Back to Journals » OncoTargets and Therapy » Volume 12
Role Of Robot-Assisted Partial Nephrectomy For Renal Cell Carcinomas In The Purpose Of Nephron Sparing
Authors Shao IH , Kan HC, Liu CY , Lin PH , Yu KJ , Pang ST, Wu CT, Chuang CK , Chang YH
Received 1 May 2019
Accepted for publication 10 September 2019
Published 4 October 2019 Volume 2019:12 Pages 8189—8196
DOI https://doi.org/10.2147/OTT.S214060
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
I-Hung Shao, 1, 2 Hung-Cheng Kan, 1, 2 Chung-Yi Liu, 1, 2 Po-Hung Lin, 1, 2 Kai-Jie Yu, 1, 2 See-Tong Pang, 1, 2 Chun-Te Wu, 3 Cheng-Keng Chuang, 1, 2 Ying-Hsu Chang 1, 2
1Division of Urology, Department of Surgery, LinKou Chang Gung Memorial Hospital, Taoyuan, Taiwan; 2College of Medicine, Chang Gung University, Taoyuan, Taiwan; 3Division of Urology, Department of Surgery, KeeLung Chang Gung Memorial Hospital, KeeLung, Taiwan
Correspondence: Cheng-Keng Chuang; Ying-Hsu Chang
Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, 5 Fu-Shing Street, Kweishan, Taoyuan 333, Taiwan
Tel +886-3-3281200 Ext 2103
Fax +886-3-3285818
Email [email protected]; [email protected]
Introduction: Surgery remains the standard treatment for localized renal cell carcinomas, and partial nephrectomy is considered before radical nephrectomy with the aim of preserving renal function. This study aimed to compare robot-assisted and open partial nephrectomy for the purpose of nephron sparing.
Materials and methods: We retrospectively enrolled consecutive patients who received partial nephrectomy at a single tertiary medical center from January 2008 to January 2015. Medical records and radiographic images were reviewed. We analyzed the patients’ general characteristics, underlying disease, complications, length of hospital stay, renal tumor complexity, surgery type, renal function, and specimen and tumor size. A comparison between open and robot-assisted nephrectomy groups was performed.
Results: A total of 136 patients were enrolled, with a male to female ratio of 2:3 and a mean age of 57.8 years. Of these, 71 and 65 patients received open and robot-assisted surgery, respectively. Compared with the open group, patients who underwent robot-assisted surgery were significantly younger (56.0 versus 60.1 years old), had a longer operative time (303 versus 224 min), and a lower kidney ischemic time (33.4 versus 46.9 min). Given similar tumor sizes, the tumor-to-excision ratio was significantly higher in the robot-assisted group (51.7% versus 39.8%), and the excisional volume loss (EVL) was smaller (12.7 versus 19.6 mL). Preoperative glomerular filtration rate and EVL were significant predictors of long-term renal function preservation in the multivariate analysis.
Conclusion: When performing partial nephrectomy, a robot-assisted procedure could increase the accuracy of excision without increasing the risk of positive surgical margin. Lower EVL could assist in better long-term postoperative renal function preservation.
Keywords: renal cell carcinoma, robotic-assisted system, partial nephrectomy, excisional volume loss, nephron sparing, renal function preservation
Corrigendum for this paper has been published
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all adult malignancies and is one of the most lethal urological cancers. Since the 1970s, radiographic imaging, including ultrasonography and computed tomography, has not only increased the RCC detection frequency1,2 but also aided early detection of asymptomatic or incidental localized RCCs.3,4
Surgery, the standard curative treatment for localized RCCs, involves the excision of the entire tumor with an adequate surgical margin. Radical nephrectomy (RN) was once widely performed in patients with localized renal tumors suspected or confirmed to be malignancy, even including T1-stage tumors. However, multiple management strategies in addition to RN are currently available; these include partial nephrectomy (PN), thermal ablation, and active surveillance. PN currently considered a standard treatment for localized renal tumors.5–8
PN is preferred over RN for small tumors or other feasible cases9–11 because the post-RN chronic kidney disease (CKD) risk is high, as reported in several longitudinal follow-up studies. These include a landmark study by the Memorial Sloan Kettering Cancer Center,12 in which Huang et al. studied 662 patients with small solitary renal tumors whose serum creatinine level and opposite kidney were normal. Nearly one-quarter of these patients had preexisting grade 3 CKD, with a 3-year probability of no new onset of grade 3 or higher CKD of 80% after PN and 35% after RN.
