interventions such as brimonidine or oxymetazoline can be utilized.49,55 Due to alpha receptor agonism, these interventions should be used with caution in patients with vascular insufficiency or spasmodic conditions. Other topical therapies, such as azelaic acid and antimicrobials, lack evidence in isolated persistent erythema. Oral pharmacology can be attempted, including clonidine, beta-blockers, selective norepinephrine reuptake inhibitors, and gabapentin.110,111 Alternatively, lasers and intense pulsed light (IPL) can be used, although the available evidence mainly comprises small and/or uncontrolled studies.
Papules and Pustules
Papulopustular rosacea is centrally distributed on the face and often accompanied by persistent facial erythema, telangiectasias, and flushing. Most patients can be successfully treated with topical metronidazole, azelaic acid, or ivermectin.112,113 If desired symptomatic control is not achieved after 8 to 12 weeks of consistent daily use, a different topical agent or a systemic agent may be implemented. Substantial evidence supports the use of doxycycline as a first-line systemic therapy.96 Lasers, IPL, and photodynamic therapy can be used, although evidence remains insufficient.114 Refractory symptoms warrant combination therapy with topical and systemic approaches or secondary systemic agents such as oral isotretinoin.104 Combinations such as topical metronidazole with subantimicrobial dose doxycycline and doxycycline 40 mg with daily application of ivermectin 1% cream are more efficacious than monotherapy with these agents.68,75,115
Phymatous Features
Most commonly affecting the nose, these cutaneous hypertrophic changes include follicle dilation and irregular nodularity. The course of these changes is uncertain, and there are limited theories regarding early intervention. Management of inflammatory aspects with systemic and topical agents can arguably achieve small progression control. Isotretinoin can be beneficial, with results likely relating to course duration and total administered dose relative to body weight. Additional evidence is needed to establish ideal post-therapy interventions and the need for multiple courses. Side effects of isotretinoin include dryness, scaling, and burning, which can limit patient adherence.116 Chronic and severe phymatous disease may require surgical therapy to remove excess tissue and recontour deformations. Procedural options include ablative lasers, electrosurgery, electrocautery, and dermabrasion. Rhinophyma can recur following surgical procedures and may require further revision.117
Ocular Involvement
Foreign body sensations, eyelid telangiectasia, frequent chalazion, blepharitis, conjunctivitis, and corneal ulcers may be features of rosacea with or without cutaneous manifestation.118,119 Patients with ocular involvement should be referred to ophthalmology to minimize the risk of vision loss. Milder presentations of disease can be managed with artificial tears and adequate lid hygiene. For moderate-to-severe symptoms, topical metronidazole or cyclosporine eye drops can be utilized after ruling out ocular infection.118,120 Doxycycline is also frequently used to treat ocular rosacea.
Atypical Features
Nodules and red-brown or yellow-brown papules on the face that are variably accompanied by typical rosacea features may indicate a rare variant named granulomatous rosacea.121 It has been described as non-inflammatory and sometimes unilateral with overlapping features that might make the diagnosis difficult. Reported treatment includes doxycycline, minocycline, and commonly used topical rosacea interventions.121 Another atypical manifestation is called rosacea fulminans (pyoderma faciale), which involves the sudden onset of inflammatory facial papules and pustules with possible abscess and sinus formation. Commonly reported triggers include hormonal shifts, emotional stress, and medications.122 Treatment includes antibiotics, isotretinoin, and prednisolone.122
Neurogenic rosacea has become increasingly recognized as another subtype of rosacea. It was first reported in 2011, and few reports have been described.59 Patients who are affected by this subtype have classical features of rosacea in addition to prominent neurologic symptoms. These neurologic and neuropsychiatric symptoms include complex regional pain syndrome, essential tremor, depression, obsessive-compulsive disorder, headaches, and Raynaud’s phenomenon. Given their limited success with typical rosacea treatments—including metronidazole, steroids, and antibiotics—gabapentin, pregabalin, tricyclic antidepressants, and duloxetine are beneficial.59 While the mechanism behind this rosacea is poorly understood and possibly multifactorial, neuronal dysregulation may play a role in the pathogenesis. Neural dysregulation includes vasomotor, inflammatory, and neuropathic components which can lead to a wide spectrum of clinical symptoms. Gabapentin, pregabalin, tricyclic antidepressants, and duloxetine can target refractory symptoms including dysesthesia, flushing, or inflammation.59 Given the limited studies, further research is needed to understand this possible subgroup of rosacea and the efficacy of these treatments.
Topical Therapies
Topical agents for papulopustular rosacea should be chosen according to the patient’s skin type, symptomatology, mode of action, efficacy, and safety. The vehicles used in popular topical formulations play a crucial role in delivering the active ingredient, enhancing therapeutic tolerability, and promoting patient adherence. The FDA-approved topical therapies are as follows: azelaic acid (15% gel), brimonidine tartrate (0.33% gel), ivermectin (1% cream), metronidazole (0.75% gel, cream, and lotion, 1% cream, and 1% gel), oxymetazoline hydrochloride (1% cream), sodium sulfacetamide (10%), sulfur (5% gel, cleanser, lotion, suspension, and cream), and calcineurin inhibitors.123
Metronidazole
Metronidazole contains anti-inflammatory properties and is used to treat erythema, pustules, and papules. It works by inhibiting protein synthesis and causing strand breakages and oxidative stress in susceptible organisms124,125 Both 1% cream and 0.75% gel formulations reduced lesion count and erythema while also improving physician’s global rosacea scores (all P = <0.05).38,39,126 When metronidazole was combined with a gentle cleanser, hydration correction, and sunscreen, patient tolerability increased.40 Consequently, metronidazole was rated as one of the most efficacious topical therapies for the major feature of papules and pustules by the National Rosacea Society Committee.21
Ivermectin
Ivermectin provides another viable therapy for the treatment of the papules and pustules of rosacea.21,91 It has anti-parasitic effects against D. folliculorum and anti-inflammatory properties that increase IL-10 and decrease IL-1β, TNF-α, and neutrophilic activity.92 In two parallel studies, once-daily use of 1% cream cleared papulopustular rosacea in 38.4% and 40.1% of subjects compared to 11.6% and 18.8% for the vehicle according to Investigator Global Assessment (IGA) (both P < 0.001).92 Ivermectin 1% cream is also more effective than metronidazole 0.75% cream in reducing inflammatory lesions by lesion count and IGA (P < 0.001).93 In a meta-analysis ivermectin demonstrated higher efficacy in clearing papulopustular rosacea and increasing quality of life compared to metronidazole, azelaic acid, and placebo measured by IGA and DLQI scores.94
Sodium Sulfacetamide/Sulfur
A combination of sodium sulfacetamide 10% and sulfur 5% decreases Demodex mite presence through antimicrobial and anti-inflammatory properties.63,64 This combination is safe and efficacious, with subject’s and physician’s global evaluations showing significant papulopustular lesion reductions of 65% versus 44% (P = 0.002) and erythema reduction of 66% vs 33% (P = 0.005) when compared to the vehicle group.127 When compared with metronidazole 0.75% cream, sodium sulfacetamide 10% and sulfur 5% with sunscreen had inflammatory lesion reduction of 80% vs 72% (P = 0.04) and erythema reduction 69% vs 45% (P = <0.001).128 Additionally, wash-off formulations offer better bioavailability and absorption with a decrease in lasting odor and irritation when compared to creams.
