Back to Archived Journals » Robotic Surgery: Research and Reviews » Volume 5
Robot-assisted vitreoretinal surgery: current perspectives
Authors Roizenblatt M, Edwards TL , Gehlbach PL
Received 18 October 2017
Accepted for publication 11 December 2017
Published 23 February 2018 Volume 2018:5 Pages 1—11
DOI https://doi.org/10.2147/RSRR.S122301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Masoud Azodi
Video abstract presented by Marina Roizenblatt.
Views: 1075
Marina Roizenblatt,1,2,* Thomas L Edwards,3,* Peter L Gehlbach2
1Department of Ophthalmology, Wilmer Eye Institute, The Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Ophthalmology, Federal University of São Paulo, São Paulo, Brazil; 3Department of Clinical Neurosciences, University of Oxford, Royal Victorian Eye and Ear Hospital, Melbourne, VIC, Australia
*These authors contributed equally to this work
Abstract: Vitreoretinal microsurgery is among the most technically challenging of the minimally invasive surgical techniques. Exceptional precision is required to operate on micron scale targets presented by the retina while also maneuvering in a tightly constrained and fragile workspace. These challenges are compounded by inherent limitations of the unassisted human hand with regard to dexterity, tremor and precision in positioning instruments. The limited human ability to visually resolve targets on the single-digit micron scale is a further limitation. The inherent attributes of robotic approaches therefore, provide logical, strategic and promising solutions to the numerous challenges associated with retinal microsurgery. Robotic retinal surgery is a rapidly emerging technology that has witnessed an exponential growth in capabilities and applications over the last decade. There is now a worldwide movement toward evaluating robotic systems in an expanding number of clinical applications. Coincident with this expanding application is growth in the number of laboratories committed to “robotic medicine”. Recent technological advances in conventional retina surgery have also led to tremendous progress in the surgeon’s capabilities, enhanced outcomes, a reduction of patient discomfort, limited hospitalization and improved safety. The emergence of robotic technology into this rapidly advancing domain is expected to further enhance important aspects of the retinal surgery experience for the patients, surgeons and society.
Keywords: robotic eye surgery, vitrectomy, retina, robotic assistance, minimally invasive surgery, precision
Introduction to robotics in surgery
Rapid development and incorporation of computerized technology into surgical systems have occurred over the last 3 decades.1 The word “robot” was originally used to describe the mass-produced “workers” assembled from artificially synthesized organic material in Karel Čapek’s 1920 play “Rossum’s Universal Robots”.2 Derived from the Czech “robota”, meaning “forced labor”, the term was subsequently popularized by Isaac Asimov,3 whose first fundamental rule of robotics – “a robot may not injure a human being or, through inaction, allow a human being to come to harm”. These rules now belie the increasing utility in medicine and surgery of modern day robotic systems and is prescient in defining a fundamental challenge in implementing the human–computer–machine interface, which enhances surgical capability while reducing surgical risk.
At present, robotic surgery is rapidly evolving, especially in the area of minimally invasive surgery (MIS); yet even during a time of well-publicized translation into human application, there remain many challenges to routine incorporation into clinical practice. Vitreoretinal microsurgery remains among the most technically challenging MIS. This work is performed with exceedingly high precision in a very limited workspace, such that robotics becomes a promising solution to the human limitations and physical challenges associated with retinal microsurgery.4,5 Yet the practical role, high impact indications and the means by which to strategically and cost effectively incorporate robotics into procedures currently performed with high levels of expertise have begun to be understood.
The first report of a robotic device in surgical practice was in 1988 when the Unimation Puma 200 robot was used to perform a computed tomography-guided brain tumor biopsy.6 This was soon followed by development and clinical trial of the first dedicated surgical robot, RoboDoc, which was used to perform total hip arthroplasty by the team of Paul, Barger and Taylor.7,8 Many of the key advances throughout this period were led by the US Military with the goal of arriving at a telepresence model of care delivery that would allow remotely controlled robotic surgery on wounded soldiers.9 The first use of a tele-operated surgical robot came in April 1997 in Brussels, where Jacques Himpens and Guy Cardiere used the da Vinci system (Intuitive Surgical Inc., Sunnyvale, CA, USA) to perform laparoscopic cholecystectomy.10 In response to this trend, hundreds of robotic systems have been sold worldwide with an increasing number of laboratories committing research effort to robotic medicine.11,12
Ocular surgery is not unique in its requirement for a highly dexterous, steady and precise surgical approach. Other current similar applications include, but are not limited to, the brain, nerves, heart and small blood vessels. In such procedures, 150- to 200- μm critical movements are routinely required.13 Therefore, MIS is coupled with an increasing demand on surgeons’ manual dexterity that robotically assisted instruments may be able to assist.14,15 As a result of recent technological advances in robotic surgery, progress has been made to reduce patient trauma, shorten hospitalizations, improve procedure safety and enhance both precision and effectiveness. However, the field of medical robotics is only now emerging as relevant and broadly applicable, and many unanswered questions remain.
Introduction to robots in retinal surgery and human limitations
Experimental models of robotic assistance in eye surgery were first introduced in the 1980s by Spitznas16 and Guerrouad and Vidal.17 This followed the breakthrough development of a handheld motor-driven vitreous cutter, suction and infusion system in the early 1970s by Machemer.18,19 This system went on to revolutionize the surgical management of a number of common vitreoretinal diseases that had previously been deemed untreatable, e.g., complex retinal detachment, macular hole, epiretinal membrane and nonclearing vitreous hemorrhage.
