Back to Journals » Drug Design, Development and Therapy » Volume 12
Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: a meta-analysis of published trials
Received 4 February 2018
Accepted for publication 10 May 2018
Published 17 September 2018 Volume 2018:12 Pages 3013—3019
DOI https://doi.org/10.2147/DDDT.S164553
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Yongping Liu, Jun Meng, Guichan Wang
Department of Gynecology, Obstetrics, Yuhuangding Hospital, Yantai, Shandong Province, People’s Republic of China
Aims: We aimed to comprehensively assess the risk of gastrointestinal toxicities associated with poly (ADP-ribose) polymerase inhibitors (PARPis) in the treatment of ovarian cancer patients.
Materials and methods: We searched several databases for relevant trials. Eligible studies included prospective Phase II and III trials of ovarian cancer patients on the four PARPis (olaparib, veliparib, niraparib and rucaparib), describing events of nausea, vomiting, diarrhea, and constipation. Summary incidence, relative risk (RR), and 95% CIs were calculated employing fixed- or random-effects models.
Results: A total of 2,286 ovarian cancer patients from 12 trials were included for analysis. Our results showed that summary incidences of all-grade gastrointestinal events in ovarian cancer patients were nausea 68.8% (95% CI, 63.5%–73.6%), vomiting 36.2% (95% CI, 30.9%–41.8%), diarrhea 25.3% (95% CI, 21.2%–29.8%), and constipation 25.3% (95% CI, 17.9%–34.5%). The RRs of all-grade nausea, vomiting, diarrhea, and constipation were 2.00 (95% CI: 1.79–2.24; P<0.001), 2.12 (95% CI: 1.75–2.58; P<0.001), 1.20 (95% CI: 1.01–1.44; P=0.044), and 1.20 (95% CI: 0.88–1.80; P=0.21); respectively. While, the RRs of high-grade nausea, vomiting, diarrhea, and constipation were 3.74 (95% CI: 1.50–9.36; P=0.005), 2.81 (95% CI: 1.17–6.74; P=0.02), 0.56 (95% CI: 0.22–1.43; P=0.23), 0.92 (95% CI: 0.34–2.49, P=0.87); respectively.
Conclusion: Our study suggests that the risk of all-grade gastrointestinal toxicities associated with PARPis, excepting constipation, is significantly increased in ovarian cancer patients. And the use of PARPis significantly increased the risk of developing high-grade nausea and vomiting, but not for diarrhea and constipation. Close clinical monitoring is recommended when administering these drugs.
Keywords: poly (ADP-ribose) polymerase inhibitors, gastrointestinal toxicities, clinical trials, meta-analysis, targeted agents, gynaecological tumors, systematic review
Introduction
Ovarian cancer is the second most common gynecologic malignancy and is the leading cause of death from gynecologic cancers worldwide.1,2 Typically, ovarian cancer presents with advanced stages at the time of diagnosis.3 Although most patients with advanced ovarian cancer initially receive platinum-based chemotherapy and achieve a clinical response, the effectiveness of the treatments diminishes over time, and most of these patients will ultimately relapse.4–6 The prognosis of these recurrent ovarian cancer patients is very poor, with a median survival ranging from 12 months to 24 months.7 Clearly, novel effective drugs or treatment strategies that can improve long-term disease control in recurrent ovarian cancer are urgently needed.8
Recent advances in the understanding of the molecular biology and mechanism of epithelial ovarian cancer have led to the development of a number of targeted therapies, including poly-ADP-ribose polymerase inhibitors (PARPis).9,10 PARPis are a novel class of therapeutic agents that target tumors with deficiencies in the homologous recombination DNA repair pathway. Until now, three PARPis olaparib,11 niraparib,12 and rucaparib13 have been approved for use in ovarian cancer by the US Food and Drug Administration. As these drugs have now entered routine clinical practice, more and more patients would receive PARPis, and concerns increase regarding the toxicities associated with the administration of PARPis. In a previously published meta-analysis conducted by Zhou et al,14 the authors found that treatment with the PARPis olaparib, veliparib, and niraparib was associated with a significant increase in the risk of developing hematologic toxicities in cancer patients. Gastrointestinal (GI) toxicities associated with PARPis have been reported in clinical trials, but the results are controversial.15,16 As a result, we conducted the present meta-analysis of all available trials to comprehensively assess the overall incidence and risk of selected GI toxicities (nausea, vomiting, diarrhea, and constipation) associated with PARPis in the treatment of ovarian cancer.