Azelaic Acid
A natural dicarboxylic acid, azelaic acid, has been approved by the FDA in the form of a 15% gel for treatment of mild-to-moderate rosacea. Dicarboxylic acid reduces erythema and inflammatory lesions through inhibition of NADPH oxidase on the neutrophilic cell membrane, which decreases the activity of reactive oxygen species (ROS).26 The utility of azelaic acid, especially for the papulopustular subtype of rosacea, has been well established. Both the 15% gel and 20% cream forms are equally effective.26 The 15% gel is significantly superior (P = 0.005) in reducing erythema and inflammatory lesions compared to placebo and more effective than metronidazole 0.75% gel in reducing erythema and inflammatory lesions in comparative trials.27,28,113 Azelaic acid 15% gel decreased erythema to a greater extent compared to metronidazole 1% gel on overall assessment and IGA.29,30 Moreover, azelaic acid 15% foam is more tolerable than metronidazole gel or cream.31 A formulation containing 15% azelaic acid and 1% dihydroavenanthramide D significantly decreased erythema after 8 weeks of treatment as measured by erythema-directed digital photography.32 Azelaic acid was rated as one of the most effective topical therapies for the major feature of papules and pustules by the National Rosacea Society Committee.21
Brimonidine Tartrate
Brimonidine tartrate is commonly employed for the treatment of erythematotelangiectatic rosacea. It stimulates alpha-adrenergic receptors and has a higher affinity for alpha-2 receptors compared to alpha-1 receptors.56 Brimonidine tartrate reduces inflammation by narrowing small blood vessels under the skin and decreasing swelling.56 The 0.5% gel form of brimonidine tartrate is effective and safe for treating rosacea and is the first and only FDA-approved topical therapy that improves the persistent facial redness and flushing of rosacea.55,56 Significant improvement has been observed as soon as 30 minutes after application, with persistent efficacy on day 29; 58.3% vs 32.0% (P < 0.001) when compared to its vehicle.57 Rated as one of the most efficacious topical therapies for persistent erythema by the National Rosacea Society Committee, brimonidine tartrate has a fast onset of action and can be used safely with other anti-inflammatory drugs.21 Frequent re-application is necessary due to its temporary action, but long-term use appears to preserve efficacy.58
Oxymetazoline Hydrochloride
Oxymetazoline is a potent alpha-1 agonist approved for treating persistent erythema in rosacea. Effects can be seen within 1 to 3 hours of application and last up to 8 to 10 hours.50 In an open-label study without a control group, clinician and patient assessments improved from day 1; after 52 weeks of oxymetazoline 1% cream, 36.7% of patients achieved a 2-grade or greater improvement from baseline in the Clinician Erythema Assessment (CEA) three and six hours after application.50 When concentrations of oxymetazoline hydrochloride 0.5%, 1%, and 1.5% were applied once (QD) or twice (BID) daily and compared with their respective vehicles, all active creams and BID application had a significantly higher number of patients achieving two-grade or greater improvement from baseline on CEA and Subject Self-Assessment of erythema (0.5% QD (P = 0.049), 1.0% QD (P = 0.006), 1.5% QD (P = 0.012), 1.0% BID (P = 0.021), and 1.5% BID (P = 0.006)).51 When applied once daily, oxymetazoline hydrochloride 1% cream had sustained efficacy, safety, and tolerability in the treatment of moderate-to-severe persistent facial erythema of rosacea.49 It is rated as one of the most efficacious topical therapies for persistent erythema by the National Rosacea Society Committee.21
Salicylic Acid
Salicylic acid (SA), a hydroxyl acid compound, has anti-inflammatory, antibacterial, and antifungal properties. SA is used for a wide range of skin conditions as a peeling agent. SA has been used to treat acne, hyperpigmentation, and other inflammatory dermatoses. Similarly, it can target the inflammatory and vascular changes that occur with papulopustular rosacea via the arachidonic acid cascade. However, some side effects reported include temporary crusting, intense exfoliation, dryness, and erythema, especially in patients with sensitive skin. A modified, recently introduced SA, supramolecular salicylic acid (SSA), has been shown to minimize the risk of irritation given its improved solubility. In a 2022 randomized controlled trial, patients treated with 30% SSA peeling treatment every three weeks for nine weeks showed improvement in erythema index.69 Another retrospective study that examined patients treated with 50 mg oral minocycline twice a day and SSA 30% chemical peels twice a month showed a significant reduction of rosacea severity (3.32 ± 0.6 at baseline to 0.89 ± 0.7 at 12 weeks (P < 0.01)) based on the Investigatory Severity Assessment after treatment. Of the 19 patients enrolled, 17 achieved “excellent improvement” in the IGA of efficacy with no significant adverse reactions reported.70
Other Topical Agents
Many topical medications have been trialed for rosacea symptom relief. Calcineurin inhibitors such as tacrolimus and pimecrolimus interfere with cellular signals, causing inhibition of T-lymphocyte activation and prevention of cytokine release.76 Azithromycin primarily works as an anti-inflammatory and antimicrobial agent in ocular rosacea.105–108 For papulopustular and erythematotelangiectatic phenotypes, silicone-encapsulated benzoyl peroxide gel in both 1% and 5% strengths provide significant benefit in reducing inflammatory lesions while simultaneously avoiding irritation.82,83 Tea tree oil in conjunction with 2.5% permethrin also reduced Demodex density through standard skin surface biopsy (P = 0.001); non-transient erythema, papules, and pustules through IGA scores (P=<.050); and dry appearance, burning, and stinging through patient report (P=<.050) when compared to placebo.37 Other naturopathic options in rosacea treatment include citron essential oils. These contain several compounds with suppressive activity of the pathophysiologic mediators of rosacea, including antimicrobial peptide LL-37, KLK5, transient receptor potential vanilloid 1 (TRPV1), and vascular endothelial growth factor (VEGF).36 For ocular rosacea, topical cyclosporine improved Schirmer scores (4.1 mm [95% CI, 1.66 to 6.54]) and quality of life.25 A 0.05% concentration of topical cyclosporine had a greater improvement in ocular surface disease index scores than artificial tears for the treatment of rosacea-associated lid and corneal changes (P < 0.022).129 Twice daily use of topical cyclosporine is also superior to 100 mg oral doxycycline in symptomatic relief and in the treatment of eyelid signs (P = 0.01).130
Systemic Therapy
Tetracyclines, macrolides, and isotretinoin are considered mainstay oral medications and first-line therapies in the treatment of inflamed phymas and papulopustular rosacea.21 Other systemic agents include metronidazole, carvedilol, clonidine, propranolol, gabapentin, hydroxychloroquine, secukinumab, rifaximin, and zinc. Though less efficacious than topical vascular agonists, oral medications can be advantageous in decreasing persistent erythema.
