Back to Journals » International Journal of General Medicine » Volume 14
Risk of Cardiovascular Disease Mortality in Relation to Depression and 14 Common Risk Factors
Received 14 November 2020
Accepted for publication 13 January 2021
Published 12 February 2021 Volume 2021:14 Pages 441—449
DOI https://doi.org/10.2147/IJGM.S292140
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Zhaoqi Jia, Sen Li
School of Life Sciences, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Correspondence: Sen Li
School of Life Sciences, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Email [email protected]
Background: Depression has been linked to a worse prognosis of Cardiovascular disease (CVD), and these two diseases share a variety of common risk factors such as unhealthy lifestyles and chronic medical conditions. However, the potential role of these common risk factors in modulating the association between depression and CVD mortality and whether the co-occurrence of depression and a specific common risk factor has a cumulative impact on CVD mortality are still largely unknown.
Methods: We pooled data from 2005– 2014 of Nation health and nutritional examination survey, leading to a study population of 22,177 adults. The Patient Health Questionnaire was employed to assess the depression symptoms, and information on CVD mortality was obtained from the linked mortality file of NHANES. Fourteen common risk factors of depression and CVD were included in this study.
Results: Based on the interaction analyses, we found overweight was protective for the risk of CVD death in depressive participants, but not in people without depression. Moreover, relative risk-based analyses indicated a mutually promotive effect of depression and baseline CVD or living alone on CVD mortality.
Conclusion: The novel findings in our study may facilitate risk stratification in the clinical programs targeting CVD mortality and help to shed light on the differential pathophysiological mechanisms in the depression-mediated elevation of CVD mortality.
Keywords: depression, cardiovascular disease mortality, epidemiology, NHANES
Introduction
Depression is a major psychiatric disorder involving emotional dysfunction and reduced life quality and has a deleterious effect on physical health.1 Cardiovascular disease (CVD) is one of the major causes of death at a global level.2 These two public health issues currently become the most common causes of disability in high-income countries according to the recent reports of World Health Organization.3
Depression can promote the occurrence of CVD, accelerate the pathogenesis, and lead to undesirable outcomes.1,3 These two diseases share a variety of common risks such as unhealthy lifestyles and chronic illnesses.4 People with depression normally present unhealthy lifestyles like sedentariness, drinking, or smoking,5 which also serve as CVD risk factors.1 Besides, living alone and self-rated health were linked with both depression and a higher risk of CVD.6–9 Moreover, people with depression are more likely to develop chronic diseases such as diabetes, high blood pressure and stroke,4,10 which may potentially contribute to increased CVD mortality.11–13 Despite the crucial role of these common risk factors played in the pathogenesis and prognosis of depression and CVD, their potential contributions in mediating and modulating the association between depression and CVD mortality are still largely unknown. Thus, we perform the current study to illustrate (1) whether the relationship between depression and CVD mortality could be modulated by a certain risk factor, and (2) whether the co-occurrence of depression and a specific risk factor has a cumulative impact on CVD mortality. Our results may shed light on the optimized allocation of scarce supportive care resources for the prevention of CVD deaths in depressed people.
Methods
Cohort
We pooled data from 2005–2014 of The Nation health and nutritional examination Survey (NHANES). Information on CVD mortality is obtained from the public-use documentation for linked mortality recorded in the national death index (NDI) death certificate. Of the 49,116 participants who were interviewed between 2005–2014, 26,517 had missing information for the depression and demographic status. Besides these participants, we also excluded individuals who lost to follow-up or did not have information about other confounders. Please see Figure 1 for participants enrollment flowchart.
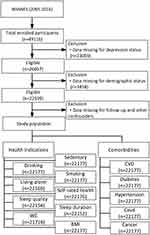 |
Figure 1 Participants enrollment flowchart including exclusion criteria-NHANES 2005–2014. |
Depression
The Patient Health Questionnaire (PHQ) was employed to examine the depression symptoms, and a cutoff score of 15 or higher has been defined as major depression.14,15
CVD Mortality
The CVD death information used in the study was based on the specific cause of death in the linked mortality file. Participants with missing death information at the end of follow-up or died from other causes were censored.
Common Risk Factors
The 14 common risk factors included in this study were reported to be associated with either depression or baseline CVD/CVD death rate. They were generalized into health indicators and status of chronic diseases, which contained 9 and 5 risk factors, respectively. Health indicators included living alone, self-rated health, sleep duration, sleep quality, smoking, alcohol use, sedentary status, BMI and waist circumference (WC). Chronic diseases included cardiovascular disease, diabetes, cancer, hypertension and cerebrovascular disease, and were derived from self-reported data.