There are limitations of unassisted human hands in terms of dexterity, tremor and precision in positioning instruments in an organ as small as the eye. The expanding scope of intraocular microsurgical interventions facilitated by modern iterations of vitrectomy machine systems has been fundamentally plateaued by the physiological limits of human hands.20,21 The eye is a small and closed space that is not tolerant to errors of instrument position that might otherwise be tolerable in general surgery. Further complicating retinal surgery, are instrument manipulations involving retina typically occur beneath the level of human tactile perception and at the limits of visual resolution. Moreover, the retina does not regenerate; hence, it is critical to avoid injury.22,23
Tremor
Chief among the challenges faced by “ultra-microsurgeons” is physiological hand tremor, a normal motion that accompanies all postures and movements24,25 that are typically involuntary, approximately rhythmic and sinusoidal in character. Pragmatically, surgery on the macula poses the highest demand on retinal surgeons requiring precision instrument control and meticulous error avoidance. Requirements to manually remove a transparent membrane, approximately 10 µm thick, from the surface of the critical and fragile macula, using only intraocular (endo) forceps and forces that are beneath the ability of a human to detect remain a rare surgical skill. Therefore, specially designed robots are a natural fit and are being evaluated to address these and other amenable human skills and limitations.26–29
Various robotic systems now suppress tremor in real time during MIS, including tele-operated systems in the present use and the Johns Hopkins “steady-hand” system based on a cooperative control scheme between the surgeon and the robot.30,31 Tremor data recorded during a standard epiretinal membrane removal operation using this type of robot, demonstrated that the root mean square amplitude of tremor at the instrument tip was 182 µm.32 In a similar study at the same center, mean tremor amplitude at the instrument tip during retinal surgery was 24, 22 and 20 µm along the x, y and z axes, respectively;33 equivalent to a peak-to-peak vector magnitude of ~100 µm.33
From an anatomical perspective, a further point of reference by which to define the current limits of retinal microsurgery precision is the caliber of retinal vessels (113±19 µm at the disk border34 from where they taper to the retinal periphery). Although there are many treatment options for retinal vein occlusions, all remain unsatisfactory in that they treat manifest complications of occlusion rather than the occlusion. The safety and efficacy of direct treatment of occlusion have not yet been tested by large studies. Vitrectomy combined with intravenous thrombolysis is a possible strategy now enabled by robotics.
While retinal vein cannulation with maintenance of cannulation and successful infusion has been achieved clinically, it is technically beyond human limitations for most surgeons to perform this procedure consistently and safely. Moreover, challenges in identifying the optimal timing for intervention as well as the best drug for delivery collectively limit the feasibility of the procedure.35–37 Robotic solutions aim to decrease vascular trauma by assisting the surgeon to position and maintain the needle in the vein for infusion. One augmenting strategy has been the development of the force-sensing microneedle, a tool developed to measure the forces required to puncture the retinal veins, limit potential movement of the tissue and hold the cannulation device securely inside the vessel.37–39
Force sensor
Forces applied to the retina during surgery are typically beneath the surgeon’s ability to detect; therefore, force-sensing instruments have emerged as an innovative technology. Their development and translational testing have further created an awareness of their potential and of novel needs not previously appreciated in vitreoretinal surgery. The integration of force sensing in microsurgical tools has a number of benefits, including but not limited to enhanced safety, instrument response to forces and the potential to create a record of forces applied during a procedure.40 To this end, a range of force-sensing instruments have been developed at Johns Hopkins University with varying degrees of freedom (DOF) and increasing robustness.
With force-sensing instruments, the forces applied to the instrument at its point of contact with the eye wall (sclerotomy) influence the forces measured at the tool tip. The concept of remote center of motion (RCM) in robotics postulates that there are only three rotational DOF at the scleral entry point and one translational DOF along the instrument axis, while all lateral translations are prohibited by mechanical constraints at point of entry through the relatively rigid eye wall.41–43 Of note is that each DOF has a significant effect on the motion accuracy of the tool during tracking and has the potential to improve motion accuracy and safety in performing robot-assisted tasks.44,45
In this context, Gonenc et al40 presented the conceptual design and optimization of three DOF force-sensing micro-forceps as the present generation of force-sensing tools. Four fiber Bragg grating (FBG) strain sensors were integrated along the tool shaft to measure tool tip forces, and the active segments were located close to the distal end in order to sense only the tip forces. Simulations revealed that the optimal design targets have been achieved based on grasping force, actuation force and various feasibility criteria. This work has led to the next step of Johns Hopkins University’s force-sensing instruments, a family of forceps that can measure the force directly at the tool tip inside the eye (Figure 1).
  | Figure 1 Conceptual design of three-DOF force-sensing micro-forceps compatible with the steady-hand robot, which will replace the previously developed two-DOF version. Note: © 2013 IEEE. Reprinted, with permission, from Gonenc B, Handa J, Gehlbach P, Taylor RH, Iordachita I. Design of 3-DOF force sensing micro-forceps for robot assisted vitreoretinal surgery. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5686–5689.40 Abbreviations: DOF, degrees of freedom; FBG, fiber Bragg grating. |
As force feedback is often entirely absent during manipulations of micron scale tissues involved in vitreoretinal surgery, excessive tissue manipulation resulting from the lack of feedback may result in iatrogenic retinal trauma during surgery.40,46 Experimental studies in rabbits have shown that a measured tool-to-tissue force of only 7 mN can induce a retinal tear in rabbit retina while surgeons can feel <20% of events performed at forces on this order of magnitude.47,48 Both, the forces detected by force sensing at the tip of the surgical instrument and those detected at the point of tool interaction with the sclera provide important intraoperative information.49,50 During membrane peeling, for instance, the experimentally measured forces at the tool tip, assisted by robotic technology, were able to limit the final applied forces on the retina to those programmed in advance.
Theoretically, this technology will prevent future iatrogenic retinal injuries due to excessive albeit imperceptible forces applied during surgery.51 Furthermore, a recent study described the development of a revolutionary robot-assisted system that minimized the forces applied on the sclera and optimized target illumination, without increasing the risk for macular light toxicity.52 A novel forcep that automatically releases tissue when force limits are met is in development.