Tetracyclines
Tetracycline compounds were the first drugs used to treat rosacea systemically and have been the primary therapy for over 40 years.97 The bacteriostatic properties of tetracyclines result from the inhibition of protein synthesis through binding to the 30S subunit of the bacterial ribosome, disrupting the binding of aminoacyl tRNA to the mRNA-ribosome complex.98 These compounds also exert immunomodulatory effects in chronic inflammatory disorders.97 The sub-antimicrobial dose of oral doxycycline (SDD) 40 mg has the strongest evidence and efficacy among oral therapies in the treatment of pustules and papules of rosacea and is the only FDA-approved systemic therapy for the treatment of inflammatory lesions in rosacea.21,96 SDD has further demonstrated avoidance of antibiotic selection pressure and bacterial resistance.99,100 Daily SDD monotherapy for 52 weeks significantly reduced relapse rate and the number of inflammatory lesions in individuals with moderate-to-severe rosacea compared to placebo (P = <0.05).96 SDD, in comparison to doxycycline 100mg, was similar in efficacy with fewer adverse gastrointestinal side effects.101 Minocycline 100 mg is comparable to doxycycline 40 mg in efficacy over a 16-week treatment period with a similar safety profile and lower risk-to-reward ratio, thus it can be a viable alternative treatment for those who cannot tolerate doxycycline 40 mg.102 Ocular rosacea also responds well to long-term therapy with lower-dose doxycycline.109
Macrolides
Macrolides such as erythromycin, azithromycin, and clarithromycin inhibit bacterial protein synthesis and exert immunomodulatory effects in chronic inflammatory disorders.84 They are effective and safe options for treating papules and pustules in rosacea, particularly for patients who are not suitable candidates for tetracycline treatment.85 Azithromycin and clarithromycin have a faster onset of action, fewer gastrointestinal side effects, and better tolerance than other macrolides.86 The safety and efficacy of azithromycin and clarithromycin has been evidenced in multiple studies.87,88 Oral azithromycin is comparable to doxycycline in reducing inflammatory lesion scores after 12 weeks of decreasing doses.89 Clarithromycin yielded similar results to doxycycline in a comparison trial based on erythema index scores and papulopustular counts (P=<.005); however, doxycycline showed better tolerability (P=<.005).90
Isotretinoin
Isotretinoin (0.5–1mg/kg/day) is primarily used to treat severe and nodulocystic acne but can also treat persistent papulopustular and erythematous rosacea. In refractory papulopustular rosacea, a 4-month course of oral isotretinoin (0.25 mg/kg/day) improves both erythema and inflammatory lesions. Remission rates do not appear to correlate with the cumulative dose of isotretinoin.33 As a result, retinoids are an efficacious option to avoid long term chronic antibiotics.34 Continuous isotretinoin therapy in microdose form (0.04–0.11 mg/kg per day) may be a viable alternative, provided there is regular laboratory monitoring and adequate contraceptive measures for childbearing women.35 This modality also improved the severity of rhinophyma, according to IGA in 47% of patients.104
Hydroxychloroquine
Hydroxychloroquine (HCQ) is commonly used for autoimmune disorders and has emerging data to support its role in the treatment of rosacea, particularly PPR and ETR subtypes. HCQ improves symptoms of rosacea due to its ability to decrease expression of MMP9 and tryptase; it also decreases mast cell infiltration and survival in tissue.131 HCQ 200mg twice daily may have comparable results to 100mg doxycycline after eight weeks of therapy, though further substantiation of its efficacy will be necessary to draw any certain conclusions.77 Because HCQ is safe for individuals who are pregnant, it may be considered as a possible treatment option throughout pregnancy.77
Gabapentin and Pregabalin
Gabapentin is an anti-epileptic agent commonly used in the treatment of neuropathic pain. It acts on voltage-gated calcium channels and induces modulation of N-methyl-D-aspartate (NMDA) receptors, protein kinase C, and inflammatory cytokines.132 Pregabalin shares a similar mechanism of action with different pharmacokinetic and pharmacodynamic characteristics.133 Rosacea patients with neuropathy and those with neurogenic rosacea can benefit from the multifactorial effects of these drugs.59 One case report of a 63-year-old female who had severe flushing and burning of her cheeks that had failed to respond to standard treatments including oral lymecycline, metronidazole gel, azelaic acid, and laser therapy was prescribed pregabalin 300 mg in the morning and 150 mg at night. Within two months of treatment, her symptoms improved dramatically.134
Rifaximin
Rifaximin is an unabsorbed antibiotic primarily active in the gastrointestinal tract that may improve symptoms of PPR, ETR, and ocular rosacea in a subset of patients with small intestinal bacterial overgrowth (SIBO). Eighty-two percent of patients with SIBO reported mild (11%), moderate (25%), or marked (46%) improvement in symptoms after 10 days of receiving rifaximin 400 mg three times daily.71 Patients with rosacea, particularly PPR, are 13 times more likely to have SIBO.72 With remission of rosacea in 64.5% of patients with SIBO who completed a regimen of rifaximin 400 mg three times daily, this is a credible therapy for this population and may warrant testing for SIBO in patients with rosacea when other therapies have failed.72
Secukinumab
This monoclonal antibody exerts its function by binding to IL-17A and is approved for psoriasis treatment. IL-17 has a pivotal role in the inflammatory cascade of rosacea and elevated serum levels of IL-17 have been observed in rosacea patients.135 An open-label, rater-blinded trial with secukinumab 300 mg weekly for five weeks then monthly for two months was conducted in 2019 for patients with papulopustular disease.65 Seventeen patients completed the study out of the initial 24. After 16 weeks, median decrease in papules evaluated by dermatologists was five fewer lesions overall (P = 0.01) from a baseline of at least 10. Global severity score was reduced by 0.3 points (P = 0.03). Improvement in quality of life based on RosaQol was observed (score reduction by 0.6 points; p = 0.001).65 It is unclear whether these results have clinical significance, therefore larger trials comparing standard treatments are needed to validate these findings.
Other Systemic Therapies
Oral beta-blockers, such as carvedilol and propranolol, rapidly reduce erythema and flushing.41 These medications inhibit beta-adrenergic receptors and reduce anxiety and tachycardia, which can exacerbate flushing reactions.52 Oral zinc sulfate may also offer relief from several symptoms of rosacea, but conflicting evidence exists. Oral zinc sulfate 100 mg three times daily significantly lowered levels of erythema, papules, and pustules with no effect on telangiectasias (P = <0.05).52 In another study, patients with moderately severe facial rosacea were given 220 mg zinc sulfate twice daily, and no significant difference in the standard grading system for rosacea was seen between this group and placebo.53
Lasers and Lights
Although the quality of evidence is narrow, light therapies and lasers have been successful in the management of rosacea features, particularly telangiectasias and phymatous change.25 The most employed therapeutics include pulsed dye laser (PDL), IPL, potassium titanyl phosphate (KTP) laser, and long-pulsed neodymium: yttrium-aluminum-garnet laser (Nd:YAG).25 Carbon dioxide (CO2) and erbium: yttrium-aluminum-garnet (Er:YAG) lasers are also helpful in treating phymatous rosacea. Light therapies are efficacious for treating flushing, erythema, and telangiectasia features. Both PDL and IPL devices are effective and satisfactory to patients given the telangiectasia component is often refractory to other treatments.
