Back to Journals » International Journal of General Medicine » Volume 14
Risk Factors for Postoperative Mortality in Patients with Acute Stanford Type A Aortic Dissection
Authors Huo Y, Zhang H, Li B, Zhang K, Li B, Guo SH, Hu ZJ, Zhu GJ
Received 23 July 2021
Accepted for publication 8 September 2021
Published 21 October 2021 Volume 2021:14 Pages 7007—7015
DOI https://doi.org/10.2147/IJGM.S330325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yan Huo, Hui Zhang, Bo Li, Kun Zhang, Bin Li, Shao-Han Guo, Zhen-Jie Hu, Gui-Jun Zhu
Department of Intensive Care Unit, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Gui-Jun Zhu
Department of Intensive Care Unit, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
Tel +86-311-86095588
Email [email protected]
Objective: The present study explored the risk factors of postoperative mortality in patients with acute Stanford type A aortic dissection (AD).
Methods: The study included 149 patients with acute Stanford type A AD who were treated at the Fourth Hospital of Hebei Medical University, China, from October 2016 to October 2018. The patients were divided into a death (n = 42) and survival group (n = 107) according to individual prognosis. Univariate analysis of all possible related risk factors was conducted; multivariate logistic regression analysis of the potential risk factors that showed statistical differences in the univariate analysis was also performed.
Results: The results of the univariate analysis showed that a body mass index (BMI) ≥ 25 kg/m2, surgery duration, duration of cardiopulmonary bypass, duration of cardiopulmonary bypass assistance, total transfusion of red blood cells, postoperative APACHE II score, sequential organ failure assessment (SOFA) score, low cardiac output, acute kidney injury (AKI), hypoxemia, diffuse intravascular coagulation (DIC), hepatic failure and other related complications, as well as postoperative stay duration in the intensive care unit (ICU), were closely correlated with a poor prognosis among patients. Multivariate logistic regression analysis showed that a BMI ≥ 25 kg/m2, SOFA score > 8, duration of cardiopulmonary bypass assistance > 70 minutes, postoperative low cardiac output, and postoperative DIC were independent risk factors for postoperative death in patients with acute Stanford type A AD.
Conclusion: A BMI ≥ 25 kg/m2, SOFA score > 8, duration of cardiopulmonary bypass assistance > 70 min, postoperative DIC, and postoperative low cardiac output were the independent risk factors for postoperative death in acute Stanford type A AD. Intraoperative blood transfusion, postoperative hepatic failure, and AKI, among others, correlated with an increased risk of death but were not independent risk factors for death.
Keywords: aortic dissection, risk factor, mortality, DIC, SOFA score
Introduction
Acute aortic syndrome is a critical, highly fatal pathological process that occurs within the aortic wall. The condition includes acute aortic dissection (AAD), intra-aortic hematoma, and penetrating atherosclerotic ulcer.1 Acute aortic dissection accounts for 85% to 95% of acute aortic syndrome cases. The condition has a high mortality rate and even with emergency surgical treatment, the total mortality is higher than 20%. The Stanford typing of aortic dissection (AD) is a commonly used clinical typing method in which AD is divided into Stanford types A and B, based on whether the ascending aorta is involved. Stanford type A AAD accounts for approximately 2/3 cases and has a high mortality rate. Currently, the treatment method for type A AD is thoracotomy and artificial blood vessel replacement. An analysis of patients with AD over the past 20 years found that the number of patients with AAD who were willing to undergo surgery had risen from 79% in the late 1990s to 90% presently.2 The in-hospital mortality among patients with type A AAD decreased from 31% to 22% over time, primarily due to a decrease in surgical mortality, from 25% to 18%.3 However, the medical mortality for type A AAD remains high (57%) and has not decreased over time. In an International Registry of Acute Aortic Dissection report, the mean interval from diagnosis to surgical intervention was 4.3 hours,4 with conservative aortic repair in 59% of patients, more extensive aortic root resection in 34%, proximal hemispherectomy in 27%, and total arch replacement in 12%; 92% of patients underwent hypothermic open surgery with circulatory arrest and a cerebral perfusion rate of 51%. For these complex procedures, patients are prone to experiencing circulation, respiration, and individual organ complications post-surgery. Accordingly, patients with acute Stanford type A AD are often required to spend the peri-operative period in the intensive care unit (ICU) following surgery. Postoperative management may also cause additional complications that can affect patient prognosis.