Demographic Variables
Demographic information that we employed in this study was collected by respondent self-report at baseline: gender, age in years, race, education, poverty income ratio.
Statistical Analysis
A Chi-square test was employed to examine the possible difference in baseline variables between people with and without depression. Logistic regression was employed to assess the association between depression/baseline CVD and each of the 14 common risk factors. Hazard ratios were calculated using the Cox proportional hazards (PH) regression without and with sequential multivariable adjustment. Schoenfeld’s residuals were used to evaluate the proportional hazards assumption in all models, and the covariates that violate the PH assumption were incorporated in the stratified COX regression. To account for the effect of non-CVD mortality, we employed fine and grey model for competing risk analysis. We used the “Survminer” package to visualize the cumulative incidence curves in R. The statistical analyses of this study were carried out using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Weighted distribution of sample characteristics at baseline is shown in Table 1. In general, the depressive individuals were more likely to be female, 40–59 years, non-white ethnicity, to have less education and income below median. The median follow-up time was 68 months in this study. We first investigated the contribution of depression on CVD mortality and found that participants with depression had higher CVD mortality (HRadjusted=1.99; 95% CI: 1.16, 3.43). Considering that a variety of risk factors may mediate and modulate the association between depression and CVD mortality, we included 14 known common risk factors of depression and CVD in the current study.1,4 A multiplicative interaction term between depression and each of the 14 risk factors was added to the main model of cox regression, which suggested a significant interaction effect between depression and BMI or WC on CVD mortality (Pinteraction=0.03 and 0.01, respectively). This was further confirmed by results of cox regression in depression-stratified subpopulations that BMI or WC was only significantly associated with CVD mortality in depressive participants, but not in people without depression (Supplementary Tables 1 and 2). Analyses of the covariate-adjusted dose-response relationship between BMI/WC, as continuous variables, and CVD mortality by three-knot restricted cubic splines consistently indicated that the relative hazards of CVD death rate decreased with increasing BMI/WC levels in depressive people (Figure 2).
 |
Table 1 Weighted Baseline Characteristics of the Study Population-NHANES 2005–2014 |
The relative risk-based cumulative effect of depression and each of the 14 risk factors on CVD mortality was subsequently analyzed. As expected, the coexistence of depression and baseline CVD presented a higher risk for CVD mortality than those with only depression or baseline CVD (Figure 3, Supplementary Table 3). Similar results were found for living status (Figure 3, Supplementary Table 3), indicating the mutually promotive effect of depression and these risk factors on CVD mortality. It is noted that the results of these analyses did not survive Bonferroni multiple testing correction. Interestingly, depressive participants with higher BMI or WC had a significantly lower relative risk for CVD mortality compared to those with depression and normal BMI or WC (Supplementary Table 3), suggesting that overweight (represented by higher BMI/WC) was a protective factor for CVD mortality in depressive people. Similar results from cox regression were obtained after accounting for potential competing risk of death from other causes (Supplementary Table 4). The cumulative incidence curves (CIC) and weighted CVD mortality according to statuses of depression and risk factors also presented trends that were consistent with cox regression (Figure 4).
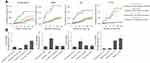 |
Figure 4 Survival curves (A) and weighted CVD mortality (B) by statuses of depression and living alone, BMI, WC or baseline CVD. |
Discussion
Depression can cause adverse effects on cardiovascular health in clinical and sub-clinical aspects,16,17 while CVD also increases the risk of depression through physical or biological changes.18 This two-way spiral relationship between depression and CVD reflected not only the onset of the disease but also its progression.1 Considering that there is no systematic analysis of how the association between depression and CVD mortality is regulated by their common risk factors, we conducted this research to explore whether this relationship is modulated by a specific factor and whether the coexistence of depression with a specific risk factor has a cumulative effect on CVD mortality.
Health indicators have been proposed to interplay with depression and cardiovascular risk.19–21 For example, people with depression are less likely to engage in healthy behaviors, which may contribute to an increased risk of CVD22–24 and its poor outcomes.6 Moreover, living alone is associated with both depression7,8 and CVD mortality.9 Interestingly, we found depression and living alone were mutually promotive for CVD mortality by relative risk-based analyses (Figure 3, Supplementary Table 3). Poor drug compliance resulting from living alone has been reported, which indirectly leads to negative health consequences in this population.25 Having family members reminded them to take their medication is beneficial to CVD treatment in people with depression.26 Thus, living alone may exaggerate CVD and elevated CVD mortality in depressive people due to insufficient drug compliance.26 Furthermore, about 70% of people with depression are reported to lack help-seeking behavior.27 Therefore, among people who live alone, depressive participants may be less likely to seek help during acute diseases, such as acute myocardial infarction, leading to a higher CVD mortality compared to people without depression.