Pulsed Dye Laser and Intense Pulsed Light
Given that rosacea can result from chronic sun exposure, PDL aims to address the photodamage response by targeting both the inflammatory vascular component of rosacea while reducing Demodex density.42 PDL uses visible (yellow) light that is selectively absorbed by oxyhemoglobin, a process known as selective photothermolysis. In 1986, PDL was approved by the US Food and Drug Administration (FDA) for treating skin vascular disorders. Initially, the PDL emitted light at 577 nm, but was later modified to emit at 585 nm and 595 nm, providing deeper injury to vascular structures, up to 1.2 mm.43 Years later, Fitzpatrick, Goldman, and Eckhouse demonstrated the potential therapeutic use of IPL, which is characterized by multiple wavelengths emitted simultaneously with an optic cutoff filter that selects the spectral output.44 Since the FDA approval of IPL in 1995, numerous devices have been created that allow for the modulation of pulse duration, energy fluence, spectral output, and area size. Both PDL and IPL have been used for decades in the treatment of rosacea due to their proven effects in reducing erythema and telangiectasia and improving quality of life.45,46
Neodymium:Yttrium-Aluminum-Garnet Lasers
Though originally designed to target deeper and darker vessels and allow for ideal thermal relaxation time, Nd:YAG is effective in the treatment of diffuse facial erythema.60 A randomized clinical trial yielded similar responses in addressing erythema when compared to PDL, and subjects who received PDL reported more significant adverse effects than those who received Nd:YAG.61
KTP laser (532 nm, 585 nm) is an effective type of Nd:YAG that enhances frequency by halving the wavelength, producing green light.66 Consequently, it is remarkably effective for telangiectasia treatment. Though allowing for a faster healing time, the halved frequency limits the effectiveness of treating erythema compared to other Nd:YAG options. Q-switched KTP achieved similar clinician’s assessment scores when compared with long-pulsed Nd:YAG. However, the latter had lower quantitative measurement of papules and erythematotelangiectatic rosacea score rate.67
CO2 Laser
Phymatous changes in the disease progression of rosacea can occur on any facial region, but the nose is the most frequently affected site. Non-inflamed lesions require mechanically abrasive, laser ablative, or radiofrequency therapies for improvement. Each modality requires meticulous care and a proficient level of training due to risks associated with permanent damage and dysfunction of treated areas. In one study, 95% of subjects (n = 124) with rhinophyma achieved good to excellent improvement after an average of one session of CO2 laser therapy.103
Other Modalities
Camouflage
Individuals with rosacea often experience discomfort and embarrassment due to the redness, papules, and pustules on their faces.78 Cosmetic camouflage such as green-tinted and yellow-tinted foundations and powders can aid in decreasing the erythematous appearance of rosacea.46 This can provide both physical and psychological benefits, such as increased self-confidence, for those who use it.
Botulinum Toxin
Botulinum toxin reduces inflammation caused by rosacea by blocking mast cell degranulation.73 Intradermal injection of botulinum toxin may be a safe and effective treatment for rosacea, particularly for patients with refractory erythema who have not responded well to other therapies. Further research is needed to confirm its effectiveness and determine the optimal dosage and frequency of treatment.74
Hyaluronic Acid
Topical and transdermal low molecular weight hyaluronic acid (LMW-HA) is safe and effective alternatives that can be used with other treatments for rosacea.79,80 The hydrophilic structure of LMW-HA allows it to penetrate the stratum corneum and interact with fibrin and collagen to support the remodeling of the extracellular matrix. This, along with the induced release of the antimicrobial peptide β-defensin 2 from keratinocytes, helps to accelerate re-epithelialization, reducing pain and edema.81
Combination Therapies
Combinative multimodal therapy is often utilized and required when treating patients with overlapping features of rosacea. Topical medications have been paired with oral therapies, light-based therapy, and other topical medications to provide more comprehensive coverage of symptom management.136 Fractional micro-needling radiofrequency (FMR), a medical device that has demonstrated anti-angiogenesis, anti-inflammatory, and dermal remodeling, in conjunction with PDL and oral isotretinoin was effective in treating recalcitrant papulopustular rosacea. Twenty-five PPR patients who had failed first-line therapies trialed three sessions of this novel therapeutic regimen at four-week intervals.95 After completion of treatment, there was a 71% decrease in the number of papules and pustules and a 54% decrease in erythema index when compared to the patient baseline; no serious side effects were noted. Notably, physician’s global assessments and patients’ subjective assessments corresponded well with these results.
In a cohort analysis of 72,173 patients diagnosed with rosacea from 2005 to 2014, over 20% were treated with a combination of topical agents, with metronidazole and azelaic acid being the most commonly used combination regimen in the United States.62 These medications generally have similar mechanisms of action, including anti-inflammatory, anti-oxidizing, and KLK5 modulation. Topical combinations such as ivermectin 1% cream with brimonidine 0.33% showed additive effect of brimonidine with superior efficacy based on IGA for erythema and inflammatory lesions in the total active group compared to vehicle (55.8% vs 36.8%, P = 0.007) at week 12 and increased treatment success based on IGA compared to ivermectin with vehicle group.137
Topical Metronidazole with Oral Doxycycline
In a randomized, double-blinded study with 72 patients (mild-to-moderate disease), doxycycline 40 mg modified release (ie, 30-mg immediate-release and 10-mg delayed-release beads) was combined with topical metronidazole 1% once daily.75 When compared to topical metronidazole 1% monotherapy, the combination group continued to have significantly higher inflammatory lesion reduction as early as week 4 and remained as such until the end of the study, 13.86 vs 8.47 lesion count reduction (P = 0.002) at week 12.75
Topical Ivermectin with Oral Doxycycline
A total of 273 subjects with severe rosacea (IGA 4) participated in a randomized, investigator-blinded trial comparing topical IVM 1% cream plus doxycycline 40 mg modified release with IVM 1% cream and placebo together. After 12 weeks, combination therapy displayed superior efficacy in reduction of inflammatory lesions: –80.3% vs –73.6% for monotherapy (P = 0.032).68 The combination group also displayed faster onset of action at week 4 and had an increased number of subjects who achieved 100% lesion reduction; 17.8% vs 7.2% (P = 0.006) at week 12.68
Oxymetazoline Cream with Light Therapy
Oxymetazoline hydrochloride cream (1%), an alpha-adrenergic agonist, used in conjunction with energy-based laser light therapy (KTP laser, IPL, PDL Vbeam Perfecta, or PDL Cynergy), is an additional regimen that has been studied in rosacea patients affected by severe facial erythema.47 After 56 days of treatment, 90.7% of evaluable patients showed an improvement in erythema by one grade or higher from baseline CEA scores.