With the standardization of emergency resuscitation and advances in auxiliary examinations, more surgical opportunities and time can be obtained for patients with acute Stanford A AD. However, many difficulties remain that require attention, such as a timely and rapid diagnosis, individualized and precise postoperative management, and a reduction in postoperative adverse events among patients. The analysis of risk factors associated with postoperative mortality in patients with Stanford type A AD may be able to identify the risk factors associated with mortality, which could assist in early detection for setting individualized targets and implementing rapid management of the condition, thereby improving the success rate of treatment among patients with acute Stanford type A AD.
This study aimed to investigate the risk factors associated with postoperative mortality in patients with Stanford A AD by prospectively observing patients with acute Stanford A AD treated at the Fourth Hospital of Hebei Medical University in China from October 2016 to October 2018 and collecting their clinical data for analysis.
Subjects and Method
Subjects
In the present study, 265 patients with AD who visited the Fourth Hospital of Hebei Medical University, China, from October 2016 to October 2018 were initially included, and 149 patients with acute Stanford type A who underwent surgical treatment were subsequently selected. The inclusion criteria were as follows: (1) patients ≥18 years old; (2) patients among whom duration from the onset of the disease to admission was <336 hours (14 days); (3) patients with a diagnosis of Stanford A AD confirmed by imaging; (4) patients that underwent surgery. The exclusion criteria were as follows: (1) patients who undertook conservative therapy, who did not undergo surgery, or who died before surgery; (2) patients with severe data deficiencies; (3) patients who abandoned treatment due to cost and other factors.
Data Collection
The preoperative general data, surgery-related data, postoperative-related data, and other possible relevant risk factors, as well as the prognosis of the enrolled patients, were retrospectively collected.
General Pre-Operative Data
General Characteristics of Patients Included in This Study
Name, gender, age, hospitalization identification, height (cm), weight (kg), body mass index (BMI), family place of origin, and a contact phone number of family members.
Patient Conditions Concerning Onset Before Admission
The specific time of onset of the disease, symptoms accompanying the onset of the disease, urinary and fecal conditions, combined with pregnancy, and others.
History Factors Included
Hypertension, diabetes mellitus, coronary heart disease, smoking history, and history of disease and surgery (particularly the history of thoracotomy and history of dissection).
Patient Diagnosis at Admission Considered the Following
The type of AD, poor tissue and organ perfusion, complications.
Data Related to Surgery
Data related to surgery included the specific method of operation, the situation regarding intra-operative exploration, difficulty disengaging the extracorporeal circulation machine during surgery, the specific cause of intra-operative death, surgery duration, the duration of cardiopulmonary bypass, the assistance duration, the degree of intra-operative blood loss, and the degree of intra-operative blood transfusion.
Postoperative Relevant Data
Relative postoperative data included staying duration in the ICU, the duration of mechanical ventilation, the duration of blood purification, post-operative complications, causes of a second surgery, and post-operative APACHE II and SOFA scores.
The Prognosis
In this study, prognosis considered whether they survived surgery and the cause of postoperative death.
Data Analysis
The SPSS Statistics 21 software package was used to conduct statistical analysis. Measurement data conforming to normal distribution were expressed as means ± standard deviations, and the independent sample t-test was used to conduct univariate analysis. Countable data were expressed based on count value, and the chi-square test was employed for univariate analysis. Multivariate logistic regression analysis was also conducted. Missing data within a 10% range were interpolated with the overall average or median value. Variables missing data at a rate higher than 10% were not included in the multivariate analysis. The measurement data were converted into binary variables according to the cut-off point of the receiver operating characteristic (ROC) curve analysis. The variables were screened using forward stepwise regression; P < 0.05 was considered statistically significant.
Definition
AKI
AKI is defined by KDIGO guidelines:5 Increase in SCr by ≥0.3mg/dl (≥26.5μmol/l) within 48 hours; or Increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or Urine volume <0.5 mL/kg/h for 6 hours.
Cardiac Output
Cardiac output was calculated by the velocity time integral(VTI) obtained by ultrasound: CO (L/min) = VTI × π(D/2)2 × HR.
Results
A total of 265 patients with AD who attended the Fourth Hospital of Hebei Medical University for treatment from October 2016 to October 2018 were selected for the present study. Among them, 98 patients with Stanford type B AD, patients who had died prior to surgery, and those who were not treated with thoracotomy for various reasons were excluded, alongside 18 cases of chronic AD. A total of 149 patients with Stanford type A AD were finally included, 42 of whom died (28.18% mortality).