Lipid disorders and abdominal obesity are important driving forces in the relationship between depression and CVD. Abdominal adipose tissue produces cytokines or hormones, some of which, such as leptin, have been shown to affect hippocampal and cortical structures,28 which is consistent with the clinical observation that metabolic abnormality caused by abdominal obesity will keep patients depressed.18 Furthermore, obesity has been linked to many risk factors of CVD, such as elevated blood pressure, and has direct effects on the structure of the heart.29 A significant interaction between depression and BMI or WC on CVD mortality had been identified in our study, indicating the influence of these common risk factors on CVD mortality varied with depression status (Supplementary Table 1). Moreover, our relative risk-based analyses indicated that CVD mortality was significantly lower in participants with both depression and high BMI/WC compared to those with depression and low BMI/WC, suggesting the protective role of overweight on CVD mortality in depressive people (Figure 3, Supplementary Table 3). One explanation of our observation comes from an epidemiologic consensus called the obesity paradox, which indicates that obese people do not show worse results when certain adverse events occur.30,31 For example, compared with thin or normal-weight patients, patients who are overweight or even moderately obese have a lower risk of death.32 Moreover, higher body weight means better nutrition or higher metabolic reserve33 and better cardiorespiratory fitness,29 which may contribute to a better CVD prognosis in depressive people. Furthermore, obese patients are generally considered to be at high risk for disease prognosis, making them have better health management or a more active treatment attitude.34
Depression has been reported as an effective predictor of CVD or diabetes.35,36 However, evidence of whether these chronic diseases combined with depression contribute to higher CVD death rate is less investigated. Thus, the potential cumulative effect of depression and five chronic diseases on CVD mortality was further analyzed. As expected, the coexistence of depression and baseline CVD presented a more substantial adverse effect on CVD death than participants with depression or baseline CVD alone, indicating depression and baseline CVD were mutually promotive for CVD mortality. It has been reported that patients with both diabetes and depression have a higher mortality rate from coronary heart disease than those who are depressed, but not diabetic (HR=1.88; 95% CI: 1.23, 2.87).10 A nested case-control study on patients with type 2 diabetes showed that depression is associated with increased risk of cardiovascular death even after adjustment of non-persistence to antidiabetic drug treatment.37 We found diabetes further increased CVD mortality in depressive participants with marginal significance after multivariable adjustment (P=0.054). A cohort study showed that patients with both hypertension and depression have increased mortality from the ischemic heart disease (HR=1.59; 95% CI: 1.08, 2.34) compared to people without hypertension and depression, which is slightly higher than those who are depressed, but not hypertensive (HR=1.54; 95% CI: 1.01, 2.36).38 We consistently observed a moderate increase in CVD mortality in sampling persons with both hypertension and depression compared to those with depression only (Figure 3). Similar results were obtained for CeVD, but participants with both depression and cancer had a non-significantly reduced CVD mortality compared to depressive participants without cancer (Figure 3). Identical results were also obtained by using a competing risk model, which excluded the possible bias from other competing events (Supplementary Table 4). Thus, CVD-related chronic diseases generally increased CVD mortality in people with depression in our study population. This is consistent with the notion that patients with depression are more likely to develop fatal CVD events after developing certain types of chronic disease.4 One explanation of our observation is that depression may hinder the pursuit of treatment in patients with chronic diseases, such as seeking medical attention promptly, taking the medication regularly, or choosing healthy behaviors,39,40 leading to an increased CVD risk as a comorbidity of these chronic diseases. Our study has several limitations. First, the study variables were categorized as binary variables, and thus the severity of chronic diseases was not considered. Second, the status of chronic diseases was based on participants’ self-reports. Another limitation of this work is that the information of common risk factors was only obtained at baseline, and the changes of these risk factors during the follow-up period were not available. Despite these limitations, the current study used nationally representative data from a 10-year follow-up cohort to comprehensively evaluate the effect of 14 risk factors on the complicated relationship between depression and CVD mortality, which were unprecedented in previous reports.