A combination of PDL and oxymetazoline 1% cream reduced both erythema and telangiectasias with at least one CEA grade of improvement and at least two-point clearance, respectively.48 The efficacy of PDL has also been explored in conjunction with intradermal botulinum toxin type-A. A quantitative reduction in erythema, measured by CEA scores and 3D Antera camera, was seen in 100% of patients (N = 20), with sustained improvements at three and nine months.54
In theory, combination therapies are more efficacious than monotherapy in rosacea through targeting multiple pathways that drive the disease process. While a variety of monotherapies have been studied to varying degrees, the permutations of combination therapies left unexplored are far greater. An assessment of the therapeutic benefit, concomitant synergy among various therapies, and the cost-effectiveness of these treatments must be more thoroughly explored.
Quality of Life and Patient Satisfaction
Rosacea can have a substantial impact on a patient’s appearance. Pain and irritation associated with the condition also places a significant burden on quality of life. Not surprisingly, patients affected by rosacea have higher reported rates of anxiety and depression. Surveys conducted by the National Rosacea Society have reported patient avoidance of social interactions and decreased workplace productivity. Although current therapies cannot cure the disease, proper management with clinical improvement of disease burden has been shown to improve patient’s quality of life.138 The National Rosacea Society cross-sectional survey evaluated the burden and impact of rosacea using Dermatology Life Quality Index (DLQI) and willingness to pay (WTP), which are well-established instruments used to assess the health-related quality of life (HRQoL). The study concluded that rosacea had a substantial impact on daily HRQoL given DLQI results, with the most bothersome symptoms being unrelenting facial erythema, blushing, and flushing features.139
Conclusion
Rosacea continues to affect a large portion of the population, but recent advances in combination therapies have resulted in more effective and long-lasting treatments. The key to effectively managing rosacea is identification of phenotype, assessment of symptom severity, and choosing treatments that meet the patient’s needs and expectations; taking efficacy, cost, and likelihood of regimen adherence into consideration. Continued research into the medical and cosmetic aspects of rosacea will help refine and improve the treatment options available to patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermato-Endocrinology. 2017;9(1):e1361574. doi:10.1080/19381980.2017.1361574
2. Gether L, Overgaard L, Egeberg A, Thyssen J. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–289. doi:10.1111/bjd.16481
3. Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51(3):327–341. doi:10.1016/j.jaad.2004.03.030
4. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the national rosacea society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46(4):584–587. doi:10.1067/mjd.2002.120625
5. Barakji YA, Rønnstad ATM, Christensen MO, et al. Assessment of frequency of rosacea subtypes in patients with rosacea: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(6):617–625. doi:10.1001/jamadermatol.2022.0526
6. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155. doi:10.1016/j.jaad.2017.08.037
7. Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72(5):749–758. doi:10.1016/j.jaad.2014.08.028
8. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–980. doi:10.1038/nm1616
9. Schwab VD, Sulk M, Seeliger S, et al.. Neurovascular and Neuroimmune Aspects in the Pathophysiology of Rosacea. Elsevier; 2011:53–62.
10. Sulk M, Seeliger S, Aubert J, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012;132(4):1253–1262. doi:10.1038/jid.2011.424
11. Seeliger S, Buddenkotte J, Schmidt-Choudhury A, et al. Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo. Am J Pathol. 2010;177(5):2563–2575. doi:10.2353/ajpath.2010.090941
12. Wladis EJ, Iglesias BV, Adam AP, Gosselin EJ. Molecular biologic assessment of cutaneous specimens of ocular rosacea. Ophthalmic Plast Reconstr Surg. 2012;28(4):246–250. doi:10.1097/IOP.0b013e31824dd9d4
13. McAleer MA, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63(1):33–39. doi:10.1016/j.jaad.2009.04.024
14. Sun Y, Chen L-H, Y-s L, et al. Identification of novel candidate genes in rosacea by bioinformatic methods. Cytokine. 2021;141:155444. doi:10.1016/j.cyto.2021.155444
15. Forton FM, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99(1):47–52. doi:10.2340/00015555-3041
16. Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(3):441–447.e6. doi:10.1016/j.jaad.2017.03.040
17. Reinholz M, Ruzicka T, Schauber J. Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann Dermatol. 2012;24(2):126–135. doi:10.5021/ad.2012.24.2.126
18. Holmes AD. Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013;69(6):1025–1032. doi:10.1016/j.jaad.2013.08.006
19. Dahl MV, Ross AJ, Schlievert PM. Temperature regulates bacterial protein production: possible role in rosacea. J Am Acad Dermatol. 2004;50(2):266–272. doi:10.1016/j.jaad.2003.05.005
20. Haber R, El Gemayel M. Comorbidities in rosacea: a systematic review and update. J Am Acad Dermatol. 2018;78(4):786–792. e8. doi:10.1016/j.jaad.2017.09.016
21. Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82(6):1501–1510. doi:10.1016/j.jaad.2020.01.077
22. Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6(7):759–764. doi:10.1016/j.cgh.2008.02.054
23. Cribier B. Rosacea under the microscope: characteristic histological findings. J Eur Acad Dermatol Venereol. 2013;27(11):1336–1343. doi:10.1111/jdv.12121
24. Aloi F, Tomasini C, Soro E, Pippione M. The clinicopathologic spectrum of rhinophyma. J Am Acad Dermatol. 2000;42(3):468–472. doi:10.1016/S0190-9622(00)90220-2
25. van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol. 2019;181(1):65–79. doi:10.1111/bjd.17590
26. Liu RH, Smith MK, Basta SA, Farmer ER. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142(8):1047–1052. doi:10.1001/archderm.142.8.1047
27. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48(6):836–845. doi:10.1067/mjd.2003.308
28. Frampton JE, Wagstaff AJ. Azelaic acid 15% gel: in the treatment of papulopustular rosacea. Am J Clin Dermatol. 2004;5:57–64. doi:10.2165/00128071-200405010-00009
29. Wolf JE
30. Gollnick H, Layton A. Azelaic acid 15% gel in the treatment of rosacea. Expert Opin Pharmacother. 2008;9(15):2699–2706. doi:10.1517/14656566.9.15.2699
31. Williamson T, LaRose A, Cameron J, et al. Rosacea treatment satisfaction: matching adjusted indirect treatment comparison analysis of metronidazole gel or cream vs azelaic acid foam. J Drugs Dermatol. 2020;19(3):295–304. doi:10.36849/JDD.2020.3679
32. Dall’Oglio F, Tedeschi A, Lacarrubba F, et al. A novel azelaic acid formulation for the topical treatment of inflammatory rosacea: a multicentre, prospective clinical trial. J Cosmet Dermatol. 2021;20:28–31. doi:10.1111/jocd.14098
33. van Zuuren EJ, Fedorowicz Z. Low-dose isotretinoin: an option for difficult-to-treat papulopustular rosacea. J Invest Dermatol. 2016;136(6):1081–1083. doi:10.1016/j.jid.2016.03.003
34. MacGibeny MA, J-h J, Kong HH. Antibiotic stewardship in dermatology—reducing the risk of prolonged antimicrobial resistance in skin. JAMA Dermatol. 2022;158(9):989–991. doi:10.1001/jamadermatol.2022.3168
35. Hofer T. Continuous ‘microdose’ isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29(2):204–205. doi:10.1111/j.1365-2230.2004.01472.x
36. Jeon HW, Na EY, Yun SJ, Lee S-C, Lee J-B. Citron essential oils alleviate the mediators related to rosacea pathophysiology in epidermal keratinocytes. Ann Dermatol. 2018;30(6):653–661. doi:10.5021/ad.2018.30.6.653
37. Ebneyamin E, Mansouri P, Rajabi M, Qomi M, Asgharian R, Azizian Z. The efficacy and safety of permethrin 2.5% with tea tree oil gel on rosacea treatment: a double-blind, controlled clinical trial. J Cosmet Dermatol. 2020;19(6):1426–1431. doi:10.1111/jocd.13177
38. Breneman D, Stewart D, Hevia O, Hino P, Drake L. A double-blind, multicenter clinical trial comparing efficacy of once-daily metronidazole 1 percent cream to vehicle in patients with rosacea. Cutis. 1998;61(1):44–47.