Analysis of General Characteristics Before Surgery
A statistical BMI difference (≥25) was observed between the two patient groups (P = 0.002) but there was no significant difference regarding age (P = 0.304) and gender (P = 0.734). There were 110 patients with a history of hypertension, including 33 patients (78.57%) in the death group and 77 patients (71.96%) in the survival group. There was no significant statistical difference between the two groups (P = 0.941). There was no statistical difference for smoking and drinking history, history of diabetes mellitus, or history of special diseases between the two groups. Two patients were pregnant and both survived following surgery. Four patients had Marfan’s syndrome, two of whom survived surgery and two who did not; no significant difference between the two groups (P = 0.316) was observed in this regard. A comparison of the specific general characteristics of the two patient groups is shown in Table 1.
 |
Table 1 The Comparison of the General Characteristics Before Surgery |
Analysis of Data Related to Surgery
Among the 149 patients included, 44 underwent Bentall surgery, 24 underwent ascending aortic replacement, 20 underwent Bentall surgery and semi-arc replacement, 94 underwent ascending aorta and aortic arch replacement, and 12 patients underwent a second operation for the endovascular exclusion of the thoracic or abdominal aorta after the initial surgery. There was no significant difference between the two groups. Four patients underwent coronary artery bypass grafting during surgery, all of whom died following the procedure (P < 0.001). The duration of the cardiopulmonary bypass (P < 0.001) and of cardiopulmonary bypass assistance (P < 0.001) in the death group were significantly higher compared with the survival group. Compared with the death group, the difference in aortic block duration in the survival group was statistically significant (P = 0.022). A total of 11 cases had intra-operative difficulty disengaging from the extracorporeal circulation machine (two cases in the survival group and nine in the death group, P < 0.001). The intra-operative massive infusion of blood products correlated with poor prognosis. The statistics of the specific intra-operative relevant factors are shown in Table 2.
 |
Table 2 The Comparison of Intra-Operative Relevant Data |
The Analysis of the Postoperative Relevant Data
There was no statistical significance regarding variances in heart rate, blood pressure, oxygen saturation, oxygenation index, blood lactic acid, level of electrolytes, and myocardial enzymology between the two groups after the patient returned to the ward post-surgery. The post-operative APACHE II score in the death group was 21.61 ± 7.63, and the SOFA score was 10.45 ± 5.82. The average APACHE II (P < 0.001) and SOFA (P < 0.001) scores in the death group were higher than those in the survival group. The ROC curve analysis showed a cut-off point of 16 for APACHE II; the cut-off point for SOFA score was 8, and the difference was statistically significant (P < 0.001)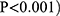 (see Figure 1). The postoperative-related conditions and complications for patients in the present study are shown in Table 3. The postoperative low cardiac output, respiratory insufficiency, cerebral dysfunction, AKI, hepatic failure, catheter-related bloodstream infection, and other indicators were risk factors for postoperative death. There was a statistical significance in the staying duration in the ICU between the survival and death groups (P < 0.001).
(see Figure 1). The postoperative-related conditions and complications for patients in the present study are shown in Table 3. The postoperative low cardiac output, respiratory insufficiency, cerebral dysfunction, AKI, hepatic failure, catheter-related bloodstream infection, and other indicators were risk factors for postoperative death. There was a statistical significance in the staying duration in the ICU between the survival and death groups (P < 0.001).
 |
Table 3 The Comparison of Post-Operative Relevant Data |
 |
Figure 1 The ROC curve. |
Analysis of the Risk Factors for Postoperative Mortality in Patients with Acute Stanford Type A AD
The cut-off point was selected using the ROC curve analysis, and part of the countable data was converted into binary variables. Then, univariate analysis was conducted and indicators with a smaller P-value were introduced into the multivariate analysis. To adjust for the confounding factors, age and gender were included in the multivariate analysis, even though the P-value was not significantly different. The results of the univariate analysis are shown in Table 4.
 |
Table 4 The Univariate Analysis of the Risk Factors |
The multivariate logistics regression analysis revealed that a BMI ≥25 kg/m2, SOFA score >8, intra-operative cardiopulmonary bypass assistance >70 min, post-operative DIC, and postoperative low cardiac output were independent risk factors for postoperative death in acute Stanford A AD in the present study (Table 5).
 |
Table 5 Multivariate Logistic Regression Analysis |
Discussion
Acute Stanford type A AD is a fatal disease that has a catastrophic impact on the life of the patient. The International AAD Registry reported the mortality rate of patients undergoing surgery as being very high (32.5%).6 Although perioperative and surgical treatment has improved, the in-hospital mortality after surgery remains between 15% and 30%.7 Early detection of the risk factors related to postoperative death can help improve treatment measures and reduce the risk of treatment for patients, thereby reducing the postoperative mortality among patients with acute Stanford type A AD.