Optical coherence tomography (OCT)
OCT technology has evolved over the last 2 decades from time domain to spectral domain and, most recently, to swept-source OCT. The applicability of such multimodal digital images for retinal study has improved in-clinic diagnostic accuracy.53 Recently, OCT has been applied as a distance sensor for intraoperative tool control and has been studied as a promising image guidance modality for real-time forward viewing during MIS.54,55 The early application of spectral domain technology for this purpose has evolved to include swept-source OCT. Smart micromanipulation-aided robotic-surgical tool is a newly developed microsurgical platform that couples swept-source common path OCT with the shaft of a surgical micro instrument. Utilizing distance sensing information to drive a compensating piezoelectric micro motor, undesirable hand tremor is canceled.56–60
A significant reduction in surgeon hand tremor, together with an enhanced ability to accurately and precisely approach a surgical target, was achieved by incorporation of OCT sensing into microsurgical tools. Improvement in micron-scale maneuvers, reduction in the freehand surgical risks and innovation of microsurgical training methods have all emerged as possible.56,60 Lack of proximity sensing is considered an important factor that contributes to surgical risk and may reduce the likelihood of achieving surgical goals. During freehand microsurgery, this issue is not solvable given human physiological limits. It is however, solved by real-time OCT scanning, producing images of local anatomy and allowing real-time and continuous calculation of the distance of the instrument from the retinal surface and also the depth of tool penetration into the retina.61
Training process and telemedicine
The recent technological advances have dramatically affected training of surgeons worldwide, especially facilitating the standardization of complex procedures through tele-mentoring systems and systematic guidance during surgery.62,63 In recent years, several virtual reality robotic systems have been developed for application in training, preoperative planning and assistance in performing surgery. Moreover, virtual simulations are a new technology that enables training under uniform conditions before entering clinical practice and provides assessment and acquisition of skills for specific surgical procedures.64,65 With an increased number of ophthalmologists adhering to the use of virtual simulators, surgical training is becoming more efficient, more realistic and more critically standardized.66
Vitreoretinal surgery with robotic assistance
Ophthalmic robots can be broadly classified into three main categories: assistive handheld instruments, comanipulation platforms, and tele-manipulation systems. Handheld instruments have some advantages, such as requiring fewer DOFs and possessing a smaller footprint, which makes it mechanically simpler and significantly less expensive to produce and implement into clinical application. Moreover, the intuitive feel of a handheld micro instrument is maintained and motion control of the tool remains in the surgeon’s hands. These attributes not only contribute to surgeon acceptance, but also may enhance safety and control for the robot, since the surgeon can respond quickly and finish the procedure in the traditional way in case of robotic failure, unexpected patient movement or they are able to simply perform the portions of the surgery that are more efficiently executed freehand.41,67 When working through a cooperative-assisted robot, the surgeon and the machine share control of a tool through a force sensor. The system “feels” the force exerted by the physician on the tool and processes this information in various modes specific to its programming to provide smooth, tremor-free, precise, positional control and force scaling to the effector-tool motion.68 Finally, in order to improve the quality of the retinal surgical procedures, tele-operated systems have been developed and implemented with the benefits of motion scaling, force feedback and better ergonomics.69
The first published report of robotic-assisted eye surgery was the Stereo-Taxical Micro-Manipulator for Ocular Surgery robot developed by Guerrouad and Vidal17 in 1989 at the Automatical Center of Lille in France. The system consisted of a “carrier” with x, y and z DOF and a “wrist” with four DOFs, permitting the device to work in spherical coordinates. Subsequent developments saw the introduction of direct current motors for smoother instrument manipulation,70 followed by integration of computer systems to control up to six DOFs mathematically. Rather than relying on physical constraints, allowing manipulation of an end effector by a multidimensional surgeon-operated joystick connected to its computer controller.71
In the early 1990s, Hunter et al72 developed a remote tele-operated system for use in a virtual operative training environment. It is comprised of a force-reflecting master and slave micro motion robot that the surgeon could manipulate remotely while wearing a virtual reality headset to control the orientation of a stereo camera system. This allowed the operator to experience simulated visual and mechanical sensations during surgery.72 The emerging tele-manipulation model comprising master and slave components was used in a collaboration between Steve Charles and the National Aeronautics and Space Administration (NASA) jet propulsion laboratory.73 The robot-assisted microsurgery device comprised of an arm with six tendon-driven joints measuring 2.5 cm in diameter and was 25 cm in length. In mock surgery testing, it was able to remove a 0.015-inch diameter particle from a simulated eyeball.73
In addition to motion scaling, a key feature of tele-manipulation systems is the RCM. To eliminate unwanted rotational eye movements during intraocular instrument manipulation, the fulcrum point, or RCM, of all x and y axes motion is positioned at a point in space beyond the tip of the “slave” instrument manipulator. This coincides in three dimensions with the sclerotomy through which instruments enter and exit the eye during retina surgery. The RCM thereby reduces unwanted forces being applied to the eye wall during instrument manipulation that would interfere with precision tracking and intraocular movement of the instrument tip. This application can be actualized using software, hardware or both.
In 1998, an Australian group led by Constable published on the efficacy of a robotic system in animal eyes that was capable of rotating about a laser-guided entry point. Four different procedures were performed: 1) drug delivery, 2) oxygen measurement, 3) micro cutting using standard endo-scissors (Grieshaber, Kennesaw, GA, USA) and 4) drainage device implantation.74 Perhaps, most significantly, they were able to place a drug-filled glass pipette into a single retinal artery and deliver a controlled dose of the drug into the vessel without significant trauma. This was made possible by the robot’s ultra-fine spatial resolution of 0.5 µm.74 A master/slave tele-operated system has also been developed by the University of Tokyo,75,76 which was used to perform microcannulation of a 70- µm diameter retinal vessel in an ex vivo porcine eye using a 30- µm glass micropipette.4
In a departure from the bulkier tele-operated systems that impose a physical separation between control input and manipulator output, Riviere et al77 designed a freehand active tremor-cancellation system called Micron, capable of motion sensing, filtering of erroneous motion and actuation of compensatory tip deflections. The handheld device kept instrument size and weight as close as possible to those of existing passive instruments. The more natural feel of this system was one of its key advantages, although unlike a tele-operated system, precise motion scaling was not possible.
Micron consisted of a motion-sensing module mounted to the back end of the steady-hand instrument handle that detected translation and rotation movements in six DOFs and used a dynamic sinusoidal model to cancel erroneous motion by estimating the time-varying frequency, amplitude and phase of surgeon tremor. This required sampling at a frequency >12 Hz to accommodate physiological tremor (typically between 8 and 12 Hz).78 Stacked piezoelectric actuators were used to effect tremor cancelation in the instrument. Experimental results showed that the prototype affected a statistically significant reduction in position error for both trained surgeons and nonsurgeons.79 Application in intraocular surgery has proven a challenge given the external guidance system in early generation systems.