39. Miyachi Y, Yamasaki K, Fujita T, Fujii C. Metronidazole gel (0.75%) in Japanese patients with rosacea: a randomized, vehicle-controlled, phase 3 study. J Dermatol. 2022;49(3):330–340. doi:10.1111/1346-8138.16254
40. Leyden JJ. Efficacy of a novel rosacea treatment system: an investigator-blind, randomized, parallel-group study. J Drugs Dermatol. 2011;10(10):1179–1185.
41. Logger JGM, Olydam JI, Driessen RJB. Use of beta-blockers for rosacea-associated facial erythema and flushing: a systematic review and update on proposed mode of action. J Am Acad Dermatol. 2020;83(4):1088–1097. doi:10.1016/j.jaad.2020.04.129
42. Bernstein EF, Schomacker K, Paranjape A, Jones CJ. Pulsed dye laser treatment of rosacea using a novel 15 mm diameter treatment beam. Lasers Surg Med. 2018;50(8):808–812. doi:10.1002/lsm.22819
43. Liu A, Moy RL, Ross EV, Hamzavi I, Ozog DM. Pulsed dye laser and pulsed dye laser–mediated photodynamic therapy in the treatment of dermatologic disorders. Dermatol Surg. 2012;38(3):351–366. doi:10.1111/j.1524-4725.2011.02293.x
44. Goldman MP, Eckhouse S. Photothermal sclerosis of leg veins. ESC medical systems, LTD photoderm VL Cooperative Study Group. Dermatol Surg. 1996;22(4):323–330. doi:10.1111/j.1524-4725.1996.tb00325.x
45. Kim BY, Moon H-R, Ryu HJ. Comparative efficacy of short-pulsed intense pulsed light and pulsed dye laser to treat rosacea. J Cosmet Laser Ther. 2019;21(5):291–296. doi:10.1080/14764172.2018.1528371
46. Tirico M, Jensen D, Green C, Ross EV. Short pulse intense pulsed light versus pulsed dye laser for the treatment of facial redness. J Cosmet Laser Ther. 2020;22(2):60–64. doi:10.1080/14764172.2020.1717540
47. Tanghetti EA, Goldberg DJ, Dover JS, et al. Oxymetazoline and energy-based therapy in patients with Rosacea: evaluation of the safety and tolerability in an open-label, interventional study. Lasers Surg Med. 2021;53(1):55–65. doi:10.1002/lsm.23253
48. Suggs AK, Macri A, Richmond H, Munavalli G, Friedman PM. Treatment of erythematotelangiectatic rosacea with pulsed-dye laser and oxymetazoline 1.0% cream: a retrospective study. Lasers Surg Med. 2020;52(1):38–43. doi:10.1002/lsm.23176
49. Kircik LH, DuBois J, Draelos ZD, et al. Pivotal trial of the efficacy and safety of oxymetazoline cream 1.0% for the treatment of persistent facial erythema associated with rosacea: findings from the first REVEAL trial. J Drugs Dermatol. 2018;17(1):97–105.
50. Draelos ZD, Gold MH, Weiss RA, et al. Efficacy and safety of oxymetazoline cream 1.0% for treatment of persistent facial erythema associated with rosacea: findings from the 52-week open label REVEAL trial. J Am Acad Dermatol. 2018;78(6):1156–1163.
51. DuBois J, Dover JS, Jones TM, Weiss RA, Berk DR, Ahluwalia G. Phase 2 randomized, dose-ranging study of oxymetazoline cream for treatment of persistent facial erythema associated with rosacea. J Drugs Dermatol. 2018;17(3):308–316.
52. Sharquie KE, Najim RA, Al‐Salman HN. Oral zinc sulfate in the treatment of rosacea: a double-blind, placebo-controlled study. Int J Dermatol. 2006;45(7):857–861. doi:10.1111/j.1365-4632.2006.02944.x
53. Bamford JT, Gessert CE, Haller IV, Kruger K, Johnson BP. Randomized, double-blind trial of 220 mg zinc sulfate twice daily in the treatment of rosacea. Int J Dermatol. 2012;51(4):459–462. doi:10.1111/j.1365-4632.2011.05353.x
54. Al-Niaimi F, Glagoleva E, Araviiskaia E. Pulsed dye laser followed by intradermal botulinum toxin type-A in the treatment of rosacea-associated erythema and flushing. Dermatol Ther. 2020;33(6):e13976. doi:10.1111/dth.13976
55. Fowler JJ, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12(6):650–656.
56. Jackson JM, Knuckles M, Minni JP, Johnson SM, Belasco KT. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;529–538. doi:10.2147/CCID.S58920
57. Jackson JM, Fowler J, Moore A, et al. Improvement in facial erythema within 30 minutes of initial application of brimonidine tartrate in patients with rosacea. J Drugs Dermatol. 2014;13(6):699–704.
58. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13(1):56–61.