Materials and methods
Data source
Several databases including PubMed, Web of Science and Cochrane library were searched for relevant trials. The search key words were PARPis, olaparib, veliparib, niraparib, rucaparib and ovarian cancer. Additionally relevant articles in the reference lists of recent meta-analyses that investigated PARPis in ovarian cancer patients were also searched. In order to avoid duplication, only the most complete or recent was considered for analysis. All results were input into Endnote X8 reference software (Thomson Reuters, Stamford, CT, USA) for duplication exclusion and further reference management. Finally, the most updated package inserts of olaparib, niraparib, and rucaparib were reviewed to identify other relevant information. Trials were chosen and reviewed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.17
Study selection
The primary objective of the present study was to evaluate the overall incidence of PARPis-related GI toxicities and the risk of GI toxicities in ovarian cancer patients treated with PARPis; therefore, only prospective phase II and III trials evaluating PARPis in ovarian cancer patients with adequate data on GI toxicities were incorporated in the analysis. Phase I trials were omitted due to multiple dose level and limited sample sizes. Clinical trials that met the following criteria were included as follows: 1) prospective phase II and III trials, expanded access protocols (EAPs); 2) participants assigned to treatment with PARPis (alone or in combination at any dosage or frequency); and 3) available data regarding events or incidence of GI toxicities and sample size. Phase I trials were excluded because of inter-study variability in drug dosing as well as the small number of patients in these trials.
Data extraction
Two investigators independently conducted data abstraction, and any discrepancy between the reviewers was resolved by consensus. Most of the incorporated trials have used the common terminology criteria of adverse events version 4.0 for grading of the relevant adverse events. A checklist of necessary data to be extracted from each study included: first author’s name, year of publication, phases of trials, number of enrolled subjects, treatment arms, number of patients in treatment and controlled groups, median age, median progression-free survival, and the number of each of the selected GI adverse events.
Statistical analysis
The primary summary measures were incidence, relative risk (RR), and corresponding 95% CIs of all grade (grade 1–4) and high-grade (grade 3–4) selected GI toxicities. All statistical analyses were performed by using Version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, NJ, USA). For the calculation of incidence, the number of patients with selected GI toxicities in PARPis alone and the total number of patients receiving PARPis alone were extracted. To calculate RR, patients assigned to PARPis were compared only with those assigned to control treatment in the same trial. Between-study heterogeneity was estimated using the χ2-based Q statistic.18 Heterogeneity was considered statistically significant when Pheterogeneity<0.1. The presence of publication bias was evaluated by using the Begg and Egger tests.19,20 A statistical test with a P-value <0.05 was considered significant.
Results
Search results
Our search yielded 240 potentially relevant citations on PARPis from the searched databases. The details for the study inclusion/exclusion procedure are illustrated in Figure 1. A total of 12 trials were considered eligible for the analysis, including three Phase III trials21–23 and nine Phase II trials.24–32 A total of 2,286 patients were included in this analysis. Most patients had a performance score between 0 and 2 and competent liver, kidney and bone marrow function. The baseline patients’ characteristics in each trial are described in Table 1.
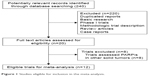  | Figure 1 Studies eligible for inclusion in the meta-analysis. |
  | Table 1 Baseline characteristics of included trials |
Overall incidence of relevant adverse events
For the incidence analysis, we considered ovarian cancer patients receiving PARPis monotherapy. The pooled incidence of all-grade nausea, vomiting, diarrhea, and constipation was 68.8% (95% CI, 63.5%–73.6%), 36.2% (95% CI, 30.9%–41.8%), 25.3% (95% CI, 21.2%–29.8%), and 25.3% (95% CI, 17.9%–34.5%), respectively. There was significant heterogeneity among trials, and we thus performed the meta-analysis by using a random-effects model (Table 2). As for high-grade GI toxicities, the pooled incidence of GI toxicities associated with PARPis was nausea 3.4% (95% CI, 2.6%–4.5%), vomiting 2.0% (95% CI, 1.4%–3.0%), diarrhea 1.7% (95% CI, 1.0%–3.0%), and constipation 1.4% (95% CI, 0.9%–2.3%), respectively. There was no significant heterogeneity among included trials, and we thus performed the meta-analysis by using a fixed-effects model (Table 2).
