Back to Journals » International Journal of Nanomedicine » Volume 18
Role of Circulating Exosomal miRNA-3976 in Early Diabetic Retinopathy
Authors Yang S , Zhang J, Zeng T, Zheng J, Min J, Chen L
Received 27 March 2023
Accepted for publication 29 June 2023
Published 4 July 2023 Volume 2023:18 Pages 3695—3709
DOI https://doi.org/10.2147/IJN.S414393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Songtao Yang,1,2 Jiaoyue Zhang,1,2 Tianshu Zeng,1,2 Juan Zheng,1,2 Jie Min,1,2 Lulu Chen1,2
1Department of Endocrinology, Union Hospital, Huazhong University of Science and Technology Tongji Medical College, Wuhan, Hubei, People’s Republic of China; 2Hubei Provincial Clinical Research Center for Diabetes and Metabolic Disorders, Wuhan, Hubei, People’s Republic of China
Correspondence: Lulu Chen, Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China, Email [email protected]
Background: Diabetic retinopathy (DR) remains as the most frequent complication of diabetes, and is the major cause of vision loss for middle-aged to elderly people. With longer life expectancies for people with diabetes, there is a significant rise in diabetic retinopathy worldwide. The treatment of DR is limited; and therefore, our study aimed to investigate the possibilities of circulating exosomal miRNAs in the early screening and prevention of DR; and to explore the function of the exosomal miRNAs in DR.
Materials and Methods: Eighteen participants were recruited and divided into two groups: the diabetes mellitus (DM) group and the DR group. We analyzed the expression profile of exosomal miRNAs derived from serum using RNA sequencing. Additionally, we conducted co-culture experiments of RGC-5 and HUVEC cells with DR-derived exosomes to examine the role of highly expressed exosomal miRNA-3976 in DR. Furthermore, we transfected RGC-5 and HUVEC cells with miRNA-3976 to investigate its effects.
Results: Among the 1059 miRNAs analyzed, we identified eighteen up-regulated exosomal miRNAs. Treatment with DR-derived exosomes resulted in increased proliferation and reduced apoptosis of RGC-5 cells, and these effects were partially reversed by the miRNA-3976 inhibitor. Moreover, over-expression of miRNA-3976 led to increased apoptosis of RGC-5 cells and indirectly reduced the abundance of NFκB1.
Conclusion: Serum-derived exosomal miRNA-3976 has the potential to serve as a biomarker for DR, primarily exerting its effects in the early stages of DR through the regulation of NFκB-associated mechanisms.
Keywords: exosomes, miRNAs, diabetes, diabetic retinopathy
Introduction
By 2045, the global population with diabetes is estimated to reach 700 million.1 With increasing number of adults with diabetes and longer life expectancies, the diabetic retinopathy (DR) population is rising worldwide. From 1990 to 2010, the rate of global visual impairment and blindness due to diabetic retinopathy increased by 64% and 27%, respectively.2 Currently, DR is one of the leading causes of preventable blindness among adults. It is the fifth leading cause of moderate and severe vision impairment (MSVI) and blindness.2,3 The disease severity and economic burden make early diagnosis and intervention necessary. However, to date, no accurate biomarkers can predict DR occurrence, and the pathophysiology of DR is highly complex, with inflammation and oxidative stress playing significant roles. NFκB, a key transcription factor regulating many inflammatory genes, plays an important role in the progression of DR. The expression of NFκB is significantly upregulated in diabetes. Excessive NFκB activity disrupts the integrity of the inner blood-retinal barrier (iBRB) by reducing the expression of tight junction proteins. Moreover, it exacerbates various cellular inflammatory responses within the retina, leading to cellular dysfunction.4,5
Screening for DR is necessary. However, the primary methods of retinal examination, such as retinal photography or fundus examination6 may not suitable for all patients, especially the elderly. For patients with other preexisting eye diseases, evaluation of the retinal condition can be more difficult. Additionally, resources for screening equipment are not entirely proficient.
Exosomes were of endosomal origin, with a diameter of 40 to 160 nm.7,8 Their membrane-coated structure is able to protect cargos from endogenous RNase activity.9 The cargos of exosomes are built of various nucleic acids and proteins,7,10 among which miRNA is the most abundant.11 Meanwhile, exosomal miRNAs have also been of focus due to their potential as disease biomarkers and therapeutic applications.12–14 miRNAs are a range of small non-coding RNAs that can widely regulate biological processes.15,16 By targeting the 3′UTR of mRNAs, miRNAs can interact with the complementary regions directly, thereby negatively regulating the abundance of specific proteins and promoting their degradation. Apart from their intracellular functions, miRNAs are also secreted into exosomes, making it possible for their transfer to distal cells to regulate gene expression.17 Studies have also found that exosomes from T2DM patients exhibit elevated levels of miRNA-20b-5p. This indicates that it is exosomal rather than serum-derived miRNAs are altered in T2DM,18 suggesting the potential interactions between exosomes and diabetes. Although several studies have discussed extracellular vesicles and diabetic retinopathy,19 the exact role of exosomal cargos in DR remains unclear.20,21 Considering the limited treatment and disease burden of DR, early screening and prevention of DM are crucial. Therefore, efforts have been focused on investigating circulating miRNAs as potential DM biomarkers,22 however DR biomarkers investigation has yet to seek further research.18,23
To date, the exact mechanism of DR progression remains largely unknown, and there is limited consensus on the reliability of biomarkers for DR. Therefore, in our study, we aimed to investigate potential biomarkers for DR by isolating exosomes from DR patients and comparing them with exosomes from DM counterparts, assessing their effects on the retinal neurovascular unit in vitro.