59. Scharschmidt TC, Yost JM, Truong SV, Steinhoff M, Wang KC, Berger TG. Neurogenic rosacea: a distinct clinical subtype requiring a modified approach to treatment. Arch Dermatol. 2011;147(1):123–126. doi:10.1001/archdermatol.2010.413
60. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69(3):438–443. doi:10.1016/j.jaad.2013.04.015
61. Seo H-M, Kim J-I, Kim H-S, Choi Y-J, Kim W-S. Prospective comparison of dual wavelength long-pulsed 755-nm alexandrite/1064-nm neodymium:yttrium-aluminum-garnet laser versus 585-nm pulsed dye laser treatment for rosacea. Ann Dermatol. 2016;28(5):607–614. doi:10.5021/ad.2016.28.5.607
62. Lev-Tov H, Rill JS, Liu G, Kirby JS. Trends in utilization of topical medications for treatment of rosacea in the United States (2005–2014): a cohort analysis. J Am Acad Dermatol. 2019;80(4):1135–1137. doi:10.1016/j.jaad.2018.09.039
63. Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30(2):158–169. doi:10.1080/09546634.2018.1473554
64. Sarac G. A comparison of the efficacy and tolerability of topical agents used in facial demodex treatment. J Cosmet Dermatol. 2019;18(6):1784–1787. doi:10.1111/jocd.12986
65. Kumar A, Chiou A, Shih Y, Li S, Chang A. An exploratory, open-label, investigator-initiated study of interleukin-17 blockade in patients with moderate-to-severe papulopustular rosacea. Br J Dermatol. 2020;183(5):942–943. doi:10.1111/bjd.19172
66. Dover JS, Arndt KA. New approaches to the treatment of vascular lesions. Lasers Surg Med. 2000;26(2):158–163. doi:10.1002/(sici)1096-9101(2000)26:2<158::aid-lsm6>3.0.co;2-o
67. Üstüner P, Balevi A, Özdemir M. The comparison of the efficacy and safety of q-switched potassium titanyl phosphate laser and long-pulsed neodymium-doped yttrium aluminum garnet laser in the treatment of erythematotelangiectatic and papulopustular rosacea. Turk J Dermatol. 2018;12(2):90–95. doi:10.4274/tdd.3535
68. Schaller M, Kemény L, Havlickova B, et al. A randomized phase 3b/4 study to evaluate concomitant use of topical ivermectin 1% cream and doxycycline 40-mg modified-release capsules, versus topical ivermectin 1% cream and placebo in the treatment of severe rosacea. J Am Acad Dermatol. 2020;82(2):336–343. doi:10.1016/j.jaad.2019.05.063
69. Xu L, Yao B, Xu T, Huang H. Assessment of the efficacy and safety of 30% supramolecular salicylic acid peeling for papulopustular rosacea treatment. Indian J Dermatol. 2022;67(5):625. doi:10.4103/ijd.ijd_353_21
70. Wang L, Li XH, Wen X, et al. Retrospective analysis of 19 papulopustular rosacea cases treated with oral minocycline and supramolecular salicylic acid 30% chemical peels. Exp Ther Med. 2020;20(2):1048–1052. doi:10.3892/etm.2020.8740
71. Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68(5):875–876. doi:10.1016/j.jaad.2012.11.038
72. Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75(3):e113–e115. doi:10.1016/j.jaad.2016.01.059
73. Choi JE, Werbel T, Wang Z, Wu CC, Yaksh TL, Di Nardo A. Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J Dermatological Sci. 2019;93(1):58–64. doi:10.1016/j.jdermsci.2018.12.004
74. Park KY, Hyun MY, Jeong SY, Kim BJ, Kim MN, Hong CK. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230(4):299–301. doi:10.1159/000368773
75. Fowler JF
76. Reitamo S, Remitz A, Kyllönen H, Saarikko J. Topical noncorticosteroid immunomodulation in the treatment of atopic dermatitis. Am J Clin Dermatol. 2002;3(6):381–388. doi:10.2165/00128071-200203060-00002
77. Wang B, Yuan X, Huang X, et al. Efficacy and safety of hydroxychloroquine for treatment of patients with rosacea: a multicenter, randomized, double-blind, double-dummy, pilot study. J Am Acad Dermatol. 2021;84(2):543–545. doi:10.1016/j.jaad.2020.05.050
78. Oussedik E, Bourcier M, Tan J. Psychosocial burden and other impacts of rosacea on patients’ quality of life. Dermatol Clin. 2018;36(2):103–113. doi:10.1016/j.det.2017.11.005
79. Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12(6):664–667.
80. Proietti I, Skroza N, Potenza C. Improving skin quality in patients with dermatologic conditions using the hyaluronic acid filler VYC-12. G Ital Dermatol Venereol. 2020;2020:06453.
81. Gariboldi S, Palazzo M, Zanobbio L, et al. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of β-defensin 2 via TLR2 and TLR4. J Immunol. 2008;181(3):2103–2110. doi:10.4049/jimmunol.181.3.2103
82. Leyden JJ. Randomized, phase 2, dose-ranging study in the treatment of rosacea with encapsulated benzoyl peroxide gel. J Drugs Dermatol. 2014;13(6):685–688.
83. Abramovits W, Jessu R, Vincent KD, Gupta AK. Epsolay™ (Microencapsulated Benzoyl Peroxide 5%) cream for the topical treatment of rosacea. Skinmed. 2023;21(2):110–111.
84. Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. doi:10.1016/j.pharmthera.2014.03.003
85. Modi S, Harting M, Rosen T. Azithromycin as an alternative rosacea therapy when tetracyclines prove problematic. J Drugs Dermatol. 2008;7(9):898–899.
86. Korting H, Schöllmann C. Current topical and systemic approaches to treatment of rosacea. J Eur Acad Dermatol Venereol. 2009;23(8):876–882. doi:10.1111/j.1468-3083.2009.03167.x
87. Kim J-H, Oh YS, Choi EH. Oral azithromycin for treatment of intractable rosacea. J Korean Med Sci. 2011;26(5):694–696. doi:10.3346/jkms.2011.26.5.694
88. Bakar Ö, Demirçay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43(2):151–154. doi:10.1111/j.1365-4632.2004.01958.x
89. Akhyani M, Ehsani AH, Ghiasi M, Jafari AK. Comparison of efficacy of azithromycin vs doxycycline in the treatment of rosacea: a randomized open clinical trial. Int J Dermatol. 2008;47(3):284–288. doi:10.1111/j.1365-4632.2008.03445.x
90. Torresani C, Pavesi A, Manara GC. Clarithromycin versus doxycycline in the treatment of rosacea. Int J Dermatol. 1997;36(12):942–946. doi:10.1046/j.1365-4362.1997.00301.x
91. van Zuuren EJ, Fedorowicz Z, Carter B, van der Linden MM, Charland L. Interventions for rosacea. Cochrane Database Syst Rev. 2015;2015(4):Cd003262. doi:10.1002/14651858.CD003262.pub5
92. Stein L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13(3):316–323.
93. Taieb A, Ortonne J, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0· 75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172(4):1103–1110. doi:10.1111/bjd.13408
94. Husein-ElAhmed H, Steinhoff M. Efficacy of topical ivermectin and impact on quality of life in patients with papulopustular rosacea: a systematic review and meta-analysis. Dermatol Ther. 2020;33(1):e13203. doi:10.1111/dth.13203
95. Kwon HH, Jung JY, Lee WY, Bae Y, Park GH. Combined treatment of recalcitrant papulopustular rosacea involving pulsed dye laser and fractional microneedling radiofrequency with low-dose isotretinoin. J Cosmet Dermatol. 2020;19(1):105–111. doi:10.1111/jocd.12982
96. Del Rosso JQ, Brantman S, Baldwin H. Long-term inflammatory rosacea management with subantibiotic dose oral doxycycline 40 mg modified-release capsules once daily. Dermatol Ther. 2022;35(1):e15180. doi:10.1111/dth.15180
97. Valentín S, Morales A, Sánchez JL, Rivera A. Safety and efficacy of doxycycline in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2009;2:129–140. doi:10.2147/ccid.s4296
98. Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75(4 Suppl):6–11.
99. Del Rosso JQ. Anti-inflammatory dose doxycycline in the treatment of rosacea. J Drugs Dermatol. 2009;8(7):664–668.
100. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. doi:10.1016/j.jaad.2006.11.021
101. Del Rosso JQ, Baum EW. Comprehensive medical management of rosacea: an interim study report and literature review. J Clin Aesthet Dermatol. 2008;1(1):20.