Conclusion
The relationship between depression and CVD mortality is complex, and the present study revealed that BMI and WC played a moderating role in this relationship. Moreover, baseline CVD or living alone promoted CVD mortality in the coexistence of depression while overweight was protective for the risk of CVD death in depressive participants. The novel findings in our study may facilitate risk stratification in the clinical programs targeting CVD mortality, and help to shed light on the differential pathophysiological mechanisms in the depression-mediated elevation of CVD mortality.
Abbreviations
NHANES, National Health and Nutrition Examination Survey; NCHS, National Centers for Health Statistics; CDC, Centers for Disease Control and Prevention; NDI, National Death Index; PH, proportional hazards; PIR, poverty income ratio; BMI, body mass index; PHQ, the Patient Health Questionnaire; WC, waist circumference; CVD, cardiovascular disease; CeVD, cerebrovascular disease; HR, Hazard ratio; OR, odds ratio; N, number; S.E., standard error; 95% CI, 95% confidence limit; CIC, cumulative incidence curves.
Data Sharing Statement
Data analyzed in this study is from NHANES (2005–2014). Data are publicly available and can be downloaded from NHANES website: http://www.cdc.gov/nchs/nhanes.htm.
Ethics Approval and Consent to Participate
Data analyzed in the present study were from NHANES. Protocols involved were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB), and consent from all participants was documented.
Funding
This study is supported by the National Natural Science Foundation of China (Grant No. 81703942 and 81973698), Young Elite Scientists Sponsorship Program by CACM (Grant No. 2019-QNRC2-B08), Science Fund for Distinguished Young Scholars in BUCM (Grant No. BUCM-2019-JCRC004) and BUCM research start-up fund (to SL).
Disclosure
All authors report no conflicts of interest.
References
1. Penninx BW. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74(PtB):277–286.
2. Lopez AD, Adair T. Is the long-term decline in cardiovascular-disease mortality in high-income countries over? Evidence from national vital statistics. Int J Epidemiol. 2019;48(6):1815–1823. doi:10.1093/ije/dyz143
3. Bucciarelli V, Caterino AL, Bianco F, et al. Depression and cardiovascular disease: the deep blue sea of women’s heart. Trends Cardiovasc Med. 2019. doi:10.1016/j.tcm.2019.05.001
4. Atlantis E, Shi Z, Penninx BJ, Wittert GA, Taylor A, Almeida OP. Chronic medical conditions mediate the association between depression and cardiovascular disease mortality. Soc Psychiatry Psychiatr Epidemiol. 2012;47(4):615–625. doi:10.1007/s00127-011-0365-9
5. Bruin MC, Comijs HC, Kok RM, Van der Mast RC, Van den Berg JF. Lifestyle factors and the course of depression in older adults: a NESDO study. Int J Geriatr Psychiatry. 2018;33(7):1000–1008. doi:10.1002/gps.4889
6. Rantanen AT, Korkeila JJA, Kautiainen H, Korhonen PE. Poor or fair self-rated health is associated with depressive symptoms and impaired perceived physical health: a cross-sectional study in a primary care population at risk for type 2 diabetes and cardiovascular disease. Eur J Gen Pract. 2019;25(3):143–148. doi:10.1080/13814788.2019.1635114
7. Fukunaga R, Abe Y, Nakagawa Y, Koyama A, Fujise N, Ikeda M. Living alone is associated with depression among the elderly in a rural community in Japan. Psychogeriatrics. 2012;12(3):179–185. doi:10.1111/j.1479-8301.2012.00402.x
8. Stahl ST, Beach SR, Musa D, Schulz R. Living alone and depression: the modifying role of the perceived neighborhood environment. Aging Ment Health. 2017;21(10):1065–1071. doi:10.1080/13607863.2016.1191060
9. Jensen MT, Marott JL, Holtermann A, Gyntelberg F. Living alone is associated with all-cause and cardiovascular mortality: 32 years of follow-up in the Copenhagen Male Study. Eur Heart J Qual Care Clin Outcomes. 2019;5(3):208–217. doi:10.1093/ehjqcco/qcz004
10. Egede LE, Nietert PJ, Deyi Z. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28(6):1339. doi:10.2337/diacare.28.6.1339
11. Roche MM, Wang PP. Sex differences in all-cause and cardiovascular mortality, hospitalization for individuals with and without diabetes, and patients with diabetes diagnosed early and late. Diabetes Care. 2013;36(9):2582–2590. doi:10.2337/dc12-1272
12. Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens. 2010;23(1):38–45. doi:10.1038/ajh.2009.191
13. Lau KK, Wong YK, Teo KC, et al. Stroke patients with a past history of cancer are at increased risk of recurrent stroke and cardiovascular mortality. PLoS One. 2014;9(2):e88283.