Research Design and Methods
Study Population
This study was approved by the Ethical Committee of Huazhong University of Science and Technology. Written informed consent was obtained from all the participants. Eighteen male patients aged 50–70 were selected from the Wuhan Union Hospital and diagnosed with T2DM with or without diabetic retinopathy. People with other eye diseases, cardiovascular diseases, or tumors are excluded. People who have a history of surgery or trauma are also excluded. The blood samples were taken from the participants when they only used an insulin pump for treatment. A study discussed the differential exosomal miRNAs between healthy individuals and patients with type 2 diabetes.18 For a more accurate investigation of DR progression factors, eighteen participants were divided into two groups: nine men with type 2 diabetes mellitus and without diabetic complications (DM group), along with nine T2DM patients with diabetic retinopathy, and without other complications (DR group) were selected in the study. The DM participants and DR participants were matched for age and BMI. To minimize the possible effects of gender, we only collected peripheral blood from the male groups. Under the preliminary screening, exosomal miRNAs were extracted from three men in the DM group and three men in the DR group. The differential miRNAs were then validated in both serum and serum exosomes among the rest of the DM and DR participants. Clinical information of the participants was presented in Table 1.
 |
Table 1 Clinical Information of the Participants |
Exosome Isolation, Identification, and Uptake
Blood samples were collected from the participants’ serum. Exosomes were obtained from serum by differential ultracentrifugation, and the isolation was performed as aforementioned.17 The isolated exosomes were stored at −80°C, then, purified exosomes were stained with 2% uranyl acetate and added to a copper grid at 4 °C for 1 min. Thereafter, transmission electron microscopy (TEM) (TECNAI G2 spititi FEI) was performed to assess exosome morphology, and NTA (Particle Metrix ZetaView PMX 110) was used for particle size analysis. The exosome surface markers were determined by Western blot. The green dye PKH67 (Sigma MINI67-1KT) was used for membrane staining and exosome tracking. The uptake of labeled exosomes was then assessed through the combination with RGC-5 or HUVEC; immunofluorescence was used for measuring uptake.
MiRNA Sequencing and Analysis
Small RNA libraries were constructed using the TruSeq Small RNA Sample Prep Kits (Illumina, San Diego, USA) following the manufacturer’s protocol. The Illumina Hiseq 2500 analyzer was used for sequencing. The data was then analyzed via the ACGT101-miR system (LC Sciences, Houston, Texas, USA). The data was uploaded to GEO database (GSE221535).
Cells Culture and Transfection with miRNA
The mouse retinal ganglion cells 5 (RGC-5) cell line was purchased from Wuhan Warner Biotechnology company, Wuhan, China (WN-10520) and cultured in F12K (BasalMedia, L450KJ) containing 10% exosome-depleted FBS (BI Israel). Human umbilical vein endothelial cells (HUVECs) were obtained from the China Center for Type Culture Collection (#GDC166, CCTCC, China) and cultured in DMEM containing 25 mmol/L glucose and 10% exosome-depleted FBS in a 37°C incubator at 5% CO2. The riboFECITM CP Transfection Kit (RiboBio 1000016) was used for transfection. miRNA-3976 mimic (1000030), mimic Negative Control (1000025), miRNA-3976 inhibitor (1000032), and inhibitor Negative Control (1000026) were all purchased from RiboBio. The transfection steps were followed accordingly with the manufacturer’s instructions.