U-Xuan Tan et al80 pursued a similar strategy in the development of a cost-effective, 3D-printed, handheld micromanipulator. The unique feature of this device was its use of flexures rather than pin and ball joints that facilitated a monolithic construction. This was not only conducive to 3D printing, but also potentially advantageous with respect to sterilization and long-term maintenance. Moreover, in order to address both controlled tremor-free motion and limitation of applied forces to the retina, Gonenc et al67 developed a system for membrane peeling that combined the active tremor-canceling handheld micromanipulator, Micron, with a force-sensing motorized micro- forcep. Three FBGs were incorporated onto the tip module to provide two-DOF force sensing capability with a resolution of 0.3 mN (Figure 2).
  | Figure 2 Left, motorized force-sensing micro-forceps magnified image. Right, (a) Force-sensing micro-forceps integrated with a handheld micromanipulator (Micron). (b) Two-DOF force-sensing micro-forceps for the steady-hand robot. (c) 23-gage disposable forceps (Alcon Laboratories, Inc., Fort Worth, TX, USA). Note: © 2014 IEEE. Reprinted, with permission, from Gonenc B, Feldman E, Gehlbach P, Handa J, Taylor RH, Iordachita I. Towards robot-assisted vitreoretinal surgery: force-sensing microforceps integrated with a handheld micromanipulator. Paper presented at: 2014 IEEE International Conference on Robotics and Automation (ICRA); May 31, 2014–June 7, 2014; 2014.66 Abbreviations: DOF, degree of freedom; FBG, fiber Bragg grating. |
The design of retinal microsurgery robots has not been restricted to single-arm devices. Wei et al81,82 presented plans for a two-armed 16-DOF hybrid robotic system comprising a frame attached to the patient’s head to which two identical hybrid robotic arms would rigidly attach. Movement of each insertion arm was restricted at the insertion point to the velocity of the eye surface at that point, plus any velocity along the z-axis of the instrument, i.e., employing the concept of RCM for each arm.
Eye Robot 2 is a novel augmented cooperative control method developed at the Johns Hopkins Hospital22,30,83 that incorporates both a significantly improved manipulator and an integrated microforce sensing tool (Figure 3). A series of experiments have been performed on the inner shell membrane of raw chicken eggs with the aim of identifying and controlling the forces associated with peeling operations. In the steady-hand robot approach, both surgeon and robot “hold” the instrument with the latter superimposing motion control and high-resolution sensing on operator-initiated movements.
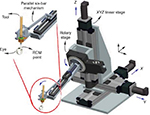  | Figure 3 Eye Robot 2 is an intermediate design toward a stable and fully capable microsurgery research platform for the evaluation and development of robot-assisted microsurgical procedures. A close-up view of its end effector is shown.83 Note: © 2010 IEEE. Reprinted, with permission, from Uneri A, Balicki MA, Handa J, Gehlbach P, Taylor RH, Iordachita I. New steady-hand eye robot with micro-force sensing for vitreoretinal surgery. Proc IEEE RAS EMBS Int Conf Biomed Robot Biomechatron. 2010;2010(26–29):814–819.83 Abbreviation: RCM, remote center of motion. |
In addition to filtering surgeon tremor, an attractive feature of this device is its force scaling or micro force-guided cooperative control. This function aims to ameliorate one of the key difficulties of retinal surgery, which is the detection of exceedingly small forces – below the threshold of tactile detection84 – applied to intraocular tissues. Overlaid robotic augmentation prevents excessive forces from being generated at the instrument tip where sensors provide “real-time” feedback to the device. Under experimental conditions with a maximum force load set at 7 mN, the operator was guided in peeling tape or egg membrane following a gradient of force toward a local minimum resistance.83 Interestingly, this resulted in a peeling angle of ~45° that corresponded to the greatest mechanical advantage.
A novel approach under development by Kummer et al85 is the use of an array of electromagnets to magnetically control untethered intraocular microbots. Proof of principle has been demonstrated for this system by its control of NdFeB permanent magnet cubes with an 800 μm (±100 μm) cube edge and a ~1.2- mm long 30-gage, glued-on needle tip. This puncturing element was placed in a silicone oil suspension covering a chicken chorioallantoic membrane, and using camera feedback, it was possible to puncture larger blood vessels (~220 μm outer diameter) of the chorioallantoic membrane under magnetic guidance.85 Magnetic control has been used in other fields of medicine, including a navigation system for endocardial catheter ablation and gastrointestinal capsule endoscopy.86
A tele-operated system developed by researchers from the Eindhoven University of Technology has been used to relieve experimental retinal vein occlusion by direct intraluminal injection of ocriplasmin into a porcine model.87,88 The Preceyes master/slave layout facilitates motion scaling and tremor filtering and has a standby function89 that could also be used to deliver slow injections into anatomical locations demanding supreme precision such as into the subretinal space for retinal gene therapy. It also features an integrated z-axis virtual boundary that can be set by the operator to limit the amplitude of advancements in that plane directed by operator input. This serves as both a precision and safety feature of the device. Together, the system represents precision improvement of 10–20 times compared to the human hand.89
Robotic augmentation in ophthalmic surgery has not been restricted to the retina; it has also been explored in the anterior segment. Using a dual instrument tele-operated master/slave system called Intraocular Robotic Interventional and Surgical System, Rahimy et al90 were able to create a continuous curvilinear anterior capsulorhexis with removal of cortical lens material from the capsular bag in porcine eyes. This was in addition to the system performing a pars plana vitrectomy and inducing a posterior vitreous detachment. They were also able to demonstrate micro cannulation of porcine retinal vessels without traumatizing surrounding tissue.90 Bimanual tele-operated penetrating keratoplasty has been performed in porcine eyes and cadaveric human eyes using three arms of a da Vinci surgical robot (Intuitive Surgical Inc.).91 Mechanical trephination, cardinal sutures, continuous 10/0 nylon sutures and suture adjustments were all managed using this robotic system.
