Back to Journals » Research and Reports in Urology » Volume 14
Risk Factors for Urologic Complications After Kidney Transplantation and Impact in Graft Survival
Authors Nino-Torres L, Garcia-Lopez A , Patino-Jaramillo N , Giron-Luque F, Nino-Murcia A
Received 4 May 2022
Accepted for publication 6 September 2022
Published 28 September 2022 Volume 2022:14 Pages 327—337
DOI https://doi.org/10.2147/RRU.S371851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Panagiotis J Vlachostergios
Laura Nino-Torres,1 Andrea Garcia-Lopez,2 Nasly Patino-Jaramillo,2 Fernando Giron-Luque,1 Alejandro Nino-Murcia1
1Department of Transplantation Surgery, Colombiana de Trasplantes, Bogotá, Colombia; 2Department of Transplantation Research, Colombiana de Trasplantes, Bogotá, Colombia
Correspondence: Alejandro Nino-Murcia, Transplant Surgeon, Colombiana de Trasplantes, Av Carrera, 30 No. 47 A-74, Bogota, 111311, Colombia, Tel +57 3112499999, Email [email protected]
Background and Purpose: Kidney transplantation (KT) is the best therapy for chronic kidney disease (CKD). Major urologic complications (MUCs) are the second etiology associated to morbidity and graft loss following KT, after rejection episodes. The objective of this study was to estimate the incidence, risk factors and impact on graft survival associated to urological complications in KT patients.
Patients and Methods: A retrospective cohort based on electronic patient files of kidney transplant recipients from Colombiana de Trasplantes was created for the period August 2008 to September 2019. Initiation of follow-up was defined as the date of transplantation up to 3 years post-transplantation. Incidence of ureteral stenosis, ureteral obstruction, and ureteral leak was measured. A logistic regression multivariate model was adjusted to determine the associated factors to MUCs (yes/no). Patient and graft survival time were analyzed using a Kaplan-Meier method.
Results: A total of 1584 KT patients were included in the cohort. MUCs were present in 195 (12.6%) KT patients. We found that dialysis duration (OR: 1.004; p = 0.02) remained significant for the incidence of MUCs in KT patients of deceased donors. Probability of graft and patient survival at 3 years of follow-up was 90.5% and 85.5%, respectively. No significant difference was found on graft and patient survival in KT patients with or without MUCs.
Conclusion: MUCs are frequent complications for KT. We did not observe significant differences in graft or patient survival according to the presence of MUCs. The identification of MUCs and risk factors may guide transplant teams for future surgical and clinical decisions.
Keywords: major urologic complications, kidney transplantation, graft survival, patient survival
Introduction
Kidney transplantation (KT) is the gold standard therapy for chronic kidney disease (CKD),1 with an important impact in quality of life.2 KT patients are immunosuppressed and may be fragile due to comorbidities associated with long standing CKD.3
Major urologic complications (MUCs) comprise the second cause of morbidity following KT4, after rejection episodes, and can be associated with graft loss,5 and mortality.3,6 MUCs are defined as a urinary leak, ureteral stenosis, bladder outlet obstruction (BOO), and graft lithiasis.6,7 The most common MUCs are urinary leak.7 The MUCs reflected longer hospital stays, higher total cost per patient,1,8 and often require additional interventions.9 They can present at any time after transplantation10 and prognosis is good if identified and treated in a timely manner.5 The most common site of MUCs location is at the ureterovesical anastomosis.7
Overall reported rate of MUCs post transplantation varies from 2.5% to 14% depending on the series2,3 and the criteria used to define it.4 Multiple MUCs causes have been documented like donor age, renal artery variants, male sex recipient, cytomegalovirus (CMV) infection, delayed graft function (DGF), and the extensive dissection of the periureteric connective tissue.11 Over the last years, this incidence has reduced due to the use of double J stents,12–14 adaptation of the extravesical Lich-Gregoir ureteroneocystostomy technique, improvement in kidney harvesting technique preserving blood supply and reduction of steroid use.10
The objective of this study is to estimate the incidence, risk factors and impact on graft survival associated to urological complications in KT patients.