Western Blotting
The lysis buffer was used to isolate the proteins, and a BCA kit (Thermo 23225) was used for the quantification of protein expression. Afterward, the proteins obtained were subjected to sodium dodecyl sulfate–polyacrylamides gel electrophoresis. The separated proteins were then transferred to a PVDF membrane and incubated overnight at 4°C with the following primary antibodies: CD9 (1:2000, Abcam, Ab92726, Cambridge, UK), TSG101 (1:2000, Abcam, Ab125011, Cambridge, UK), and CD63 (1:2000, Abcam, Ab216130, Cambridge, UK). Horseradish peroxidase-conjugated secondary antibodies were used to probe the blots, and the proteins were detected with a Bio-Rad (Hercules, CA, USA) imaging system.
qRT-PCR Analysis
RNA was isolated and reversed using commercial kits (Takara Biological Incorporated, Japan). The mRNA levels were tested by qPCR using the SYBR Green I Master Mix (Takara Biological Incorporated, Japan) on a Light Cycler 480 system (Roche, USA). A miRNeasy Micro Kit (QIAGEN 217084) was used to extract exosomal miRNAs, followed by using a miRNA cDNA synthesis kit (by stem-loop)(Vazyme, MR-101) for cDNA synthesis. Lastly, a Light Cycler 480 system (Roche, USA) was used for qPCR reactions. The primers applied to real-time qPCR can be found in Table 2.
 |
Table 2 miRNAs and mRNA Primer Sequence |
CCK8 Assays and Cell Apoptosis Assay
For the Cell Counting Kit 8 (CCK8) assay, 3×103 RGC-5 were added to 96-well plates, and 5×103 HUVECs were added to 96-well plates for 24, 48, or 72 h respectively. Next, the CCK-8 reagent was carried out into the cells for 2 h. The microplate reader (Tecan, Spark 10M) was then used to measure the absorbance at 450 nm. An Annexin V and PI apoptosis kit (Proteintech, PF00005, USA) was used to assess cell apoptosis through flow cytometry.
Dual-Luciferase Reporter Assay
The WT or mutated treatments of the 3′-UTR of NFκB1 were carried out in the pMIR luciferase reporter plasmid. The mutated version of the plasmid had a deletion of the predicted miRNA-3976 binding site. The 293T cells were cotransfected with different constructs and appropriate miRNA-3976 mimic using Lipofectamine 2000 (11668019, Invitrogen). The Dual-Luciferase Reporter Assay System (E1960, Promega) was then used to quantify luciferase activity after 48 h.
Statistics
The entirety of the experiments was repeated at least three times, and the data were presented as the mean ± SEM. The analysis of the data was performed using R v4.0.3. Comparisons of the two groups were carried out with Student’s t-tests, with P < 0.05 considered significant.
Results
Characterization of Extracellular Particles from Men with T2DM or DR
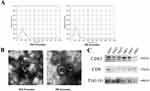
The characteristics of the 18 participants are shown in Table 1. The average age of the participants is 54, and other parameters including BMI, and blood pressure were matched accordingly between the two groups. Exosomes are known for carrying RNAs, including miRNAs.24 We determined the miRNA expression profile of serum exosomal miRNA from men with T2DM or DR. The current industry standard of isolation, differential ultracentrifugation, was used to isolate extracellular particles containing exosomes from serum to determine the exosomal miRNA expression profile. By using Nanoparticle Tracking Analysis (NTA), transmission electron microscopy (TEM), and Western blotting (WB), we began the assessment of the isolated exosomes. NTA revealed the size of the extracellular particles to range between 40 to 160 nm (average ~100 nm), which was consistent with previous findings concerning the size of the exosomes (Figure 1A).7 TEM revealed isolated extracellular particles to have a cup- or sphere-shaped morphology (Figure 1B). WB further confirmed that these extracellular particles contained exosomal surface biomarkers: CD63, CD9, and TSG101 (Figure 1C). These results confirmed that the isolated extracellular particles were exosomes.
Differentially Expressed Exosomal miRNAs from Men with DR
The previous study showed that miRNA profiles varied in different stages of the disease.25 However, little is known about the exact period of DR alteration in circulating exosomal miRNA expression. In the preliminary screening, we selected three men with mild non-proliferative DR (NPDR) using fundoscopic examination from the DR group. The three men were randomly selected from the DM group and matched accordingly for age and BMI. The fundoscopic examination showed mild NPDR with microaneurysms and hemorrhage (Figure 2A). In this screening, compared with the DM group, 18 exosomal miRNAs were identified up-regulated among the 1059 miRNAs in the DR group (Table 3 and Figure 2B), and two exosomal miRNAs (hsa-miRNA-494-3p, hsa-let-7f-5p) were down-regulated, however with no significance (P= 0.0826, P= 0.094).
 |
Table 3 Differential Expression Analysis of Exosomal miRNA |
For further understanding of gene function, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed to analyze the function of predicted miRNAs target genes (Figure 2C and D). We discovered that the most enriching biological process terms related to the differentially expressed miRNAs prediction of target genes were signal transduction, regulation of transcription, DNA-template, and positive regulation of transcription via RNA polymerase II. The top enriched cellular component terms were the membrane, cytoplasm, and nucleus, whilst the top three enrichment of molecular function terms were protein binding, metal iron binding, and transferase activity. KEGG analysis showed that differentially expressed miRNAs targeted genes were mainly correlated with the Sphingolipid signaling pathway, Neurotrophin signaling pathway, and Hippo signaling pathway.