Present limitations, challenges and future directions
A rapidly growing body of evidence is emerging that robotic devices, strategically applied may provide a higher level of surgical capability than is possible using freehand procedures. This observation strongly supports the premise that research in the field should be continued and suggests that the return on such investments of time and resources will be rewarded. As part of the emergence and relative infancy of the field, it is also expected that a number of well-defined, as well as yet undiscovered risks and complications remain to be addressed. The points of maximum impact in surgical care have not yet been fully defined and there will be aspects of freehand microsurgery for which robotic implementation is cost prohibitive or is simply not advantageous. Moreover, identifying these points of high value in implementation will be of great utility in creating surgeon, payer and patient acceptance.92,93 Questions relating to comparative efficacy, cost, safety and relevant use remain unanswered at this time, given the lack of long-term comparative studies available to date.94,95
In this context, a recent study directed at understanding surgeon perception of importance of various aspects of vitreoretinal surgical systems found that precision and safety remain very important as has been previously reported in the literature. High importance was also ascribed to mechanism stability, excellent visualization, collision avoidance and compatibility with the surgical environment. These attributes were valued relatively higher than positioning control and cost of the device, as is the case with robotic technology.96
There are already several surgical procedures for which robotic intervention has been considered as the new gold standard.97 Recent advances in robot-assisted vitreoretinal surgery reflect innovative engineering developments in the field, including but not limited to, tools that are progressively smaller in diameter, high-speed cutters, better visualization systems and wide-field illumination probes. The rapid advances in instrumentation and mechanization in this very challenging field have positioned it as an ideal point of entry for novel robotic systems.98 This is made most apparent in recent news reports of the Preceyes team performing the world’s first robotic intraocular surgery. At the time of writing, this clinical trial involved 12 patients. In the initial study, the robot was used for membrane peeling, and in the second part, robot-assisted with subretinal injection in preparation for retinal gene therapy.99
Conclusion
Robotics technology has begun to have a significant impact on many surgical specialties. An increasing number of robotic systems and task-specific applications are now being sold worldwide. Against this backdrop, the small but highly technical field of robotic retinal surgery is rapidly evolving and has witnessed an exponential growth in technological development and capabilities over the last three decades. With the emergence of relevant capabilities including, but not limited to, tremor cancelation, enhanced dexterity, haptic feedback, micron-scale distance sensing and sensor-servo functions and others. Robotics has become among the most promising trends in advancing the field of retinal microsurgery. Currently, there is a broad lack of clinical experience among potential users, and medical robotics is still associated with real challenges to implementation, including but not limited to learning curves, costs, risks and complications. However, given the emerging advantages of robotic medicine, continuous support and investment in the field are warranted.
Acknowledgments
MR was supported by Lemann Foundation, Instituto de Visao-IPEPO, São Paulo, Brazil. PLG was supported by Research to Prevent Blindness, New York, NY, USA, and gifts by the J. Willard and Alice S. Marriott Foundation, the Gale Trust, Mr. Herb Ehlers, Mr. Bill Wilbur, Mr. & Mrs. Rajandre Shaw, Ms. Helen Nassif and Mr. Ronald Stiff.
Disclosure
The authors report no conflicts of interest in this work.
References
Panait L, Doarn CR, Merrell RC. Applications of robotics in surgery. Chirurgia (Bucur). 2002;97(6):549–555. | ||
Moran ME. Rossum’s universal robots: not the machines. Journal of endourology. 2007;21(12):1399–1402. | ||
Asimov I1-1. I, robot. New York: Gnome Press. 1950. | ||
Ida Y, Sugita N, Ueta T, Tamaki Y, Tanimoto K, Mitsuishi M. Microsurgical robotic system for vitreoretinal surgery. Int J Comput Assist Radiol Surg. 2012;7(1):27–34. | ||
Thakor NV, Shanbao T. Therapeutic technologies in neuroengineering. IEEE Engineering in Medicine and Biology Magazine. 2006;25(5):30–31. | ||
Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE transactions on bio-medical engineering. 1988;35(2):153–160. | ||
Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clinical orthopaedics and related research. 1992(285):57–66. | ||
Bargar WL, Bauer A, Borner M. Primary and revision total hip replacement using the Robodoc system. Clinical orthopaedics and related research. 1998(354):82–91. | ||
Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surgical laparoscopy, endoscopy & percutaneous techniques. 2002;12(1):6–16. | ||
Himpens J, Leman G, Cadiere GB. Telesurgical laparoscopic cholecystectomy. Surgical endoscopy. 1998;12(8):1091. | ||
Jeganathan VS, Shah S. Robotic technology in ophthalmic surgery. Current opinion in ophthalmology. 2010;21(1):75–80. | ||
Channa R, Iordachita I, Handa JT. Robotic Vitreoretinal Surgery. Retina. 2016. | ||
Cleary K, Nguyen C. State of the art in surgical robotics: clinical applications and technology challenges. Computer aided surgery: official journal of the International Society for Computer Aided Surgery. 2001;6(6):312–328. | ||