  | Table 2 Incidence of adverse outcomes for clinical trials included in the meta-analysis |
RR of all-grade relevant GI toxicities
A meta-analysis of the RR of all-grade adverse events was performed on the included randomized controlled trials (RCTs), which contained a direct comparison between PARPis and control treatment. The RRs of all-grade nausea, vomiting, diarrhea, and constipation were 2.00 (95% CI: 1.79–2.24; P<0.001, Figure 2A), 2.12 (95% CI: 1.75–2.58; P<0.001, Figure 2B), 1.20 (95% CI: 1.01–1.44; P=0.044, Figure 2C), 1.26 (95% CI: 0.88–1.20); P<0.001, Figure 2D); respectively. Thus, patients treated with PARPis had an increased risk of developing all-grade nausea, vomiting, diarrhea, and constipation. The fixed effects model was used for all the evaluated toxicities, except for constipation.
RR of high-grade relevant GI toxicities
RCTs directly comparing PARPis with control treatment were included to calculate RRs of high-grade GI toxicities. The RR of high-grade nausea, vomiting, diarrhea, and constipation were 3.74 (95% CI: 1.50–9.36; P=0.005, Figure 3A), 2.81 (95% CI: 1.17–6.74; P=0.02, Figure 3B), 0.56 (95% CI: 0.22–1.43; P=0.23, Figure 3C), 0.92 (95% CI: 0.34–2.49, P=0.87, Figure 3D); respectively. Thus, patients treated with PARPis had an increased risk of developing high-grade nausea and vomiting, but not for high-grade diarrhea and constipation. The fixed effects model was used for all evaluated toxicities.
Discussion
To the best of our knowledge, this is the most comprehensive and up-dated meta-analysis to provide an investigation of overall incidence and risk of GI toxicities in ovarian cancer patients treated with PARPis. Our analysis of data demonstrates that the risk of all-grade gastrointestinal toxicities associated with PARPis is significantly increased in the treatment of ovarian cancer. In addition, the use of PARPis in ovarian cancer significantly increases the risk of developing high-grade nausea and vomiting, but not for diarrhea and constipation. The four PARPis evaluated within our analysis are olaparib, veliparib, niraparib, and rucaparib. And three of them olaparib, niraparib, and rucaparib have been approved for use in ovarian cancer due to their survival benefits. Additionally, a number of ongoing Phase II and III studies are assessing these agents in multiple other solid tumors indications.33,34 Therefore, the use of PARPis is anticipated to increase in the near future, and clinicians should pay particular attention to regular monitoring of GI toxicities related to PARPis during the administration of these drugs.
Until now, GI toxicities are considered an important cause for treatment interruption and/or permanent discontinuation in the clinical trials. Although high-grade GI toxicities are relatively uncommon, there are currently no methods to predict patients at highest risk, and thus regular monitoring of clinical parameters during the administration of these drugs is warranted. Generally, administration of supportive therapy should be undertaken with the earliest manifestation of nausea and vomiting experienced by ovarian cancer patients treated by PARPis.
However, as far as we know, there are no specific guidelines for the treatment of PARPis-induced GI toxicities due to lack of controlled studies addressing the subject. The philosophy of management of anticancer-related GI toxicities extends to employing some nutritional interventions. In a recent Cochrane review evaluating how to minimize the frequency of diarrhea associated with pelvic radiotherapy, the authors found that combinations of modified fat, lactose-restriction, fat-restriction and fiber supplementation was recommended to reduce to the frequency of diarrhea.35 Additionally, according to the package insert of approved PARPis,36–38 if grade three or higher non-hematological toxicity occurs, temporary dose interruption and/or dose reduction to half the dose previously administered should be considered based on tolerability and severity.