Currently, the results of the analysis of risk factors for postoperative death in patients with AD can vary significantly. Furthermore, there is a lack of multicenter studies that include large samples. Goda et al8 posit that in univariate analysis, the significant preoperative risk factors for in-hospital mortality include cardiopulmonary resuscitation, coagulation dysfunction, renal insufficiency, elevated aspartate aminotransferase levels, myocardial ischemia, and lower-limb ischemia. The risk factors related to surgery include the duration of cardiopulmonary bypass, the duration of the aortic block, and the need for substantial blood transfusion. In the multivariate analysis, the independent preoperative risk factors were cardiopulmonary resuscitation, renal insufficiency, and lower-limb ischemia. In the present study, a comparative analysis was conducted regarding general conditions on admission and patient history, intra-operative-related influencing factors, postoperative complications, and postoperative testing indicators. The results of the univariate analysis indicated differences in many indicators between the death and survival groups.
A BMI ≥25 kg/m2 was an independent risk factor for death, which was consistent with the results of a recent study.9 Mechanical ventilation under sedation and analgesia was generally required for patients after AD surgery. For all patients included in the present study, mechanical ventilation was performed after surgery. The higher BMI, which mean the thicker chest wall, caused lower thoracic compliance, and greater resistance to overcome during the breathing exercise. Furthermore, with an increase in oxygen demand, severe hypoxemia will develop. Meanwhile, overweight patients will be more likely to have poor wound healing and develop infections after surgery due to excessive subcutaneous fat, poor skin elasticity, and long thoracotomy wounds.
Cardiac surgery requires cardiopulmonary bypass support technology. In the case of cardiopulmonary bypass, even if the operation is successful, there will still be risks such as injury to the central nervous and other organs, eg, the kidneys, liver, and intestines.8 In the present study, surgery duration, duration of the cardiopulmonary bypass, duration of the aortic block, and duration of the cardiopulmonary bypass assistance in the death group were longer than those in the survival group.
Surgery conducted for the Stanford type A AD has a long duration and inflicts significant trauma, and the advanced management of postoperative patients is particularly important. In the 149 cases enrolled in the present study, postoperative low cardiac output, respiratory insufficiency, cerebral dysfunction, AKI, catheter-related bloodstream infection, and hepatic failure affected the mortality of patients; among these factors, low cardiac output and DIC were independent risk factors for death.
Various unfavorable factors in Stanford A AD impact the lungs, which can easily cause postoperative hypoxemia. When this happens, tissue cell hypoxia can easily increase the risk of ventilator-related lung injury. In the present study, although hypoxemia was not an independent risk factor affecting the prognosis of patients, its incidence reached 40%, and the difference between the survival group and the death group was statistically significant (P = 0.009). Liu et al10 showed that a time ≥72 h from the onset of symptoms to the start of surgery, preoperative PaO2/FiO2 ≤ 300, and a duration of hypothermic circulatory arrest >25 min closely correlated with the occurrence of postoperative hypoxemia. This may be due to the formation of an aortic pseudocavity in the early stage of the disease. Furthermore, a large number of active components in the blood were activated, which may have induced systemic inflammatory response syndrome and coagulation system diseases, which will lead to further continuous multiple organ injury. These results suggest that early surgery may improve and stabilize hemodynamics and effectively prevent the occurrence of postoperative hypoxemia.
For Stanford type A AD, patients will undergo a cardiopulmonary bypass and significant blood transfusion; as such, the tissue will be fragile, increasing the risk of severe complications such as cardiac dysfunction, tissue edema, and decreased myocardial contractility. It seems desirable for these patients to receive extracorporeal membrane oxygenation (ECMO) to reduce mortality after surgery.11 However, a meta-analysis found that the use rate of ECMO after thoracic aortic disease repair was as high as 12%, and the use of ECMO correlated with 65% of hospitalization mortality.12 Ibrahim et al also suggest that ECMO support in AAD correlates with extremely high mortality rates. In the present study, two patients who received ECMO treatment died. As such, the use of ECMO after surgery requires further research.
For post-operative patients with Stanford type A AD, hepatic failure accounts for only 4% of gastrointestinal complications but the mortality can be as high as 70%.13 In the present study, seven patients with postoperative hepatic failure had a mortality of 100%. However, postoperative hepatic function often has a poor prognosis, and there is currently no satisfactory alternative treatment for hepatic insufficiency. Thus, the protection of hepatic functioning is particularly important.
The dominant DIC in coagulation dysfunction after surgery can cause hemorrhage, microcirculation disorder, microvascular embolism, and microvascular hemolytic disease, resulting in refractory shock, respiratory failure, impaired consciousness, kidney injury, and, ultimately, multiple organ dysfunction. The incidence of post-operative DIC was 8%, while the mortality rate reached 75%.