102. van der Linden MMD, van Ratingen AR, van Rappard DC, Nieuwenburg SA, Spuls PI. DOMINO, doxycycline 40 mg vs minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176(6):1465–1474. doi:10.1111/bjd.15155
103. Madan V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161(4):814–818. doi:10.1111/j.1365-2133.2009.09317.x
104. Gollnick H, Blume-Peytavi U, Szabó EL, et al. Systemic isotretinoin in the treatment of rosacea – doxycycline- and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8(7):505–514. doi:10.1111/j.1610-0387.2010.07345.x
105. Opitz DL, Tyler KF. Efficacy of azithromycin 1% ophthalmic solution for treatment of ocular surface disease from posterior blepharitis. Clin Exp Optom. 2011;94(2):200–206. doi:10.1111/j.1444-0938.2010.00540.x
106. Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Article. Cornea. 2013;32(1):44–53. doi:10.1097/ICO.0b013e318254205f
107. Yildiz E, Yenerel NM, Turan-Yardimci A, Erkan M, Gunes P. Comparison of the clinical efficacy of topical and systemic azithromycin treatment for posterior blepharitis. Article. J Ocul Pharmacol Ther. 2018;34(4):365–372. doi:10.1089/jop.2017.0095
108. Zandian M, Rahimian N, Soheilifar S. Comparison of therapeutic effects of topical azithromycin solution and systemic doxycycline on posterior blepharitis. Article. Int J Ophthalmol. 2016;9(7):1016–1019. doi:10.18240/ijo.2016.07.14
109. Pfeffer I, Borelli C, Zierhut M, Schaller M. Treatment of ocular rosacea with 40 mg doxycycline in a slow release form. J Dtsch Dermatol Ges. 2011;9(11):904–907. doi:10.1111/j.1610-0387.2011.07723.x
110. Guarrera M, Parodi A, Cipriani C, Divano C, Rebora A. Flushing in rosacea: a possible mechanism. Arch Dermatol Res. 1982;272(3–4):311–316. doi:10.1007/bf00509061
111. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;9:Cd004923. doi:10.1002/14651858.CD004923.pub2
112. Sahni DR, Feldman SR, Taylor SL. Ivermectin 1% (CD5024) for the treatment of rosacea. Expert Opin Pharmacother. 2018;19(5):511–516. doi:10.1080/14656566.2018.1447562
113. Elewski BE, Fleischer AB, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139(11):1444–1450. doi:10.1001/archderm.139.11.1444
114. Katz B, Patel V. Photodynamic therapy for the treatment of erythema, papules, pustules, and severe flushing consistent with rosacea. J Drugs Dermatol. 2006;5(2 Suppl):6–8.
115. Sanchez J, Somolinos AL, Almodóvar PI, Webster G, Bradshaw M, Powala C. A randomized, double-blind, placebo-controlled trial of the combined effect of doxycycline hyclate 20-mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. J Am Acad Dermatol. 2005;53(5):791–797. doi:10.1016/j.jaad.2005.04.069
116. Engin B, Özkoca D, Kutlubay Z, Serdaroğlu S. Conventional and novel treatment modalities in rosacea. Clin Cosmet Investig Dermatol. 2020;13:179–186. doi:10.2147/ccid.S194074
117. Schweinzer K, Kofler L, Spott C, et al. Surgical treatment of rhinophyma: experience from a German cohort of 70 patients. Eur J Dermatol. 2017;27:281–285. doi:10.1684/ejd.2017.2987
118. Stone DU, Chodosh J. Ocular rosacea: an update on pathogenesis and therapy. Curr Opin Ophthalmol. 2004;15(6):499–502. doi:10.1097/01.icu.0000143683.14738.76
119. Tanzi EL, Weinberg JM. The ocular manifestations of rosacea. Cutis. 2001;68(2):112–114.
120. Barnhorst DA
121. Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33(3):447–455. doi:10.1016/j.det.2015.03.009
122. Walsh RK, Endicott AA, Shinkai K. Diagnosis and treatment of rosacea fulminans: a comprehensive review. Am J Clin Dermatol. 2018;19:79–86. doi:10.1007/s40257-017-0310-0
123. Wollina U, Jelin AC, Thiet M-P. Recent advances in the understanding and management of rosacea. F1000prime Rep. 2014;6:6. doi:10.12703/P6-6
124. Edwards DI. Reduction of nitroimidazoles in vitro and DNA damage. Biochem Pharmacol. 1986;35(1):53–58. doi:10.1016/0006-2952(86)90554-x
125. Edwards DI. Nitroimidazole drugs--action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31(1):9–20. doi:10.1093/jac/31.1.9
126. Nielsen PG. Treatment of rosacea with 1% metronidazole cream. A double-blind study. Br J Dermatol. 1983;108(3):327–332. doi:10.1111/j.1365-2133.1983.tb03972.x
127. Sauder D, Miller R, Gratton D, Danby W, Griffiths C, Phillips S. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8(2):79–85. doi:10.3109/09546639709160276
128. Torok HM, Webster G, Dunlap FE, Egan N, Jarratt M, Stewart D. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75(6):357–363.
129. Schechter BA, Katz RS, Friedman LS. Efficacy of topical cyclosporine for the treatment of ocular rosacea. Adv Ther. 2009;26:651–659. doi:10.1007/s12325-009-0037-2
130. Arman A, Demirseren DD, Takmaz T. Treatment of ocular rosacea: comparative study of topical cyclosporine and oral doxycycline. Int J Ophthalmol. 2015;8(3):544–549. doi:10.3980/j.issn.2222-3959.2015.03.19
131. Marchitto MC, Chien AL. Mast cell stabilizers in the treatment of rosacea: a review of existing and emerging therapies. Dermatol Ther. 2021;11(5):1541–1549. doi:10.1007/s13555-021-00597-7
132. Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharmacal Res. 2013;36:237–251. doi:10.1007/s12272-013-0057-y
133. Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108–113. doi:10.1016/j.coph.2005.11.003
134. Parkins G, Maan A, Dawn G. Neurogenic rosacea: an uncommon and poorly recognized entity?. Clin Exp Dermatol. 2015;40(8):930–931. doi:10.1111/ced.12630
135. Hayran Y, Şen O, Fırat Oğuz E, et al. Serum IL-17 levels in patients with rosacea. J Cosmet Dermatol. 2022;21(3):1147–1153. doi:10.1111/jocd.14169
136. Zhang H, Tang K, Wang Y, Fang R, Sun Q. Rosacea treatment: review and update. Dermatol Ther. 2021;11:13–24. doi:10.1007/s13555-020-00461-0
137. Gold LS, Papp K, Lynde C, et al. Treatment of rosacea with concomitant use of topical ivermectin 1% cream and brimonidine 0.33% gel: a randomized, vehicle-controlled study. J Drugs Dermatol. 2017;16(9):909–916.
138. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6(6):348–354.
139. Baldwin HE, Harper J, Baradaran S, Patel V. Erythema of rosacea affects health-related quality of life: results of a survey conducted in collaboration with the National Rosacea Society. Dermatol Ther. 2019;9:725–734. doi:10.1007/s13555-019-00322-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.