Of the 18 miRNAs identified as highly expressed in exosomes from the men with DR, miRNA-3976, PC-5p-39533, and PC-3p-37421 showed the larger fold change. To further confirm the source of the differentially expressed miRNA, we explored the possibility of alteration in the total serum for the expression of miRNA-3976, PC-5p-3933, and PC-3p-3721. The result of qPCR showed no significant differences in total serum miRNA obtained from the same individuals (Figure 3A), suggesting that exosomal rather than serum-derived miRNAs incurred alteration in the DR group. A similar finding was found in another study discussing serum exosomes between T2DM patients and healthy control.18 It has been known that exosomes were released from the donated cells. Though the exact mechanism of how to alter exosomal cargo remain unclear, changing the circumstance of the donated cells has the possibility of manipulating the types of exosomal cargo.26 This indicates that exosomal cargo differed among different stages of disease, making it possible for exosomal miRNAs to be a potential disease biomarker. However, further evaluation should be conducted through blood examination.
Correlation Between Clinical Parameters and Exosomal miRNA Content
miRNAs have been considerable biomarkers in the progression of various diseases.27 Therefore, our study aimed to determine whether exosomal miRNAs expression content correlated with clinical parameters of the study cohorts, the association between exosomal miRNA-3976, PC-5p-39533, and PC-3p-37421 content. The clinical parameters was investigated by the Pearson correlation coefficient test (Table 4). The content of PC-5p-39533 and PC-3p-37421 were not correlated with clinical features in the study cohorts (data not shown), while miRNA-3976 content negatively correlated with HDL-C in the men with DR (P<0.05) (Table 4). This correlation was not significant within the DM group (r = −0.318, P = 0.794), however, there was found to be an inverse correlation between miRNA-3976 and fasting C-peptide in the group of men with DR (r = −0.995, P = 0.015).
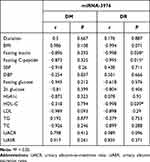 |
Table 4 Correlation of Exosomal miRNA-3976 Content with Clinical Parameters |
Exosome Tracing and the Effect of Exosomal miRNA-3976 on RGC-5 and HUVEC
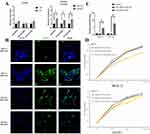
The previous studies confirmed that exosomes could be secreted from cells and released into circulation.28 Afterwards, exosome uptake occurs by the recipient cells.29 New studies on retinal physiology suggest that DR-oriented retinal dysfunction may be viewed as an impairment in the retinal neurovascular unit.30,31 Therefore, we selected RGC-5 and HUVEC to assess the neural dysfunction and vascular changes, respectively. To determine whether the circulating exosomes from the DR group targeted the retina, we evaluated the uptake ability of RGC-5 and HUVECs in vitro by labeling exosomes with PKH67 dye, and then proceeding to add them into the culture medium of RGC-5 and HUVEC separately. After 24h co-culture, we discovered that both RGC-5 and HUVECs had the properties to take up exosomes from the DR group (Figure 3B), based on the green fluorescence staining in the cells. Notably, RGC-5 took up more DR-exosomes than HUVEC. It still remains unclear on how exosomes are specifically targeted to recipient cells or how exosome uptake occurs. However, previous studies have suggested that exosome uptake occurs in a cell-specific manner.32 Thus, it is thought that RGC-5 instead of HUVEC acts as one of the recipient cells of DR-exosomes. However, when treated with DM-exosomes, HUVEC took up more DM-exosomes than RGC-5. RGC-5 took up more DR-exosomes than DM-exosomes. In contrast, HUVEC took up more DM-exosomes than DR-exosomes, indicating that changing conditions could alter the exosome uptake in cells (Figure 3B).
We investigated the effects of exosomes and miRNA-3976 from the DR group on RGC-5 or HUVEC functionality. To determine the success of miRNA-3976 overexpression, we evaluated the transfection efficiency of miRNA-3976 mimic (Figure 3C). Additionally, by using CCK-8 assays assessment on RGC-5 and HUVEC proliferation, we observed that treatment of RGC-5 with 20 µg/mL DR-exosomes could increase RGC-5 proliferation compared to the DM-exosome treatment. Conversely, further treatment with the miRNA-3976 inhibitor dramatically decreased RGC-5 proliferation (Figure 3D), while treatment of HUVEC with 20 µg/mL DR-exosomes hindered HUVEC proliferation (Figure 3D). We also used flow cytometry to determine apoptosis rates in treated cells. We found that RGC-5 apoptosis decreased by 24% and 40%, respectively, compared to the NC group, following 10 and 20µg/mL DR-exosomes incremental treatments (Figure 4A). On the other hand, DM-exosome treatment led to a higher apoptosis rate in a dose-dependent manner (Figure 4A). Furthermore, we transfected 20 µg/mL of DR-exosome-treated cells with miRNA-3976 inhibitor. The results showed an increase in RGC-5 apoptosis compared to the iNC group. However, when transfected with the miRNA-3976 mimic in an exosome non-treated condition, there was a higher percentage of RGC-5 apoptosis (Figure 4B). As for HUVEC, there were no significant changes in HUVEC apoptosis (Figure 4C and D). Together, these results indicate that DR-exosomes have the potential to regulate RGC-5 functionality.