A number of limitations are needed to be acknowledged: first, as with any similar meta-analysis, the pooled results of our study are significantly affected by the confounding factors of individual studies that are chosen. Second, this meta-analysis only includes published trials, and a meta-analysis of individual level data might define more clearly treatment benefits in specific subgroups. Third, heterogeneity is observed in some of the RR analyses. This may be related to the different PARPis for the RR analysis, and differences in sample size, although we have tried to minimize its influence by using the random-effect model. Finally, as the present meta-analysis excludes Phase I trials, the impact of PARPis dose/schedules on the risk of gastrointestinal toxicities could not be fully assessed in this analysis.
Conclusion
Our study suggests that the risk of all-grade gastrointestinal toxicities associated with PARPis is significantly increased in ovarian cancer patients receiving these drugs. And the use of PARPis significantly increased the risk of developing high-grade nausea and vomiting, but not for diarrhea and constipation. Although the rate of clinically high-grade GI toxicities is very low, clinicians should be aware of these risks and perform regular assessment for such toxicities during administration of these drugs. Additionally, further research into the pathogenesis of these toxicities is still needed.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Siegel R, Ma J, Zou Z, Jemal A, Statistics C. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Bookman MA. Optimal primary therapy of ovarian cancer. Ann Oncol. 2016;27(Suppl. 1):i58–i62. | ||
Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81(1):17–38. | ||
Marth C, Reimer D, Zeimet AG. Front-line therapy of advanced epithelial ovarian cancer: standard treatment. Ann Oncol. 2017;28(Suppl. 8):viii36–viii39. | ||
Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14(10):1020–1026. | ||
Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology. 2013;27(4):288298–294. | ||
Mariappan L, Jiang XY, Jackson J, Drew Y. Emerging treatment options for ovarian cancer: focus on rucaparib. Int J Womens Health. 2017;9:913–924. | ||
Bitler BG, Watson ZL, Wheeler LJ, Behbakht K. PARP inhibitors: clinical utility and possibilities of overcoming resistance. Gynecol Oncol. 2017;147(3):695–704. | ||
Sisay M, Edessa D. PARP inhibitors as potential therapeutic agents for various cancers: focus on niraparib and its first global approval for maintenance therapy of gynecologic cancers. Gynecol Oncol Res Pract. 2017;4:18. | ||
Kim G, Ison G, Mckee AE, et al. FDA Approval summary: Olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21(19):4257–4261. | ||
Scott LJ. Niraparib: first global approval. Drugs. 2017;77(9):1029–1034. | ||
Balasubramaniam S, Beaver JA, Horton S, et al. FDA Approval summary: Rucaparib for the treatment of patients with deleterious BRCA mutation-associated advanced ovarian cancer. Clin Cancer Res. 2017;23(23):7165–7170. | ||
Zhou JX, Feng LJ, Zhang X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2017;11:3009–3017. | ||
Moore KN, Monk BJ. Patient counseling and management of symptoms during olaparib therapy for recurrent ovarian cancer. Oncologist. 2016;21(8):954–963. | ||
Samol J, Ranson M, Scott E, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Invest New Drugs. 2012;30(4):1493–1500. | ||
Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8(12):e83138. | ||
Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. | ||
Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–371. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. | ||
Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–1961. | ||
Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. | ||
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. | ||
Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–379. | ||
Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–861. | ||
Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. | ||
Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199–203. | ||
Bell-McGuinn KM, Konner JA, Tew WP, et al. A phase 2, single arm study of iniparib in patients with BRCA1 or BRCA2 associated advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer. Int J Gynecol Cancer. 2016;26(2):255–260. | ||
Coleman RL, Sill MW, Bell-McGuinn K, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation – An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137(3):386–391. | ||
Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–1214. | ||
Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. | ||
Lowery MA, Kelsen DP, Capanu M, et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19–26. | ||
Han HS, Diéras V, Robson M, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29(1):154–161. | ||
Henson CC, Burden S, Davidson SE, Lal S. Nutritional interventions for reducing gastrointestinal toxicity in adults undergoing radical pelvic radiotherapy. Cochrane Database Syst Rev. 2013;11(11):CD009896. | ||
Zejula (niraparib) FDA prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208447Orig1s000Lbl.pdf. Accessed January 6, 2018. | ||
Rubraca (rucaparib) FDA prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/209115Orig1s000Lbl.pdf. Accessed January 6, 2018. | ||
LYNPARZA (olaparib) FDA prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206162s008lbl.pdf. Accessed January 6, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