Material and Methods
We conducted a retrospective cohort study at Colombiana de Trasplantes. To give a context it must be said that Colombiana de Trasplantes is a transplant network in Colombia with 4 centers with around 21% of the annual national kidney transplant activity. Electronic records of kidney transplant recipients who were transplanted between August 2008 and September 2019 were reviewed. Patients requiring auto transplantation, with evidence of arterial or venous renal thrombosis requiring nephrectomy were excluded. Donor, recipient, and surgical variables were collected from institutional medical records for our data base. Recipients were followed up to graft failure, death, or end of follow-up at 3 years post transplantation, whichever was earliest. Expanded criteria donors (ECD) were defined as any donor ≥60 years old, or between 50 and 59 years old with at least 2 of the following: serum creatinine >1.5 mg/dl, history of hypertension or death from cerebrovascular accident.15 This research was conducted in accordance with the Declaration of Helsinki, and that organ donations were conducted in congruence with the Declaration of Istanbul.
Recipients were divided into two groups, one group with and one group without urological complications within 3 years after KT.
Pre- Transplant Recipient Evaluation
All patients were evaluated prior to transplantation, including a mental health, nephrology, and transplant surgery evaluation. Etiology of CKD was defined as history of CKD and native kidney biopsy, and urine production volume was noted. Abdominal ultrasonography was performed. For patients with previous congenital abnormalities and long-standing diabetic patients further urologic studies included urodynamic testing and/or cystography.
Organ Harvesting, Kidney Donor Allocation and Storage
During organ harvesting, it is emphasized the importance of avoiding excessive dissection of the “golden triangle”: ureter, kidney’s lower pole and renal artery as well as preservation of lower pole accessory arteries.
For deceased brain donors (DBD), the National Health Institute in Colombia allocates organs according to geographical location within the nation, blood type, accumulated time on the waiting list, HLA mismatch, sensitization and loss of vascular access.16 Diverse preservation solutions are used by different transplant centers during procurement. Kidneys are preserved in cold static storage. Donation after circulatory death is not performed in Colombia. Kidney donor profile index (KDPI) online calculator was used for research purposes and not for organ allocation.
Surgical Technique
Kidney anatomy (vascular multiplicity, ureteral duplication), laterality, cold and warm ischemia times were recorded. For arterial multiplicity a side to side or end to side anastomosis is performed at the bench (Living donor kidney transplant (LDKT)) or included in Carrel patch (deceased donor kidney transplant (DDKT)). For polar arteries of <2 mm diameter ligation is performed. All ureteroneocystostomies were performed under extravesicular Lich-Gregoir technique: trimming and spatulation of the ureter with a previous assessment of its irrigation and a cystotomy on the bladder dome. A running absorbable suture around the ureter border and the vesical mucosa ensuring a tension-free anastomosis, followed by closure of the detrusor muscle over the anastomosis as a tunnel with a continuous absorbable suture. Double J ureteral stents were not routinely used for the initial procedure, unless intraoperative findings such as a very small, atrophic bladder, a witnessed leak or a ureter-ureterostomy was performed. It is also used at a second procedure following a urological complication. Ureter-ureterostomy was reserved for difficult bladders or kidneys with shorter ureter. For some time, stents were tied to the foley catheter tip for removal as an outpatient, this was then discontinued. Intraoperative urological complications were recorded.
Postoperative Care
Most of our patients were included in our protocol: immunosuppression medication is based upon induction mainly with polyclonal anti-thymocyte globulin (6 mg/kg dose divided in three days) for every patient regardless of the risk. Protocol of maintenance immunosuppression included tacrolimus (tailored according to trough levels 5–8 ng/dl) and mycophenolate mofetil (1000 mg twice daily) without steroids. This regimen is started on day 1 after transplantation.
Prophylaxis is given with preemptive therapy for Cytomegalovirus with valganciclovir 3 times a week for 3 months (450 mg), trimethoprim sulfamethoxazole 3 times a week for 3 months (960 mg) and albendazole 400 mg the first two days after transplantation and a similar dose 15 days after as Strongyloides stercoralis prophylaxis. Patients are discharged day 2 postoperative and followed closely as an outpatient by a multidisciplinary team.