Overexpression of miRNA-3976 Could Down-Regulated NFκB1 Expression
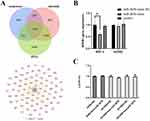
To further understand the molecular mechanisms by which miRNA-3976 affects RGC-5 functionality, we then identified possible targets of miRNA-3976 by using Miranda, Targetscan, and PITA. The prediction found 1176 genes as miRNA-3976 target genes (Figure 5A), of which these genes were further ranked into the top 80 by scores. Hyperglycemia could trigger the activation of NFκB, which then is able to modulate a pro-apoptotic signal to cells in the retina. Recent studies have demonstrated the association between NFκB signaling and DR.33–35 We hypothesised whether exosomal miRNA-3976 functions through the NFκB pathway, and discovered that NFκB1 may be a potential target gene worth considering (Figure 5A). Other previous studies have confirmed that NFκB signaling pathways were closely related to cell survival.36 We found that the overexpression of miRNA-3976 could lead to a suppression of NFκB1 expression (Figure 5B). Hence, a dual-luciferase reporter assay was performed to confirm whether miRNA-3976 is able to directly bind to the predicted target region of the NFκB1 mRNA and regulate NFκB1 specifically. However, the result of the dual-luciferase reporter assay indicated that miRNA-3976 had no effect on luciferase expression with NFκB1 3’UTR (Figure 5C).
Discussion
In our findings, we demonstrated that RNA expression of miRNA-3976, PC-5p-39533, and PC-3p-37421 increased in serum exosomes from DR patients. However, the total serum miRNA abundance of these miRNAs was not significantly altered compared with the T2DM patients. In order to explore the role of miRNA-3976 enriched exosomes, we treated RGC-5 and HUVEC with DR-exosomes. We found that these exosomes could increase RGC-5 proliferation and reduce RGC-5 apoptosis, and these changes could be partly reversed via the miRNA-3976 inhibitor. To further understand the role of miRNA-3976 on gene expression, signal transduction, and similar properties, we overexpressed miRNA-3976 in RGC-5 and found that miRNA-3976 overexpression suppressed the expression of NFκB1.
Exosomes are secreted by all cells and released into body fluid, which can be absorbed by recipient cells to regulate their functionalities.7,37 Therefore, exosomes have been considered potential biomarkers or therapeutic applications of various diseases.7,38–42 In addition to disease diagnosis, the protection of their cargo makes them ideal candidates for vehicles in drug delivery.43 However, the precise origins of exosomal cargo and exosome uptake remain uncertain. Some studies have suggested that almost all types of cells can take up exosomes, whereas others advocated cell-specific uptake. In our study, we noticed that RGC-5 internalized more DR-exosomes than HUVEC after 24h co-culture. This indicates that cell-specific exosome uptake occurred in the early stage of DR, and these cells may have been the first to react. The previous study also revealed that neural impairment occurred before overt microangiopathy, and RGCs were the first neurons to detect apoptosis caused by diabetes.31 However, our results showed that RGC-5 apoptosis had decreased following the DR-exosomes treatment, and an increase in RGC-5 proliferation had also been observed. Additionally, we found that RGC-5 took up more DR-exosomes than DM-exosomes. These results suggest RGC-5’s protective ability of circulating DR-exosome, or the possibility of a compensated stage for limited rescue prior to overt microangiopathy. Nonetheless, these assumptions require further research and evidence. We theorized that circulating exosomes were involved in the pathological process of DR.
MiRNAs are small non-coding RNAs;44,45 and have raised concerns as they regulate gene expression making them ideal candidates for disease biomarkers.27,46 Collective efforts have been made to identify the miRNA profiles for diagnosis and therapeutic potential, and the findings of exosomes containing nucleic acid make it highly appealing. Some reports have discussed the relationship between specific exosomal miRNAs and DR.34,47 However, the exosomal miRNAs they found come from specific types of cells, and usually, the exosomes are from cell supernatants. Our study contained T2DM and DR patients through strict standards, and exosomes were isolated from participants’ serum. It not only has the potential for diagnosis but also can observe the combined effect on the whole body. We found that miRNA-3976 and the two novel miRNAs (PC-5p-39533 and PC-3p-37421) were up-regulated in DR-exosomes for the first time. To further explore the effect of miRNA-3976, we transfected RGC-5 with a miRNA-3976 inhibitor and discovered lower RGC-5 proliferation and higher RGC-5 apoptosis rates. These findings indicate that miRNA-3976 from DR-exosome may exert a protective role on RGC-5. However, we observed that the overexpression of miRNA-3976 in RGC-5 aggravated the cell apoptosis rate. Although the overexpression of miRNA-3976 suppressed NFkB1 expression, it may have also protected cell proliferation and reduced cell apoptosis. Further, we found the regulation of miRNA-3976 was indirect to NFkB1 (Figure 5). One possible explanation was that the proper content of miRNA-3976 could exert a protective role partly via NF‑κB axis repression, and an excess of miRNA-3976 could regulate multiple pathways. The combined effects of these are potentially harmful. Our subsequent study aims to further investigate the exact molecular mechanism behind our current findings.