14. Gara MA, Minsky S, Silverstein SM, Miskimen T, Strakowski SM. A naturalistic study of racial disparities in diagnoses at an outpatient behavioral health clinic. Psychiatr Serv. 2019;70(2):130–134.
15. Werremeyer A, Maack B, Strand MA, Barnacle M, Petry N. Disease control among patients with diabetes and severe depressive symptoms. J Prim Care Community Health. 2016;7(2):130–134. doi:10.1177/2150131915627423
16. Seldenrijk A, Vogelzangs N, Hout H, Marwijk H, Diamant M, Penninx BW. Depressive and anxiety disorders and risk of subclinical atherosclerosis: findings from the Netherlands Study of Depression and Anxiety (NESDA). J Psychosom Res. 2010;69(2):203–210.
17. Hamer M, Kivimaki M, Lahiri A, Marmot MG, Steptoe A. Persistent cognitive depressive symptoms are associated with coronary artery calcification. Atherosclerosis. 2010;210(1):209–213. doi:10.1016/j.atherosclerosis.2010.01.038
18. Penninx BWJH. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2016;74(PtB):277–286.
19. Barton DA, Dawood T, Lambert EA, et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25(10):2117–2124. doi:10.1097/HJH.0b013e32829baae7
20. Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57(2):531–553.
21. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi:10.1097/PSY.0b013e3181907c1b
22. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease. Arch Intern Med. 2005;165(21):2508. doi:10.1001/archinte.165.21.2508
23. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depressive symptoms and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523–532. doi:10.1007/s12160-016-9777-9
24. Tang HY, Sayers SL, Weissinger G, Riegel B. The role of depression in medication adherence among heart failure patients. Clin Nurs Res. 2014;23(3):231–244. doi:10.1177/1054773813481801
25. Park HY, Seo SA, Yoo H, Lee K. Medication adherence and beliefs about medication in elderly patients living alone with chronic diseases. Patient Prefer Adherence. 2018;12:175–181. doi:10.2147/PPA.S151263
26. Kusaslan Avci D. Evaluation of the relationship between loneliness and medication adherence in patients with diabetes mellitus: a cross-sectional study. J Int Med Res. 2018;46(8):3149–3161. doi:10.1177/0300060518773223
27. Kim Y, Kim H-Y, Jang SJ. Factors and help-seeking behaviors associated with depression in Korean adults: review of data from 2014 and 2016 Korea national health and nutrition examination surveys. Psychiatry Res. 2019;275:10–19. doi:10.1016/j.psychres.2019.03.013
28. Paz-Filho G, Wong M-L, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract. 2010;64(13):1808–1812. doi:10.1111/j.1742-1241.2010.02536.x
29. Lavie CJ, De Schutter A, Parto P, et al. Obesity and prevalence of cardiovascular diseases and prognosis – the obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–547. doi:10.1016/j.pcad.2016.01.008
30. Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk? Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12(9):680–687. doi:10.1111/j.1467-789X.2011.00879.x
31. Goyal A, Nimmakayala KR, Zonszein J. Is there a paradox in obesity? Cardiol Rev. 2014;22(4):163. doi:10.1097/CRD.0000000000000004
32. Doehner W, Clark A, Anker SD The obesity paradox: weighing the benefit. 2010:146.
33. Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165(1):55–61. doi:10.1001/archinte.165.1.55
34. Amundson DE, Djurkovic S, Matwiyoff GN. The obesity paradox. Crit Care Clin. 2010;26(4):583–596. doi:10.1016/j.ccc.2010.06.004
35. Koen VDK, Hein VH, Harm M, Haan M, Coen S, Aartjan B. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2010;22(7):613–626.
36. Gara MA, Minsky S, Silverstein SM, et al. A naturalistic study of racial disparities in diagnoses at an outpatient behavioral health clinic. Psychiatr Serv. 2019;70:130–134.
37. Lunghi C, Zongo A, Tardif I, É D, Guénette L. Depression but not non-persistence to antidiabetic drugs is associated with mortality in type 2 diabetes: a nested case-control study. Diabetes Res Clin Pract. 2020;171:108566. doi:10.1016/j.diabres.2020.108566
38. Axon RN, Zhao Y, Egede LE. Association of depressive symptoms with all-cause and ischemic heart disease mortality in adults with self-reported hypertension. Am J Hypertens. 2010;23(1):30–37. doi:10.1038/ajh.2009.199
39. Penninx BW, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–1726. doi:10.1001/jama.279.21.1720
40. Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53(4):0–902.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