Foley catheters were routinely removed on postoperative day 5 unless the surgeon considered a friable mucosa and left it up to 10 days. Clinical and laboratory follow up was performed but no routine image was obtained unless there was a clinical suspicion of a complication.
Urological Complications
Urological complications (all dichotomous variables: absent or present) were identified after clinical suspicion, by radiology and surgical records. Urine leak was defined as drainage or collection formation of urine around the transplant kidney and ureter requiring treatment with surgery and/or a percutaneous nephrostomy (PCN). Ureteral necrosis was defined as perfusion impairment of the ureter as evidenced intra operatively. Ureteral stenosis was defined as an initial ultrasound urinary system dilation of the graft, confirmed by a percutaneous antegrade pyelography which required PCN followed by surgical correction. Ureteral stenosis was differentiated for ureteral external obstruction by imaging or intraoperatively and timing of presentation.
Bladder outlet obstruction (BOO) was defined as evidence of urinary retention followed by urology consultation and imaging evidence of prostate or urethral obstruction. Early treatment and diagnosis of BOO occurring in the lower urinary tract is fundamental for the maintenance of graft functions.17
Graft lithiasis was defined as presence of lithiasis identified either in the donor before procurement or during imaging evaluation at post transplantation. However, our center does not perform imaging evaluation in deceased donors before procurement and we accept live kidney donors with a unique kidney stone. Therefore, it could correspond to an incidental finding.
MUCs were defined as ureteral stenosis, ureteral obstruction, and ureteral leak.18 They were also classified according to the timing of their postoperative presentation in early (<1 month), medium (1–6 months) and late (>6 months).
Surgical Management of Urologic Complications
Surgical corrections depend on the urological complication. For urine leak or ureteral stenosis, a new ureteroneocystostomy with double-J stent is performed. If there is evidence of a higher leak point in the pelvis or there is a necrotic ureter a uretero-pyelostomy or uretero-ureterostomy may be performed. Small bowel interposition may be executed. If the leak is from the parenchyma a suture may be performed. Other complications may require prostatectomy or urethral dilation.
Statistical Analysis
Descriptive statistics were used to report the population characteristics: frequencies and percentages for categorical variables, numerical variables were reported according to their distribution using mean and standard deviation for normally distributed variables, and median and inter-quartile range (IQR) for non-normally distributed variables. There were few missing values (49 was the maximum value in dialysis duration), and those were randomly distributed. Therefore, we performed a complete available case analysis in the univariate and multivariate models.
Comparisons between the two groups (urologic complications yes/no) were performed using Pearson’s chi-square test for categorical variables and the t-test for numerical variables.
Logistic regression analysis was applied to model the outcome (urologic complications) as dichotomous variable. We calculated odds ratios (OR) with 95% confidence intervals (CI), to identify independent risk factors for urological complications. Variables with p-values less than or equal to 0.20 and those with clinical importance were included in a full multivariate logistic regression model. The full model was subjected to variable selection, removing those with a highest p-value one by one. A p-value of <0.05 in our multivariate model was considered statistically significant.
Statistical analysis was performed using R version 4.0.3, using the following libraries: tidyverse, ggplot2, ISLR, survival, KMsurv.
 |
Table 1 Donor Characteristics and Frequency of MUCs After KT |
Graft and patient survival were analyzed using a Kaplan–Meier curve for survival distribution and compared using a Log rank test.
Donor Characteristics
DDKT (n = 781, 60.6%) and LDKT (n = 507, 39.4%) were performed. Donor age mean was 39.5 ± 13.7 years. Overall, the bivariate analysis found that the incidence of MUCs was significant comparing DDKT and LDKT (p = 0.02). No significant associations of any other donor baseline factors such as donor age, BMI grade, expanded criteria donor, or KDPI with the development of urologic complication was observed. Data of baseline donor characteristics are shown in Table 1.
Ethics Considerations
This study was approved by the Institutional Research Committee (Ethics Committee DexaDiab), acting in concordance with local and national regulations. The informed consent from the transplant recipients in this study was waived by the Ethics Committee due to its retrospective design. None of the transplant donors were from a vulnerable population and all donors or next of kin provided written voluntary informed consent.