CKD is associated with cardiovascular events and death, with their relative risk increasing with upstaging of CKD.13 The favorable outcomes of PN, such as reduced risk of CKD and overall mortality, in the treatment of T1-stage renal tumors have been confirmed.14 Moreover, in some patients, the oncological outcomes of PN can be equivalent to those of RN.5
Minimally invasive approaches to perform PN have provided encouraging results.15,16 Laparoscopic and robot-assisted PN (RPN) appear to have an equivalent margin status and oncological outcomes compared with open PN (OPN), provided the surgeon is experienced and patient selection is sensible.16–18
Main goal of this study is to compare renal function preservation between OPN and RPN, and to further find the possible predictors. We also compared the surgical and oncological outcome in these two groups. compared surgical and oncological outcomes and renal function changes after open and robot-assisted PN and identified factors that can predict consequent renal function preservation.
Materials And Methods
Patients
We conducted this retrospective study of consecutive patients who received PN at a single tertiary medical center from January 2008 to January 2015. We reviewed the medical charts and radiographic images of patients diagnosed as having RCC and were eligible for PN according to evaluation by our uro-oncology team. Patients who decided to receive PN (either OPN or RPN) after discussion with the surgeons were enrolled. The benefits and risks of OPN and RPN were both explained and discussed with the patients in the outpatient department. The choice between OPN and RPN depended on the patients’ preference. In OPN, we routinely used cold ischemia during tumor resection. Before standard renorrhaphy with renal parenchyma primary closure, we use small-size suture (for example, dexon 4-0) to perform collecting system repair in possible defect and all visible cut surface of vessels in the parenchymal incisional plane. In RPN group, collecting system repair is only performed in obvious defect, and then primary closure of renal parenchyma was done.
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. The patient consent to review their medical records was waived by the Institutional Review Board of Chang Gung Memorial Hospital due to retrospective study. The patient data confidentiality and compliance fulfilled the Declaration of Helsinki, which is approved by the board.
Data Collection
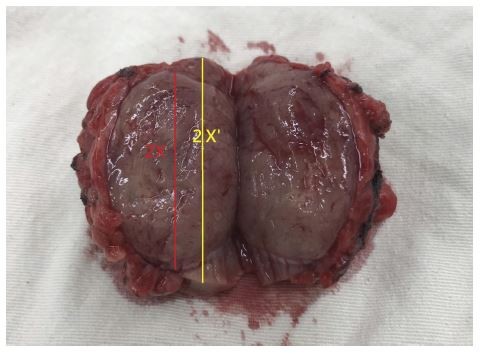
General preoperative characteristics, including sex, age, PADUA score, RENAL score, tumor side and stage, diabetes mellitus, hypertension, serum creatinine concentration, and estimated glomerular filtration rate (eGFR), were recorded. Surgical characteristics, including surgical methods, operation time, blood loss, kidney ischemic time, major perioperative complications (defined as Clavien-Dindo Classification ≥ grade III), hospital stay, and surgical margin status, were also collected. We calculated the whole excisional specimen size and actual tumor volume based on the measurements reported by pathologists. The estimated volume was calculated by the formula for an ellipsoid 4πXYZ/3 (actual tumor volume) and 4πX′Y′Z′/3 (excisional volume), respectively (Figures 1 and 2). We then defined the tumor-to-excision ratio as (actual tumor volume/excisional volume) and excisional volume loss (EVL) as (excisional volume − actual tumor volume).
 |
Figure 1 Illustration of actual tumor volume, excisional volume, and resection ratio. |
 |
Figure 2 Photograph of specimen to illustrate the measurement of length. |
Renal function was measured as the serum creatinine levels immediately post-operation (within 1 week of surgery) and 3 and 12 postoperative months. Renal function change was presented as the glomerular filtration rate (GFR) preservation (GFR-P), given by (eGFR at third postoperative month) or (eGFR at 12th postoperative month/preoperative eGFR).