Vitiello V, Lee SL, Cundy TP, Yang GZ. Emerging robotic platforms for minimally invasive surgery. IEEE reviews in biomedical engineering. 2013;6:111–126. | ||
Mylonas GP, Kwok KW, James DR, et al. Gaze-Contingent Motor Channelling, haptic constraints and associated cognitive demand for robotic MIS. Medical image analysis. 2012;16(3):612–631. | ||
Spitznas M. Motorized teleguided stereotactic micromanipulator for vitreous microsurgery. Archives of ophthalmology (Chicago, Ill: 1960). 1983;101(4):623–630. | ||
Guerrouad A, Vidal P. SMOS: stereotaxical microtelemanipulator for ocular surgery. Paper presented at: Engineering in Medicine and Biology Society, 1989. Images of the Twenty-First Century., Proceedings of the Annual International Conference of the IEEE Engineering in1989; 9–12 Nov, 1989. Seattle, WA, USA. | ||
Machemer R, Parel J-M, Buettner H. A new concept for vitreous surgery: 1. Instrumentation. American journal of ophthalmology. 1972;73(1):1–7. | ||
Machemer R. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol. 1971;75:813–820. | ||
Vander Poorten E, Esteveny L, Gijbels A, et al. Use Case for European Robotics in Ophthalmologic Micro-Surgery. Paper presented at: Proceedings of the 5th Joint Workshop on New Technologies for Computer/Robot Assisted Surgery2015; 10–12 September, 2015. Brussels, Belgium. | ||
He X, van Geirt V, Gehlbach P, Taylor R, Iordachita I. IRIS: Integrated Robotic Intraocular Snake. IEEE Int Conf Robot Autom. 2015;2015:1764–1769. | ||
Mitchell B, Koo J, Iordachita I, et al. Development and application of a new steady-hand manipulator for retinal surgery. Paper presented at: Robotics and Automation, 2007 IEEE International Conference on2007. | ||
Morris M, Tosunoglu S. Robotic Ocular Surgery. Florida Conference on Recent Advances in Robotics. 2015; 10–14 April 2007. Rome, Italy. | ||
Eshner AA. A graphic study of tremor. The Journal of experimental medicine. 1897;2(3):301–312. | ||
Harwell RC, Ferguson RL. Physiologic tremor and microsurgery. Microsurgery. 1983;4(3):187–192. | ||
Gao A, Gonenc B, Guo J, Liu H, Gehlbach P, Iordachita I. 3-DOF force-sensing micro-forceps for robot-assisted membrane peeling: Intrinsic actuation force modeling. Paper presented at: 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob); 26–29 June 2016 Singapore. | ||
Gonenc B, Gehlbach P, Taylor RH, Iordachita I. Effects of Micro-Vibratory Modulation during Robot-Assisted Membrane Peeling. Rep U S. 2015;2015:3811–3816. | ||
Balicki M, Uneri A, Iordachita I, Handa J, Gehlbach P, Taylor R. Micro-force sensing in robot assisted membrane peeling for vitreoretinal surgery. Medical image computing and computer-assisted intervention: MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2010;13(Pt 3):303–310. | ||
He X, Gehlbach P, Handa J, Taylor R, Iordachita I. Toward robotically assisted membrane peeling with 3-DOF distal force sensing in retinal microsurgery. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6859–6863. | ||
Taylor R, Jensen P, Whitcomb L, et al. A steady-hand robotic system for microsurgical augmentation. Int J Robot Res. 1999;18(12):1201–1210. | ||
Gonenc B, Handa J, Gehlbach P, Taylor RH, Iordachita I. A Comparative Study for Robot Assisted Vitreoretinal Surgery: Micron vs. the Steady-Hand Robot. IEEE Int Conf Robot Autom. 2013:4832–4837. | ||
Riviere CN, Jensen PS. A study of instrument motion in retinal microsurgery. Paper presented at: Engineering in Medicine and Biology Society, 2000. Proceedings of the 22nd Annual International Conference of the IEEE2000; 23–28 July 2000. Chicago, IL, USA. | ||
Singhy SPN, Riviere CN. Physiological tremor amplitude during retinal microsurgery. Paper presented at: Proceedings of the IEEE 28th Annual Northeast Bioengineering Conference (IEEE Cat. No.02CH37342); 21–21 April 2002. Philadelphia, PA, USA. | ||
Jonas J, Gusek G, Naumann G. Parapapillary diameter of retinal vessels. I. Estimating the size of the optic papilla (a papillometric study of over 264 normal eyes). Klinische Monatsblatter fur Augenheilkunde. 1988;192(4):325–328. | ||
Shahid H, Hossain P, Amoaku WM. The management of retinal vein occlusion: is interventional ophthalmology the way forward? The British journal of ophthalmology. 2006;90(5):627–639. | ||
Karia N. Retinal vein occlusion: pathophysiology and treatment options. Clinical Ophthalmology (Auckland, NZ). 2010;4:809–816. | ||
Gonenc B, Tran N, Riviere CN, Gehlbach P, Taylor RH, Iordachita I. Force-Based Puncture Detection and Active Position Holding for Assisted Retinal Vein Cannulation. IEEE/SICE/RSJ International Conference on Multisensor Fusion and Integration for Intelligent Systems IEEE/SICE/RSJ International Conference on Multisensor Fusion and Integration for Intelligent. 2015;2015:322–327. | ||
Gonenc B, Tran N, Gehlbach P, Taylor RH, Iordachita I. Robot-assisted retinal vein cannulation with force-based puncture detection: Micron vs. the steady-hand eye robot. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:5107–5111. | ||
Gonenc B, Gehlbach P, Handa J, Taylor RH, Iordachita I. Force-Sensing Microneedle for Assisted Retinal Vein Cannulation*. Proc IEEE Sens. 2014;2014:698–701. | ||
Gonenc B, Handa J, Gehlbach P, Taylor RH, Iordachita I. Design of 3-DOF force sensing micro-forceps for robot assisted vitreoretinal surgery. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5686–5689. | ||
Kuru I, Gonenc B, Balicki M, et al. Force sensing micro-forceps for robot assisted retinal surgery. Paper presented at: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Aug. 28 2012-Sept. 01 2012. San Diego, CA, USA. | ||
Taylor RH, Funda J, Grossman DD, Karidis JP, LaRose DA. Remote center-of-motion robot for surgery. Google Patents; 1995. | ||
He X, Balicki MA, Kang JU, et al. Force sensing micro-forceps with integrated fiber bragg grating for vitreoretinal surgery. Paper presented at: Proc. SPIE2012; 30 January, 2012. San Francisco, CA, USA. | ||