Results
Patient Demographics and Clinical Characteristics
After excluding patients with graft thrombosis (n = 82) and auto-transplantation (n = 2), a total of 1584 KT patients were analyzed during the study period. Most patients were male, with an overall mean age of 41.7 ± 14.7 years. Female proportion was lower (n = 627, 39.6%), with 73 (37.4%) MUCs cases in this population. MUCs were present in 195 (12.6%). Twenty kidney recipients had more than one KT. There was no significant difference in the bivariate analysis of gender, age, underlying cause of CKD, type of dialysis, anuria, diabetes history and BMI grades. Intraoperative findings reported cold ischemia time mean in 13.5 hours. Lower cold ischemia time was associated with MUCs in the bivariate analysis (p = 0.0008). In the anatomical findings, there were 1209 (76.3%) KT patients with a single renal artery and 375 (23,7%) KT patients with arterial multiplicity. Among those, 153 KT patients with single renal artery and 42 KT patients with arterial multiplicity developed MUCs. No differences were found between single renal artery or multiple renal arteries with the risk of MUCs. Sixty-four KT patients required intraoperative double J stent placement. Of those, 7 KT patients had MUCs. Cystostomy was performed in 27 KT patients, and 3 out of these 27 KT patients had MUCs. Dialysis duration, cold ischemia time, and Operation Room Time (ORT) were included in the multivariate analysis. Table 2 summarizes the recipient characteristics, intraoperative findings, and the incidence of MUCs after KT.
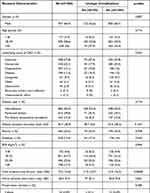 |
Table 2 Recipient Baseline Characteristics, Intraoperative Findings, and Frequency of MUCs After KT |
MUCs After KT
The MUCs were classified according to the time of presentation after KT in early (<1 month) was 71.2% (n = 139), medium (1–6 months) 14.8% (n = 29) and late (>6 months) with 17.9% (n = 35).
Urologic Complications by Donor Type and Graft Loss
A 3-year follow-up of KT patients was analyzed for the incidence of MUCs and its association with graft loss by donor type. A total of 152 (9.5%) cases of graft loss were registered. Twenty-five (16.4%) cases of graft loss were associated to MUCs. Urinary leak was present in 134 (8.5%) KT patients with 78 (7.2%) cases in DDKT and 56 (11%) cases in LDKT. Among them, there were 14 (10.4%) cases of graft loss. Other urologic complications were ureteral stenosis (n = 58, 3.7%) with 9 (15.5%) KT patients with graft loss, graft lithiasis (n = 15, 0.9%), and Bladder Outlet Obstruction (BOO) (n = 15, 0.9%) with an incidence of one (6.6%) graft loss case in each complication, respectively. However, no significant differences were found between graft loss and the type of MUCs. There was a significant difference between DDKT and LDKT in the bivariate analysis for urinary leak (p = 0.04). Table 3 depicts donor type and frequency of MUCs after KT.
 |
Table 3 Donor Type and Frequency of MUCs After KT |
Impact of MUCs on Long-Term Graft and KT Patient Survival
Probability of graft and patient survival at 3 years of follow-up was 90.5% and 85.5% respectively (see Figure 1). Figure 1 depicts the Kaplan–Meier survival analysis for global graft failure and patient survival in the KT patients at 3 years of follow-up.
 |
Figure 1 Kaplan-Meier survival analysis: (A) Global graft survival of KT patients at 3 years of follow-up. (B) Patient graft survival at 3 years of follow-up. |
Our KT cohort registered 166 (10.4%) deaths in the period of the study. We did not find any significant difference between patient or graft survival and the incidence of urologic complications (see Figure 2).