The present study also found that postoperative APACHE II scores and the SOFA score in the death group were significantly higher than those in the survival group. The postoperative SOFA score emphasizes the early and dynamic monitoring of indicators including the assessment of breathing, blood, liver, cardiovascular, central nervous, and renal systems. Among these, multivariate regression analysis revealed that a post-operative SOFA score >8 was an independent risk factor for death.
In addition, a recent meta-analysis showed that Elevated NT‐proBNP levels on admission are associated with an increased risk of short‐term mortality in AAD.14 Cardiac troponin elevation at the time of admission for AAD has also been shown associated with an increased risk of in-hospital mortality.15 It can be seen that the cardiac biomarkers have potential for predictive value for the prognosis of patients with AAD. As this study was prospective, the study design failed to take this into account, which needs to be further explored in future studies.
According to existing studies and the results of the present study, AD is a life-threatening and critical illness. Early detection and diagnosis, and early surgical treatment based on the clinical manifestations of the patient to develop an individualized postoperative management plan can potentially improve the success rate of therapy and reduce the occurrence of postoperative complications. The SOFA score can assess the evaluation and prognosis of various organs, thereby improving the cure rate of surgery performed for AD.
There were some limitations to the present study. First, it represents a single-center retrospective study with small sample size. A specific type of patients who had undergone thoracotomy was selected, which increased the homogeneity of the study population. Currently, there is a lack of large-sample multi-center studies in China to determine the independent risk factors for death in patients with AD; a larger focus on such studies can help to support and guide early clinical intervention to improve the cure rate among patients.
Conclusion
A BMI ≥25 kg/m2, a SOFA score >8, an intra-operative cardiopulmonary bypass assistance duration >70 min, post-operative DIC, and postoperative low cardiac output were independent risk factors for postoperative death in acute Stanford type A AD patients. Intra-operative blood transfusion, postoperative hepatic failure, AKI, and other factors correlated with the increased risk of death but were not independent risk factors for death.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of The Fourth Hospital of Hebei Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sheikh AS, Ali K, Mazhar S. Acute aortic syndrome. Circulation. 2013;128(10):1122–1127. doi:10.1161/CIRCULATIONAHA.112.000170
2. Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846–1860. doi:10.1161/CIRCULATIONAHA.117.031264
3. Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015;66(4):350–358. doi:10.1016/j.jacc.2015.05.029
4. Harris KM, Strauss CE, Eagle KA, et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011;124(18):1911–1918. doi:10.1161/CIRCULATIONAHA.110.006320
5. Kidney International Supplements. Kidney Int Suppl. 2012 Mar;2(1):1
6. Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation. 2002;105(2):200–206. doi:10.1161/hc0202.102246
7. Russo CF, Mariscalco G, Santé P. [Acute aortic dissection type A: from the past to the present]. G Ital Cardiol. 2016;17(11):908–914. Italian.
8. Goda M, Imoto K, Suzuki S, et al. Risk analysis for hospital mortality in patients with acute type a aortic dissection. Ann Thorac Surg. 2010;90(4):1246–1250. doi:10.1016/j.athoracsur.2010.05.069
9. Wu Y, Jiang R, Xu P, Wang G, Wang J, Yang S. Perioperative results and risk factors for in-hospital mortality in patients with Stanford type a aortic dissection undergoing sun’s procedure - a Single Center Study. Heart Surg Forum. 2018;21(6):E432–7. doi:10.1532/hsf.1909
10. Liu N, Zhang W, Ma W, Shang W, Zheng J, Sun L. Risk factors for hypoxemia following surgical repair of acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2017;24(2):251–256.
11. Khorsandi M, Dougherty S, Bouamra O, et al. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg. 2017;12(1):55. doi:10.1186/s13019-017-0618-0
12. Sultan I, Habertheuer A, Wallen T, Siki M, Szeto W, Bavaria JE, et al. The role of extracorporeal membrane oxygenator therapy in the setting of Type A aortic dissection. J Card Surg. 2017 Dec;32(12):822–5.
13. Hessel EA. Abdominal organ injury after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8(3):243–263. doi:10.1177/108925320400800306
14. Vrsalovic M, Vrsalovic Presecki A, Aboyans V. N-terminal pro-brain natriuretic peptide and short-term mortality in acute aortic dissection: a meta-analysis. Clin Cardiol. 2020;43(11):1255–1259.
15. Vrsalovic M. Prognostic effect of cardiac troponin elevation in acute aortic dissection: a meta-analysis. Int J Cardiol. 2016;214:277–8.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