Diabetic retinopathy (DR) is one of the leading causes of preventable blindness in adults.3 T2DM patients have a 50–60% risk of developing DR in their lifetime, while the risk in T1DM increases up to 90%.48 Currently, anti-VEGF therapy is the primary treatment for DR, however, the effects are limited and costly. Therefore, early identification and intervention are crucial. By exploring the correlation between clinical parameters and miRNA-3976 content, we found that miRNA-3976 content was significantly negatively correlated with the HDL-C level of DR patients. GO analysis showed the predicted miRNAs target genes were enriched in the lipid metabolism process. Compared to previous studies, fenofibrate treatment in DR patients with dyslipidemia can slow down retinopathy progression, particularly in patients with extremely mild NPDR.49 Fenofibrate improved the lipid profile, notably the TG and HDL-C levels in patients with dyslipidemia,50 and the protective effect may have been due to the HDL-C level improvement. If Fenofibrate was to improve the HDL-C level, this would eventually lead to lowered levels of exosomal miRNA-3976. This was consistent with our findings given that miRNA-3976 overexpression resulted in RGC-5 apoptosis, whereas the appropriate level of miRNA-3976 had a protective effect. However, these findings were found in the early stage of DR, and further research is needed for the later period of DR. Our study provided new evidence for the relationship between HDL-C and DR progression. We also observed a negative correlation between miRNA-3976 and fasting C-peptide in the group of men with DR (r = −0.995, P = 0.015). One study demonstrated that miRNA-3976 was significantly upregulated in serum-derived exosomes from patients with pancreatic cancer (PaCa) compared with the control group.51 Patients with pancreatic cancer usually had impaired pancreatic islets function, and it was reported that approximately 25% of PaCa patients had diabetes at diagnosis and a further 40% had impaired glucose tolerance.52 Overall, we took into consideration whether poor pancreatic islets function may be a risk factor for DR, and further verification is required. One primary aspect was to determine where exosomes originate from and where they travel. In our study, we demonstrated that RGCs were one of the recipient cells of DR-exosomes, however, their origin remained unclear. The previous study showed that miRNA-3976 enriched exosomes from both serum of PaCa patients and the PaCa cell culture supernatant.51 Thus, we considered pancreatic islets may be one of the possible origins of exosomal miRNA-3976.
It is undeniable that our study has certain limitations and unresolved issues that need further attention. One major limitation is the limited sample size, which posed challenges in acquiring a sufficient number of exosomes for animal research. We are currently working on increasing the sample volume for secondary sequencing and further verification. Additionally, our study only encompassed in vitro studies, necessitating further in vivo validation. Furthermore, the precise mechanism underlying the function of miRNA-3976 remains unclear. Although treatment with DR-exosomes has shown to enhance RGC-5 proliferation and reduce apoptosis, the miRNA-3976 inhibitor partially reverses this protective effect. We observed that miRNA-3976 overexpression increases RGC-5 apoptosis, contradicting the expected protective effects of miRNA-3976. The direct downstream targets regulated by miRNA-3976 are still unknown. Lastly, the role of the newly identified PC-5p-39533 and PC-3p-37421 also requires exploration. While these miRNAs may be associated with DR, gaining a deeper understanding of the physiological functions of PC-5p-39533 and PC-3p-37421 is essential.
Our study provided evidence that miRNA-3976 is highly expressed in serum exosomes isolated from DR patients, and exosomal miRNA-3976 has the potential to serve as a potential DR biomarker. Moreover, suitable contents of miRNA-3976 can promote RGCs proliferation and reduce RGCs apoptosis in the early stage of DR. Although, since exosomal miRNA-3976 content was found to be negatively correlated with HDL-C and C-peptide levels in the DR group, low-levels of HDL-C and poor pancreatic islets function may contribute to being a risk factor for DR progression. In summary, our study highlighted the potential interactions between circulating exosomal miRNAs and DR.
Ethical Statements
Approval date of registry and the registration No. of the study: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by The Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571) on 28/07/2021 (2021S189).
Informed Consent: Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Acknowledgments
Dr Songtao Yang and Dr Jiaoyue Zhang contributed equally as co-first authors.