Associated Risk Factors of MUCs
We performed binary logistic regression to identify factors associated for the occurrence of MUCs within 3 years after transplantation. In the regression analysis, dialysis duration was significantly associated with the occurrence of urological complications in DDKT and LDKT (see Tables 4 and 5). The multiple regression model found cold ischemia time significant for LDKT in the development of urological complications (see Table 5). Tables 4 and 5 depict multiple predictor regression analysis for urological complications within 3 years after KT in DDKT, and LDKT, respectively.
 |
Table 4 Multiple Predictor Regression Analysis for Urological Complications Within 3 Years After KT in DDKT |
 |
Table 5 Multiple Predictor Regression Analysis for Urological Complications Within 3 Years After KT in LDKT |
Discussion
MUCs are the most common pathology after KT and are related to significant morbidity and mortality.19 New surgical techniques have decreased the incidence of MUCs.8 We analyzed a cohort of 1584 KT and determined its MUCs incidence, risk factors and their influence with graft loss and KT recipients’ mortality. MUCs occurred in 195 (12.6%) of our KT patients which is comparable to the findings reported by other publications.7,11,20 Urinary leak is the most common of the MUCs in literature and in our study, with a presentation of 8.5% followed by ureteral stenosis, graft lithiasis and BOO, this last one was less than 1% in our study. Comparable results in the BOO incidence (0.25%) were published in a research with 3951 KT patients,21 duration of dialysis and age are important risk factors in men for the development of urinary retention after KT.17 In a Colombian cohort with 3060 KT patients, there were 119 (3.8%) cases of urinary leak.22
Urinary stenosis is the second most common MUCs after KT. This presentation can be related to a previous urinary leak or as an independent presentation. A total of 58 urinary stenosis cases were recorded (3.75%), and can be diagnosed within 1–6 months (14.8%) or at a late presentation (17.9%). Ciancio et al identified the urinary leak and ureteral stenosis as the main MUCs in 500 KT.8 Another research with 635 KT supported similar results.4 Urinary leak and BOO are evident at early time of presentation being the most common with 71.2%. Graft lithiasis can be present any time during transplantation and comprised less than 1%. Graft lithiasis in renal transplantation has been described with a frequency from 0.4% to 4.4%.23
At our study, MUCs were surgically repaired with a new ureteroneocystostomy with double J catheter for either ureteral leak or stenosis (n = 146). If the urinary leak was evident intraoperative with a pelvic leak, a pelvic suture was performed additionally (n = 3). For 2 cases, a Boari flap was necessary. When ureteral necrosis was associated to the urinary fistula and was extensive, a pyelocystostomy (n = 6) or pyeloureterostomy (n = 1) was required. For one case, a ureteroureterostomy was executed to restore the urinary continuity. We used the surgical techniques designed to correct MUCs.24
For bladder outlet obstruction, a urinary catheter was placed in the emergency room and urology follow-up was completed with anatomic studies and correction when needed (Prostatectomy n = 1). If during the reoperation an external compression of the transplanted ureter was evident, the round ligament or the epigastric vessels were ligated (n = 2). Lithiasis did not present with complete obstruction and medical management was our approach. For non-surgical candidates, a definitive PCN was placed with timed exchanges by interventional radiology (n = 1).
Urinary leak seemed to be significant associated to LDKT in the bivariate analysis. A study with 193 LDKT patients with laparoscopic nephrectomy found similar results.25 This can theoretically be explained by intraoperative technique in living donor nephrectomy in which the ureter dissection tends to retrieve less periureteral tissue which can be associated to a diminished irrigation. This could be a risk factor for MUCs when compared to DDKT, however, it did not remain significant in the multivariate analysis. Bruintjes et al evaluated in KT patients the importance of preservation of the peri-ureteric connective tissue to prevent ischemia of the distal ureter and MUCs in LDKT without a significant association.7 A study with 901 KT patients did not report any association between type of donor and the incidence of MUCs.10
On bivariate analysis, risk factors for MUCs were the duration of the dialysis, cold ischemia time and ORT for the KT recipients. On multivariate analysis, the duration of dialysis for DDKT and LDKT (p = 0.02 and p = 0.009, respectively) remained significant. This could theoretically relate to a diminished urinary output before transplantation which may limit the bladder size and therefore make the ureteral anastomosis technically complex, leading to MUCs.17
For LDKT recipients, cold ischemia time (p = 0.002) remained significant in the multivariate analysis. At our center, LDKT tends to have lower cold ischemia time than DDKT. Some studies have found this association in the bivariate analysis, but it was not significant in the regression model.7 Comparable to the literature, ORT was not significant in the multivariate model.26
As shown in other studies, arterial multiplicity has shown to have repercussions when investigating MUCs.4 This is related to the perfusion of the ureter and the preservation of the irrigation for an adequate anastomotic healing. Further evaluation of whether multiple arteries may be an indication for rutinary doble J catheter placement may be advised. However, in our study, arterial multiplicity was not significantly associated with MUCs.