Samad MD, Hu Y, Sutherland GR. Effect of force feedback from each DOF on the motion accuracy of a surgical tool in performing a robot-assisted tracing task. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2093–2096. | ||
He X, Gehlbach P, Handa J, Taylor R, Iordachita I. Development of A Miniaturized 3-DOF Force Sensing Instrument for Robotically Assisted Retinal Microsurgery and Preliminary Results. Proceedings of the IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics. 2014;2014:252–258. | ||
He X, Balicki M, Gehlbach P, Handa J, Taylor R, Iordachita I. A Multi-Function Force Sensing Instrument for Variable Admittance Robot Control in Retinal Microsurgery. IEEE Int Conf Robot Autom. 2014;2014:1411–1418. | ||
Sunshine S, Balicki M, He X, et al. A force-sensing microsurgical instrument that detects forces below human tactile sensation. Retina. 2013;33(1):200–206. | ||
Gupta PK, Jensen PS, de Juan E. Surgical forces and tactile perception during retinal microsurgery. Lect Notes Comput Sc. 1999;1679:1218–1225. | ||
Cutler N, Balicki M, Finkelstein M, et al. Auditory force feedback substitution improves surgical precision during simulated ophthalmic surgery. Investigative ophthalmology & visual science. 2013;54(2):1316–1324. | ||
He X, Balicki M, Gehlbach P, Handa J, Taylor R, Iordachita I. A Novel Dual Force Sensing Instrument with Cooperative Robotic Assistant for Vitreoretinal Surgery. IEEE Int Conf Robot Autom. 2013;2013:213–218. | ||
He X, Handa J, Gehlbach P, Taylor R, Iordachita I. A submillimetric 3-DOF force sensing instrument with integrated fiber Bragg grating for retinal microsurgery. IEEE transactions on bio-medical engineering. 2014;61(2):522–534. | ||
Horise Y, He X, Gehlbach P, Taylor R, Iordachita I. FBG-based sensorized light pipe for robotic intraocular illumination facilitates bimanual retinal microsurgery. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:13–16. | ||
Lavinsky F, Lavinsky D. Novel perspectives on swept-source optical coherence tomography. Int J Retina Vitreous. 2016;2:25. | ||
Cheon GW, Huang Y, Cha J, Gehlbach PL, Kang JU. Accurate real-time depth control for CP-SSOCT distal sensor based handheld microsurgery tools. Biomed Opt Express. 2015;6(5):1942–1953. | ||
Song C, Park DY, Gehlbach PL, Park SJ, Kang JU. Fiber-optic OCT sensor guided “SMART” micro-forceps for microsurgery. Biomed Opt Express. 2013;4(7):1045–1050. | ||
Song C, Gehlbach PL, Kang JU. Active tremor cancellation by a “smart” handheld vitreoretinal microsurgical tool using swept source optical coherence tomography. Optics express. 2012;20(21):23414–23421. | ||
Song C, Gehlbach PL, Kang JU. CP-OCT sensor guided SMART micro-forceps. Paper presented at: Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications XIV, February 1, 2014 - February 2, 20142014; San Francisco, CA, United states. | ||
Song C, Gehlbach PL, Kang JU. Swept source optical coherence tomography based smart handheld vitreoretinal microsurgical tool for tremor suppression. Paper presented at: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Aug. 28 2012-Sept. 01 2012. San Diego, CA, USA. | ||
Song C, Gehlbach PL, Kang JU. Ball Lens Fiber Optic Sensor based Smart Handheld Microsurgical Instrument. Proceedings of SPIE--the International Society for Optical Engineering. 2013;8576. | ||
Song C, Gehlbach PL, Kang JU. Ball Lens Fiber Optic Sensor based Smart Handheld Microsurgical Instrument. In: Gannot I, ed. Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications Xiii. Vol 8576. Bellingham: Spie-Int Soc Optical Engineering; 2013; p 85760I. | ||
Balicki M, Han JH, Iordachita I, et al. Single fiber optical coherence tomography microsurgical instruments for computer and robot-assisted retinal surgery. Medical image computing and computer-assisted intervention: MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2009;12(Pt 1):108–115. | ||
Lee JH, Tanaka E, Woo Y, et al. Advanced real-time multi-display educational system (ARMES): An innovative real-time audiovisual mentoring tool for complex robotic surgery. Journal of surgical oncology. 2017. | ||
Antoniou SA, Antoniou GA, Franzen J, et al. A comprehensive review of telementoring applications in laparoscopic general surgery. Surgical endoscopy. 2012;26(8):2111–2116. | ||
Cook DA, Hatala R, Brydges R, et al. Technology-enhanced simulation for health professions education: a systematic review and meta-analysis. Jama. 2011;306(9):978–988. | ||
Someya Y, Omata S, Hayakawa T, et al. Training system using Bionic-eye for internal limiting membrane peeling. Paper presented at: 2016 International Symposium on Micro-NanoMechatronics and Human Science (MHS); 28–30 Nov. 2016. Nagoya, Japan. | ||
Mukai N, Harada M, Muroi K, Miyamoto Y, Uratani A, Yano T. Development of a PC-based real-time surgical simulator. Systems and Computers in Japan. 2002;33(7):11–20. | ||
Gonenc B, Feldman E, Gehlbach P, Handa J, Taylor RH, Iordachita I. Towards robot-assisted vitreoretinal surgery: Force-sensing micro-forceps integrated with a handheld micromanipulator. Paper presented at: 2014 IEEE International Conference on Robotics and Automation (ICRA); May 31 2014-June 07 2014. Hong Kong, China. | ||
Fleming I, Balicki M, Koo J, et al. Cooperative robot assistant for retinal microsurgery. Medical image computing and computer-assisted intervention: MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2008;11(Pt 2):543–550. | ||
Gijbels A, Poorten EBV, Stalmans P, Brussel HV, Reynaerts D. Design of a teleoperated robotic system for retinal surgery. Paper presented at: 2014 IEEE International Conference on Robotics and Automation (ICRA); May 31 2014-June 7 2014. Hong Kong, China. | ||
Pournaras CJ, Shonat RD, Munoz J-L, Petrig BL. New ocular micromanipulator for measurements of retinal and vitreous physiologic parameters in the mammalian eye. Experimental eye research. 1991;53(6):723–727. | ||