Statistical Analyses
We compared general and surgical characteristics between open and robot-assisted PN groups and analyzed factors possibly affecting short- and long-term renal function deterioration. The chi-square and independent t-tests were used to examine associations between nominal and continuous variables, respectively. We regarded p values of less than 0.05 as significant. All statistical analyses were performed using IBM SPSS Statistics (version 22).
Results
A total of 136 patients were included, all of whom were diagnosed as having RCC and received PN for tumor excision. The mean age was 57.8 years, with a male-to-female ratio of 2:3. The average PADUA and RENAL scores were 8.38 and 6.93, respectively. T1a- and T1b-stage tumors accounted for 78% and 19% of the cases, respectively, whereas T2a or higher–stage tumors accounted for only 3% of the cases.
Open and robot-assisted PN was administered to 71 (52.2%) and 65 (47.8%) patients, respectively. The average surgical time was 261 mins, with an average ischemic time of 39.1 mins. The average excisional volume and actual-tumor volumes were 34.4 and 18.6 mL, respectively; thus, the average tumor-to-excisional ratio was 45.5%. Detailed general and surgery-related characteristics are listed in Table 1.
 |
Table 1 Patients’ General Characteristics |
Patients in the open group were significantly older than those in the robot-assisted group (60.1 versus 56.0 years) and had a shorter surgical time (224.3 versus 302.5 mins) and longer kidney ischemic time (46.9 versus 33.4 mins). The actual tumor volume was similar in both the open and robotic-assisted groups (18.8 ± 36.4 and 18.3 ± 31.9 mL, respectively; p = 0.943); however, the tumor-to-excision ratio was significantly lower in the open group (39.8% versus 51.7%; p = 0.001), thus resulting in a significantly smaller EVL in the robot-assisted group. A detailed comparison of parameters between the open and robot-assisted groups is listed in Table 2.
 |
Table 2 Comparison Of Patients Receiving Robotic-Assisted Or Open Partial Nephrectomy |
Table 3 lists parameters possibly correlated with the tumor-to-excision ratio. In the univariate analysis, robot-assisted PN, higher PADUA score, higher preoperative GFR, and larger actual tumor volume were significantly correlated with a higher tumor-to-excision ratio, whereas in the multivariate analysis, only robot-assisted PN and larger actual tumor volume were significant predictors of a higher ratio. Tumor-to- excision ratio was not significantly correlated with positive surgical margin (PSM).
 |
Table 3 Factors Correlated To Tumor-To-Excision Ratio |
Renal function change was defined using GFR-P. Predictors for GFR-P 3 and 12 months after PN are listed in Table 4. In the univariate analysis, robot-assisted surgery, PADUA score, RENAL score, preoperative GFR, and EVL were significant predictors for GFR-P 3 months after PN, whereas only robot-assisted surgery and preoperative GFR remained significant predictors in the multivariate analysis. At 12 months after PN, PADUA score, RENAL score, preoperative GFR, excisional volume, and EVL were significant predictors of GFR-P, whereas they were only preoperative GFR and EVL in the multivariate analysis.
 |
Table 4 Predictors For GFR-P |
Figure 3 illustrates the trend of GFR and GFR-P preoperatively, immediately postoperatively, and at 3 and 12 months postoperatively in all patients (Figure 3A and B). The trend of GFR and GFR-P in subgroups with an EVL of ≥16 mL or <16 mL is illustrated in Figure 3C and D.
 |
Figure 3 (A-B) The trend of GFR and GFR-P before and after partial nephrectomy in all patients. (C-D) The trend of GFR and GFR-P in patients with an EVL of ≥16 mL or <16 mL. |
Discussion
PN is currently the gold standard for surgical treatment of patients with small renal tumors. With equivalent oncological outcomes, the key benefits of PN are preserving renal function and limiting the risks of CKD, cardiovascular morbidity, and death.13,19 A large proportion of PN is performed using a minimally invasive approach,20 even in patients with complex renal masses,21 and this trend is increasing.8
During PN, nephron injury can result from a prolonged warm ischemia time, sacrificed benign parenchyma during surgical excision, or iatrogenic injury during reconstruction.22 Lower accuracy of surgical excision during PN may contribute to long-term renal dysfunction. Srinath et al demonstrated that non-neoplastic parenchyma volume removed in PN (i.e., EVL) was associated with the upstaging of CKD.23 In our study, higher EVL was a significant predictor for lower long-term renal function preservation in multivariate analysis; however, it was not associated with the short-term outcome significantly. Similarly, ischemic time was not associated with renal function preservation—consistent with findings that EVL is the predominant factor affecting ultimate renal function.24,25
In theory, minimizing the gap between the surgical and actual-tumor margins can considerably reduce as much EVL as possible and thus preserve renal function. Thompson reported that a 5% increase in preserved renal volume could reduce the risk of stage-4 CKD by 17%.26 This result supports the currently prevalent use of a closer surgical resection margin, but increase PSM risk.