Funding
This work was supported by the Ministry of Science and Technology under Grants (NO: 2016YFC0901203) and the National Natural Science Foundation of China Grants (NO: 81770843 and 82070809), and the Natural Science Foundation of Hubei Province (NO: 2016CFA025).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Saeedi P, Petersohn I, Salpea P, et al. Committee, Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
2. Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39(9):1643–1649. doi:10.2337/dc15-2171
3. Teo ZL, Tham YC, Yan Yu MC, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591.
4. Tang L, Zhang C, Yang Q, et al. Melatonin maintains inner blood-retinal barrier via inhibition of p38/TXNIP/NF-κB pathway in diabetic retinopathy. J Cell Physiol. 2021;236(8):5848–5864. doi:10.1002/jcp.30269
5. Zhu SH, Liu BQ, Hao MJ, et al. Paeoniflorin suppressed high glucose-induced retinal microglia MMP-9 expression and inflammatory response via inhibition of TLR4/NF-κB pathway through upregulation of SOCS3 in diabetic retinopathy. Inflammation. 2017;40:1475–1486. doi:10.1007/s10753-017-0571-z
6. Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8(4):337–347. doi:10.1016/S2213-8587(19)30411-5
7. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
8. Mahbubfam S, Rezaie J, Nejati V. Crosstalk between exosomes signaling pathway and autophagy flux in senescent human endothelial cells. Tissue Cell. 2022;76:101803. doi:10.1016/j.tice.2022.101803
9. Feghhi M, Rezaie J, Akbari A, et al. Effect of multi-functional polyhydroxylated polyhedral oligomeric silsesquioxane (POSS) nanoparticles on the angiogenesis and exosome biogenesis in human umbilical vein endothelial cells (HUVECs). Mater Des. 2021;197:109227. doi:10.1016/j.matdes.2020.109227
10. Shaban SA, Rezaie JA-O, Nejati VA-O. Exosomes derived from senescent endothelial cells contain distinct pro-angiogenic miRNAs and proteins. Cardiovasc Toxicol. 2022;22(6):592–601. doi:10.1007/s12012-022-09740-y
11. Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derivedexosomal RNAs by deep sequencing. BMC Genomics. 2013;14(319). doi:10.1186/1471-2164-14-319
12. Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022;20(1):145. doi:10.1186/s12964-022-00959-4
13. Rezaie J, Etemadi T, Feghhi M. The distinct roles of exosomes in innate immune responses and therapeutic applications in cancer. Eur J Pharmacol. 2022;933:175292. doi:10.1016/j.ejphar.2022.175292
14. Nemati M, Singh B, Mir RA, et al. Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Commun Signal. 2022;20. doi:10.1186/s12964-022-00889-1
15. Faramin Lashkarian M, Hashemipour N, Niaraki N, et al. MicroRNA-122 in human cancers: from mechanistic to clinical perspectives. Cancer Cell Int. 2023;23. doi:10.1186/s12935-023-02868-z
16. Shirvani H, Ghanavi J, Aliabadi A, et al. MiR-211 plays a dual role in cancer development: from tumor suppressor to tumor enhancer. Cell Signal. 2023;101:110504. doi:10.1016/j.cellsig.2022.110504
17. Xiong Y, Chen L, Yan C, et al. Circulating exosomal miR-20b-5p inhibition restores Wnt9b signaling and reverses diabetes-associated impaired wound healing. Small. 2020;16(3):e1904044. doi:10.1002/smll.201904044
18. Katayama M, Wiklander OPB, Fritz T, et al. Circulating Exosomal miR-20b-5p Is Elevated in Type 2 Diabetes and Could Impair Insulin Action in Human Skeletal Muscle. Diabetes. 2019;68(3):515–526. doi:10.2337/db18-0470
19. Martins B, Amorim M, Reis F, Ambrosio AF, Fernandes R. Extracellular Vesicles and MicroRNA: putative role in diagnosis and treatment of diabetic retinopathy. Antioxidants. 2020;9:8.