We have identified some limitations in our study. First, it is a retrospective analysis. In our electronic records arterial multiplicity is documented but the actual anatomy, whether there are to renal arteries, or polar arteries and their location are not routinely recorded for deceased donors, for living donors a contrast Angio tomography (CTA) was routinely performed preoperative assessment as performed worldwide.27 Likewise, identification of patients with nephrostomy was not possible. Information was incomplete for some variables even when triangulating information of multiple sources. Some old records were hard to find. Information regarding congenital genitourinary malformations and previous urological surgeries such as bladder augmentation was not available at the time of the study.
Conclusion
MUCs are frequent complications for KT and represent an important burden for the patient and the health system. Even though it has shown no significant difference in graft or patient survival, it may require invasive procedures or reoperations.
Abbreviations
KT, Kidney Transplantation; CKD, Chronic kidney disease; MUC, Major urologic complications; ECD, Expanded criteria donors; SCD, Standard Criteria Donors; SD, Standard Deviation; KDPI, Kidney donor profile index; DBD, Deceased brain donors; LDKT, Living donor kidney transplant; DDKT, Deceased donor kidney transplant; PCN, Percutaneous nephrostomy; BOO, Bladder outlet obstruction; IQR, Inter-quartile range; OR, Odds ratios; CI, 95% confidence intervals; BMI, Body mass index; CTA, Contrast Angio tomography.
Acknowledgments
To the Colombiana de Trasplantes working team for allowing us to research and perform this study, and their support.
To Dr. Esther de Vries for her valuable contributions in the final review of this article.
Funding
This work was supported by Colombiana de Trasplantes.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Araújo JC, Barbosa RWDS, Machado MF, et al. Clinical impact of surgical complications in kidney transplant recipients in a reference hospital in Salvador, Bahia, Brazil. Transplant Proc. 2016;48(7):2301–2305. doi:10.1016/j.transproceed.2016.06.017
2. Slagt I. Urological complications in kidney transplantation. Transl Androl Urol. 2015;45(1):25–31.
3. Di Carlo HN, Darras FS. Urologic considerations and complications in kidney transplant recipients. Adv Chronic Kidney Dis. 2015;22(4):306–311. doi:10.1053/j.ackd.2015.04.003
4. Rahnemai-Azar AA, Gilchrist BF, Kayler LK. Independent risk factors for early urologic complications after kidney transplantation. Clin Transplant. 2015;29(5):403–408. doi:10.1111/ctr.12530
5. Buttigieg J, Agius-Anastasi A, Sharma A, Halawa A. Early urological complications after kidney transplantation: an overview. World J Transplant. 2018;8(5):142–149. doi:10.5500/wjt.v8.i5.142
6. Nie Z, Zhang K, Huo W, Li Q, Zhu F, Jin F. Comparison of urological complications with primary ureteroureterostomy versus conventional ureteroneocystostomy. Clin Transplant. 2010;24(5):615–619. doi:10.1111/j.1399-0012.2009.01134.x
7. Bruintjes MHD, D’ancona FCH, Zhu X, Hoitsma AJ, Warlé MC. An update on early urological complications in kidney transplantation: a national cohort study. Ann Transplant. 2019;24:617–624. doi:10.12659/AOT.920086
8. Ciancio G, Farag A, Gonzalez J, Vincenzi P, Gaynor JJ. Results of a previously unreported extravesical ureteroneocystostomy technique without ureteral stenting in 500 consecutive kidney transplant recipients. PLoS One. 2021;16:1–20. doi:10.1371/journal.pone.0244248
9. Choate HR, Mihalko LA, Choate BT. Urologic complications in renal transplants. Transl Androl Urol. 2019;8(2):141–147. doi:10.21037/tau.2018.11.13