The accuracy of tumor excision during PN can be presented as the tumor-to-excision ratio. This study demonstrated that factors associated with excision accuracy were robot-assisted surgery, excisional and tumor volume, and tumor complexity. With a similar tumor size and tumor complexity, excision accuracy was higher in the robot-assisted group than in the open group, resulting in significantly lower EVL. Neither robot-assisted surgery nor tumor-to- excision ratio increased PSM risk.
Higher excision accuracy could be explained by the advantages of the robot-assisted system (da Vinci Surgical System, Intuitive Surgical). In addition to the general benefits of minimally invasive surgery, a robot-assisted system can provide increased magnification, precise camera control, seven degrees of freedom, tremor filtration, and three-dimensional vision. These advantages can be particularly useful when the tumor location is difficult to access or when performing resection and renorrhaphy. Intraoperative ultrasonography is more frequently used to identify the depth and location of tumors, particularly in endophytic or small tumors. The benefits of a robot-assisted system can help clinicians perform more precise resection and preserve as much healthy renal parenchyma as possible.
Parenchyma quality may be an essential factor affecting renal function, although there may be a difference between CKD resulting from medical causes (CKD-M) or from surgery (CKD-S). In a large population study, the mean annual decline in the renal function was 4.7% in patients with CKD-M compared with that of only 0.7% in patients with CKD-S. However, because parenchyma quality is a nonmodifiable factor, clinicians have focused more on the leading potentially modifiable factor, namely EVL.24
Toshio reported that a solitary kidney significantly affects excision accuracy when performing PN but without increasing PSM risk.27 This finding indicates that EVL can be minimized through the surgeon’s caution and the use of instruments, regardless of tumor size or complexity.
A limitation of this study is its retrospective design and relatively small sample size. Although we demonstrated the possible benefits of a robot-assisted system for surgical precision during PN, the results still require confirmation in a prospective large-scale study. Examination of longer-term renal function trends also requires a longer follow-up period.
Conclusion
PN has become the mainstay for treatment of small renal tumors. Its main advantage is nephron preservation, thus decreasing the risk of CKD development or upstaging. Clinicians should make every effort to minimize nephron injury during surgery, including lower EVL and shorter ischemic time. A robot-assisted PN system not only can ensure minimal postoperative discomfort, shorter hospital stays, and more rapid recovery compared with open surgery but also may improve excision accuracy during PN and thus preserve more healthy parenchyma without affecting the surgical margin. Lower EVL represents the only major modifiable predictor for better renal function preservation after PN.
Acknowledgments
We would like to thank Research Services Center for Health Information, Chang Gung University, Taoyuan, Taiwan, for the funding: CMRP-MK 103 No: CIRPD1D0031 and CMRP-MK 104 No: CIRPD1D0032. CORPG3F0291 Kidney Cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Decastro GJ, McKiernan JM. Epidemiology, clinical staging, and presentation of renal cell carcinoma. Urol Clin North Am. 2008;35(4):
2. Kummerlin IP, Ten Kate FJ, Wijkstra H, et al. Changes in the stage and surgical management of renal tumours during 1995–2005: an analysis of the Dutch national histopathology registry. BJU Int. 2008;102(8):946–951. Epub 02008 Jun 07728. doi:10.1111/j.1464-1410X.2008.07770.x
3. Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166(5):1611–1623.
4. Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113(1):78–83. doi:10.1002/cncr.23518
5. Van Poppel H, Becker F, Cadeddu JA, et al. Treatment of localised renal cell carcinoma. Eur Urol. 2011;60(4):662–672. Epub 2011 Jun 1029. doi:10.1016/j.eururo.2011.1006.1040
6. Volpe A, Cadeddu JA, Cestari A, et al. Contemporary management of small renal masses. Eur Urol. 2011;60(3):501–515. Epub 2011 Jun 1011. doi:10.1016/j.eururo.2011.1005.1044
7. Kim SP, Thompson RH. Approach to the small renal mass: to treat or not to treat. Urol Clin North Am. 2012;39(2):171–179. doi:10.1016/j.ucl.2012.01.003
8. Chang YH, Chang SW, Liu CY, et al. Demographic characteristics and complications of open and minimally invasive surgeries for renal cell carcinoma: a population-based case-control study in Taiwan. Ther Clin Risk Manag. 2018;14:1235–1241. eCollection 162018. doi:10.2147/TCRM.S164592
9. Nakada SY. Surgical removal of small renal tumors–going, going, gone? J Urol. 2005;174(1):9. doi:10.1097/1001.ju.0000167236.0000190062.0000167295
10. Nguyen CT, Campbell SC, Novick AC. Choice of operation for clinically localized renal tumor. Urol Clin North Am. 2008;35(4):
11. Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–1279. Epub 2009 Aug 1214. doi:10.1016/j.juro.2009.1207.1004
12. Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–740. doi:10.1016/S1470-2045(1006)70803-70808
13. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi:10.1056/NEJMoa041031
14. MacLennan S, Imamura M, Lapitan MC, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61(5):972–993. Epub 2012 Feb 1025. doi:10.1016/j.eururo.2012.1002.1039
15. Gill IS. Towards the ideal partial nephrectomy. Eur Urol. 2012;62(6):
16. Lane BR, Golan S, Eggener S, et al. Differential use of partial nephrectomy for intermediate and high complexity tumors may explain variability in reported utilization rates. J Urol. 2013;189(6):2047–2053. Epub 2013 Jan 2049. doi:10.1016/j.juro.2013.2001.2007
17. Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177(1):
18. Mullins JK, Feng T, Pierorazio PM, et al. Comparative analysis of minimally invasive partial nephrectomy techniques in the treatment of localized renal tumors. Urology. 2012;80(2):316–321. Epub 2012 Jun 1013. doi:10.1016/j.urology.2012.1003.1043
19. Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4–7 cm. BJU Int. 2006;97(5):939–945. doi:10.1111/j.1464-1410X.2006.06060.x
20. Ghani KR, Sukumar S, Sammon JD, Rogers CG, Trinh Q-D, Menon M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: results from the nationwide inpatient sample. J Urol. 2014;191(4):907–912. Epub 2013 Oct 1029. doi:10.1016/j.juro.2013.1010.1099
21. Borghesi M, Schiavina R, Gan M, et al. Expanding utilization of robotic partial nephrectomy for clinical T1b and complex T1a renal masses. World J Urol. 2013;31(3):499–504. Epub 02013 May 00345. doi:10.1007/s00345-00013-01095-00342
22. Mir MC, Ercole C, Takagi T, et al. Decline in renal function after partial nephrectomy: etiology and prevention. J Urol. 2015;193(6):1889–1898. Epub 2015 Jan 1829. doi:10.1016/j.juro.2015.1801.1093
23. Kotamarti S, Rothberg MB, Danzig MR, et al. Increasing volume of non-neoplastic parenchyma in partial nephrectomy specimens is associated with chronic kidney disease upstaging. Clin Genitourin Cancer. 2015;13(3):239–243. Epub 2014 Nov 1015. doi:10.1016/j.clgc.2014.1011.1005
24. Simmons MN, Fergany AF, Campbell SC. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol. 2011;186(2):405–410. Epub 2011 Jun 1015. doi:10.1016/j.juro.2011.1003.1154
25. Mir MC, Campbell RA, Sharma N, et al. Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology. 2013;82(2):263–268. Epub 2013 Jun 1020. doi:10.1016/j.urology.2013.1003.1068
26. Thompson RH, Lane BR, Lohse CM, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012;79(2):356–360. doi:10.1016/j.urology.2011.1010.1031
27. Takagi T, Mir MC, Campbell RA, et al. Predictors of precision of excision and reconstruction in partial nephrectomy. J Urol. 2014;192(1):30–35. Epub 2013 Dec 1025. doi:10.1016/j.juro.2013.1012.1035
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