20. Liu J, Jiang F, Jiang Y, et al. Roles of exosomes in ocular diseases. Int J Nanomedicine. 2020;15:10519–10538. doi:10.2147/IJN.S277190
21. Gu S, Liu Y, Zou J, et al. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp Eye Res. 2020;201:108271. doi:10.1016/j.exer.2020.108271
22. Dehghan M, Ghorbani F, Najafi S, et al. Progress toward molecular therapy for diabetes mellitus: a focus on targeting inflammatory factors. Diabetes Res Clin Pract. 2022;189:109945. doi:10.1016/j.diabres.2022.109945
23. Jiang L, Cao H, Deng T, et al. Serum exosomal miR-377-3p inhibits retinal pigment epithelium proliferation and offers a biomarker for diabetic macular edema. J Int Med Res. 2021;49(4):3000605211002975. doi:10.1177/03000605211002975
24. Chang W, Wang J. Exosomes and their noncoding RNA cargo are emerging as new modulators for diabetes mellitus. Cells. 2019;8(8):853. doi:10.3390/cells8080853
25. Hiratsuka I, Yamada H, Munetsuna E, Hashimoto S, Itoh M. Circulating MicroRNAs in graves’ disease in relation to clinical activity. Thyroid. 2016;26(10):1431–1440. doi:10.1089/thy.2016.0062
26. Xiong L, Chen L, Wu L, et al. Lipotoxicity-induced circGlis3 impairs beta cell function and is transmitted by exosomes to promote islet endothelial cell dysfunction. Diabetologia. 2022;65(1):188–205. doi:10.1007/s00125-021-05591-4
27. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. doi:10.1016/j.jaci.2017.08.034
28. Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017;19:137–146. doi:10.1111/dom.13027
29. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
30. Antonetti DA, Klein R, Bronson SK. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi:10.1056/NEJMra1005073
31. Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61(9):1902–1912. doi:10.1007/s00125-018-4692-1
32. Naslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK, Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. 2014;28(2):171–180. doi:10.1097/QAD.0000000000000159
33. Liang WJ, Yang HW, Liu HN, Qian W, Chen XL. HMGB1 upregulates NF-kB by inhibiting IKB-alpha and associates with diabetic retinopathy. Life Sci. 2020;241:117146. doi:10.1016/j.lfs.2019.117146
34. Li W, Jin L, Cui Y, Nie A, Xie N, Liang G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-kappaB axis repression. J Endocrinol Invest. 2021;44(6):1193–1207. doi:10.1007/s40618-020-01405-3
35. Alomar SY, B MB, Eldosoky M, et al. Protective effect of metformin on rat diabetic retinopathy involves suppression of toll-like receptor 4/nuclear factor-k B expression and glutamate excitotoxicity. Int Immunopharmacol. 2021;90:107193. doi:10.1016/j.intimp.2020.107193
36. Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168(1–2):37–57. doi:10.1016/j.cell.2016.12.012
37. Nikfarjam S, Rezaie J, Zolbanin NM, Jafari RA-O. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18. doi:10.1186/s12967-020-02622-3
38. Vahabi A, Rezaie J, Hassanpour M, et al. Tumor Cells-derived exosomal CircRNAs: novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochem Pharmacol. 2022;200:115038. doi:10.1016/j.bcp.2022.115038
39. Joo HS, Suh JH, Lee JM, Bang ES, Lee JM. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727. doi:10.3390/ijms21030727
40. Rezaie J, Ahmadi M, Ravanbakhsh R, et al. Tumor-derived extracellular vesicles: the metastatic organotropism drivers. Life Sci. 2022;289:120216. doi:10.1016/j.lfs.2021.120216
41. Almohammai A, Rahbarghazi R, Keyhanmanesh R, Rezaie J, Ahmadi M. Asthmatic condition induced the activity of exosome secretory pathway in rat pulmonary tissues. J Inflamm. 2021;18. doi:10.1186/s12950-021-00275-7
42. Rahbarghazi R, Jabbari N, Sani NA, et al. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell Commun Signal. 2019;17. doi:10.1186/s12964-019-0390-y
43. Tian Y, Zhang F, Qiu Y, et al. Reduction of choroidal neovascularization via cleavable VEGF antibodies conjugated to exosomes derived from regulatory T cells. Nat Biomed Eng. 2021;5:968–982. doi:10.1038/s41551-021-00764-3
44. Aghaei-Zarch SM, Alipourfard I, Rasoulzadeh H, et al. Non-coding RNAs: an emerging player in particulate matter 2.5-mediated toxicity. Int J Biol Macromol. 2023;235:123790. doi:10.1016/j.ijbiomac.2023.123790
45. Bahari Khasraghi L, Nouri M, Vazirzadeh M, et al. MicroRNA-206 in human cancer: mechanistic and clinical perspectives. Cell Signal. 2023;101:110525. doi:10.1016/j.cellsig.2022.110525
46. Mori MA, Ludwig RG, Garcia-Martin R, Brandao BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30(4):656–673. doi:10.1016/j.cmet.2019.07.011
47. Cao X, Xue LD, Di Y, Li T, Tian YJ, Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021;272:119232. doi:10.1016/j.lfs.2021.119232
48. Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi:10.1038/nrdp.2016.12
49. American Diabetes. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S135–S151. doi:10.2337/dc20-S011
50. McKeage K, Keating GM. Fenofibrate: a review of its use in dyslipidaemia. Drugs. 2011;71(14):1917–1946. doi:10.2165/11208090-000000000-00000
51. Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2015;136(11):2616–2627. doi:10.1002/ijc.29324
52. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi:10.1016/S0140-6736(10)62307-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.