Grace KW, Colgate JE, Glucksberg MR, Chun JH. A six degree of freedom micromanipulator for ophthalmic surgery. Paper presented at: [1993] Proceedings IEEE International Conference on Robotics and Automation; 2–6 May 1993. Atlanta, GA, USA. | ||
Hunter IW, Doukoglou TD, Lafontaine SR, et al. A teleoperated microsurgical robot and associated virtual environment for eye surgery. Presence: Teleoperators & Virtual Environments. 1993;2(4):265–280. | ||
Charles S, Das H, Ohm T, et al. Dexterity-enhanced telerobotic microsurgery. Paper presented at: Advanced Robotics, 1997. ICAR’97. Proceedings., 8th International Conference on 1997; 7–9 July 1997. Monterey, CA, USA. | ||
Yu DY, Cringle SJ, Constable IJ. Robotic ocular ultramicrosurgery. Australian and New Zealand journal of ophthalmology. 1998;26 Suppl 1:S6–8. | ||
Takahashi H, Yonemura T, Sugita N, et al. Master manipulator with higher operability designed for micro neuro surgical system. Paper presented at: Robotics and Automation, 2008. ICRA 2008. IEEE International Conference on 2008; 9–23 May 2008. Pasadena, CA, USA. | ||
Nakano T, Sugita N, Ueta T, Tamaki Y, Mitsuishi M. A parallel robot to assist vitreoretinal surgery. Int J Comput Assist Radiol Surg. 2009;4(6):517–526. | ||
Riviere CN, Wei Tech A, Khosla PK. Toward active tremor canceling in handheld microsurgical instruments. IEEE Transactions on Robotics and Automation. 2003;19(5):793–800. | ||
Elble RJ, WC. K. Tremor. Baltimore: University Press. | ||
Maclachlan RA, Becker BC, Tabares JC, Podnar GW, Lobes LA, Jr., Riviere CN. Micron: an Actively Stabilized Handheld Tool for Microsurgery. IEEE transactions on robotics: a publication of the IEEE Robotics and Automation Society. 2012;28(1):195–212. | ||
Tan U-X, Latt WT, Shee CY, Ang WT. Design and development of a low-cost flexure-based hand-held mechanism for micromanipulation. Paper presented at: Robotics and Automation, 2009. ICRA’09. IEEE International Conference on2009. | ||
Wei W, Goldman R, Simaan N, Fine H, Chang S. Design and theoretical evaluation of micro-surgical manipulators for orbital manipulation and intraocular dexterity. Paper presented at: Robotics and Automation, 2007 IEEE International Conference on2007. | ||
Wei W, Goldman RE, Fine HF, Chang S, Simaan N. Performance evaluation for multi-arm manipulation of hollow suspended organs. IEEE transactions on robotics. 2009;25(1):147–157. | ||
Uneri A, Balicki MA, Handa J, Gehlbach P, Taylor RH, Iordachita I. New Steady-Hand Eye Robot with Micro-Force Sensing for Vitreoretinal Surgery. Proceedings of the IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics. 2010;2010(26–29):814–819. | ||
Jensen P, Gupta P, De Juan E. Quantification of microsurgical tactile perception. Paper presented at: [Engineering in Medicine and Biology, 1999. 21st Annual Conference and the 1999 Annual Fall Meetring of the Biomedical Engineering Society] BMES/EMBS Conference, 1999. Proceedings of the First Joint1999; 13–16 Oct 1999. Atlanta, GA, USA. | ||
Kummer MP, Abbott JJ, Kratochvil BE, Borer R, Sengul A, Nelson BJ. OctoMag: An electromagnetic system for 5-DOF wireless micromanipulation. IEEE Transactions on Robotics. 2010;26(6):1006–1017. | ||
Carpi F, Pappone C. Stereotaxis Niobe® magnetic navigation system for endocardial catheter ablation and gastrointestinal capsule endoscopy. Expert review of medical devices. 2009;6(5):487–498. | ||
de Smet MD, Meenink TC, Janssens T, et al. Robotic Assisted Cannulation of Occluded Retinal Veins. PloS one. 2016;11(9):e0162037. | ||
de Smet MD, Stassen JM, Meenink TC, et al. Release of experimental retinal vein occlusions by direct intraluminal injection of ocriplasmin. British Journal of Ophthalmology. 2016;100(12):1742–1746. | ||
Meenink T, Naus G, de Smet M, Beelen M, Steinbuch M. Robot assistance for micrometer precision in vitreoretinal surgery. Investigative ophthalmology & visual science. 2013;54(15):5808–5808. | ||
Rahimy E, Wilson J, Tsao TC, Schwartz S, Hubschman JP. Robot-assisted intraocular surgery: development of the IRISS and feasibility studies in an animal model. Eye (London, England). 2013;27(8):972–978. | ||
Bourges JL, Hubschman JP, Burt B, Culjat M, Schwartz SD. Robotic microsurgery: corneal transplantation. The British journal of ophthalmology. 2009;93(12):1672–1675. | ||
Pitcher JD, Wilson JT, Tsao T-C, Schwartz SD, Hubschman J-P. Robotic eye surgery: Past, present, and future. Journal of Computer Science & Systems Biology. 2012;5(2):1. | ||
Zorn KC, Gofrit ON, Orvieto MA, et al. Da Vinci Robot Error and Failure Rates: Single Institution Experience on a Single Three-Arm Robot Unit of More than 700 Consecutive Robot-Assisted Laparoscopic Radical Prostatectomies. Journal of endourology. 2007;21(11):1341–1344. | ||
Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. The New England journal of medicine. 2010;363(8):701–704. | ||
Salman M, Bell T, Martin J, Bhuva K, Grim R, Ahuja V. Use, cost, complications, and mortality of robotic versus nonrobotic general surgery procedures based on a nationwide database. The American surgeon. 2013;79(6):553–560. | ||
Xu H, Yang Y. Customer Requirements for a Vitreoretinal Robot. International Conference on Management Science and Management Innovation (MSMI 2014) 2014; 14–15 June, 2014. Changsha, China. | ||
Wedmid A, Llukani E, Lee DI. Future perspectives in robotic surgery. BJU international. 2011;108(6 Pt 2):1028–1036. | ||
Molaei A, Abedloo E, de Smet MD, et al. Toward the Art of Robotic-assisted Vitreoretinal Surgery. Journal of ophthalmic & vision research. 2017;12(2):212–218. | ||
MacLaren R. World first for robot eye operation. [Last accessed on 2017 Jun 27]. http://www.ox.ac.uk/news/2016–09-12-world- first-robot-eye-operation2016. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