10. Saeb-Parsy K, Kosmoliaptsis V, Sharples LD, et al. Donor type does not influence the incidence of major urologic complications after kidney transplantation. Transplantation. 2010;90(10):1085–1090. doi:10.1097/TP.0B013E3181F7C031
11. Slagt IKB, IJzermans JNM, Visser LJ, Weimar W, Roodnat JI, Terkivatan T. Independent risk factors for urological complications after deceased donor kidney transplantation. PLoS One. 2014;9(3):e91211. doi:10.1371/JOURNAL.PONE.0091211
12. Bruintjes MHD, Langenhuijsen JF, Kusters A, Hilbrands LB, d’Ancona FCH, Warlé MC. Double J stent is superior to externally draining ureteric stent in enhancing recovery after kidney transplantation – a prospective cohort study. Int J Surg. 2019;71:175–181. doi:10.1016/j.ijsu.2019.09.031
13. Vogel T, Utech M, Schmidt F, et al. Double-J versus external ureteral stents in kidney transplantation: a retrospective analysis. Nephrourol Mon. 2015;7(4):0–4. doi:10.5812/numonthly.27820
14. Sarier M, Yayar O, Yavuz A, Turgut H, Kukul E. Update on the management of urological problems following kidney transplantation. Urol Int. 2021;105(7–8):541–547. doi:10.1159/000512885
15. Metzger R, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–125. doi:10.1034/j.1600-6143.3.s4.11.x
16. Nino-Murcia A, Pinto Ramirez JL, Nino-Torres L. Organ transplantation in Colombia. Transplantation. 2018;102(11):1779–1782. doi:10.1097/tp.0000000000002409
17. Sarıer M, Duman İ, Demir M, Yüksel Y, Emek M, Kukul E. The outcomes of transurethral incision/resection of the prostate (TUIP/TURP) performed early after renal transplantation. Turkish J Urol. 2018;44(2):172–177. doi:10.5152/TUD.2018.98404
18. Cai JF, Wang W, Hao W, et al. Meta-analysis of early versus late ureteric stent removal after kidney transplantation. Transplant Proc. 2018;50(10):3411–3415. doi:10.1016/j.transproceed.2018.08.033
19. Haberal M, Boyvat F, Akdur A, Kırnap M, Özçelik Ü, Karakayalı FY. Surgical complications after kidney transplantation. Exp Clin Transplant. 2016;14(6):587–595. doi:10.6002/ect.2016.0290
20. Neri F, Tsivian M, Coccolini F, et al. Urological complications after kidney transplantation: experience of more than 1000 transplantations. Transplant Proc. 2009;41(4):1224–1226. doi:10.1016/J.TRANSPROCEED.2009.03.044
21. Whang M, Benson M, Salama G, et al. Urologic complications in 4000 kidney transplants performed at the Saint Barnabas health care system. Transplant Proc. 2020;52(1):186–190. doi:10.1016/j.transproceed.2019.10.008
22. Ospina AV. Organ transplantation in the center HUSVP-U de A at Medellín. Rev Colomb Cir. 2005;2005:3.
23. Harraz AM, Kamal AI, Shokeir AA. Urolithiasis in renal transplant donors and recipients: an update. Int J Surg. 2016;36(PD):693–697. doi:10.1016/j.ijsu.2016.11.032
24. Berli JU, Montgomery JR, Segev DL, et al. Surgical management of early and late ureteral complications after renal transplantation: techniques and outcomes. Clin Transplant. 2015;29(1):26–33. doi:10.1111/ctr.12478
25. Philosophe B, Kuo PC, Schweitzer EJ, et al. Laparoscopic versus open donor nephrectomy: comparing ureteral complications in the recipients and improving the laparoscopic technique. Transplantation. 1999;68(4):497–502. doi:10.1097/00007890-199908270-00009
26. Choi YS, Kim KS, Choi SW, et al. Ureteral complications in kidney transplantation: analysis and management of 853 consecutive laparoscopic living-donor nephrectomies in a single center. Transplant Proc. 2016;48(8):2684–2688. doi:10.1016/j.transproceed.2016.06.054
27. Sarier M, Callioglu M, Yuksel Y, Duman E, Emek M, Usta SS. Evaluation of the renal arteries of 2144 living kidney donors using computed tomography angiography and comparison with intraoperative findings. Urol Int. 2020;104(7–8):637–640. doi:10.1159/000507796
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

