Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Risk Factors for Perioperative Respiratory Adverse Events in Children with Recent Upper Respiratory Tract Infection: A Single-Center-Based Retrospective Study
Authors Lee HJ , Woo JH , Cho S, Oh HW, Joo H, Baik HJ
Received 17 September 2020
Accepted for publication 30 November 2020
Published 14 December 2020 Volume 2020:16 Pages 1227—1234
DOI https://doi.org/10.2147/TCRM.S282494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Hyun Jung Lee,1 Jae Hee Woo,2 Sooyoung Cho,3 Hye-Won Oh,1 Hyunyoung Joo,3 Hee Jung Baik2
1Department of Anesthesiology and Pain Medicine, Ewha Womans University Seoul Hospital, Seoul, South Korea; 2Department of Anesthesiology and Pain Medicine, College of Medicine, Ewha Womans University, Seoul, South Korea; 3Department of Anesthesiology and Pain Medicine, Ewha Womans University Mokdong Hospital, Seoul, South Korea
Correspondence: Hee Jung Baik
Department of Anesthesiology and Pain Medicine, College of Medicine, Ewha Womans University, 25, Magokdong-ro 2-gil, Gangseo-gu, Seoul 07804, Republic of Korea
Tel +82-2-2650-2868
Fax +82-2-2655-2924
Email [email protected]
Purpose: In pediatric patients, the most common reason for delaying surgical intervention is an upper respiratory tract infection (URI). To date, there has been no consensus regarding the optimal timeframe for deferring surgery in children with URI. We conducted this study to evaluate whether a URI symptom-free period and other risk factors affect the incidence of perioperative respiratory adverse events (RAEs).
Patients and Methods: The study population included 267 pediatric patients (aged 0 to 13 years) with a recent URI episode who underwent surgery under general anesthesia. Following a retrospective review of medical records, several risk factors including a URI symptom-free period for intra- and postoperative RAEs were analyzed using univariate and multivariate logistic regression analyses.
Results: RAEs occurred in 23 of 267 patients (8.6%). Univariate analysis revealed that abnormal preoperative chest images (odds ratio [OR], 7.48; 95% confidence interval [CI], 2.46– 22.68, p < 0.001) and emergency operations (OR, 2.84; 95% CI, 1.03– 7.81, p = 0.04) were associated with RAEs. Four variables (abnormal preoperative chest images, emergency operations, age under 1 year and symptom-free period of 7– 13 days) with a significance of < 0.20 in the univariate logistic regression analysis were selected as candidate risk factors for the multivariate model. Among the four variables, abnormalities in preoperative chest images (OR, 7.60; 95% CI, 2.28– 25.3, p = 0.001) and a symptom-free period of 7– 13 days (OR, 0.13; 95% CI, 0.02– 0.88, p = 0.04) were independently associated with RAEs in multivariate logistic regression analysis.
Conclusion: For pediatric patients who require surgery and have a recent history of URI, procedures should be performed after a URI symptom-free period of at least 1– 2 weeks. Confirming the absence of abnormalities in preoperative chest images can reduce the incidence of perioperative RAEs.
Keywords: pediatric, general anesthesia, upper respiratory tract infection, respiratory adverse event
Introduction
Perioperative pulmonary complications contribute a substantial proportion of the risks associated with surgery and anesthesia, and are a major cause of postoperative morbidity, mortality, and prolonged hospital stays.1 One systematic review found that the incidence of postoperative pulmonary complications ranged from 2% to 19%.2 In particular, respiratory adverse events (RAEs) are major complications during the intraoperative and postoperative periods in children with upper respiratory tract infection (URI).3 Thus, URIs are a common reason for delays in surgical interventions.4
Most URIs are viral in etiology and are self-limiting, although some patients are predisposed to bacterial complications such as acute sinusitis, acute otitis media, and lower respiratory tract infections. Symptoms of URIs include sore throat, runny nose, nasal congestion, sneezing, dry cough, mild fever, and a degree of malaise.5
The incidence of perioperative RAEs is reportedly two to seven times higher in children with a URI than in children without a URI, and the incidence increases to 11 times higher if the trachea is intubated.6 This difference may be attributed to alterations in pulmonary function including diminished diffusion capacity, decreased lung compliance, increased airway resistance, and abnormities in lung clearance mechanisms due to the involvement of the lower respiratory tract; as well as airway hyperreactivity to stimuli, which can persist for up to 6 weeks after a URI. Epithelial damage in the pharynx during a URI is postulated to induce sensitization of oropharyngeal receptors, which may reflexively mediate airway hyperreactivity.7
Independent risk factors for RAEs in children with active URIs reportedly include the use of an tracheal tube, age under 5 years old, history of premature birth (<37 weeks), history of reactive airway disease, parental smoking, airway-related surgery, presence of massive secretions, and nasal congestion.8,9 The decision to proceed with anesthesia for a child with a URI is dependent on the presence of these risk factors and must be evaluated together with the need for emergent surgery and the experience of the anesthesiologist.
One review article concluded that children with complicated URI should have elective surgery postponed for more than 4 weeks, whereas children with uncomplicated URI should have elective surgery postponed for up to 2 weeks.10 However, there is no consensus regarding the optimal timing for surgical delay in children with URI.
Therefore, it is necessary to evaluate whether the URI symptom-free period affects the incidence of perioperative RAEs. The purpose of this retrospective study was to investigate risk factors associated with perioperative RAEs, including the URI symptom-free period, age, surgical procedures involving the airways, abnormal findings on chest imaging, underlying respiratory disease, intubation, and emergency operation.
Patients and Methods
Patient Characteristics
The study population included 267 pediatric patients (aged up to 13 years) with recent URI (within 2 months) who underwent surgery under general anesthesia at Ewha Womans University Mokdong Hospital during the period from 1 January 2014 to 31 December 2015. The study protocol was approved by the Institutional Review Board at Ewha Womans University Mokdong Hospital Seoul, Korea (Chairperson, Professor W. B. Pyun) on 11 June 2018 (EUMC 2018–05–040–001). The trial was registered by the clinical research information service (http://cris.nih.go.kr, KCT0003094). Written consent was specifically waived by the approval from the institutional review board because of the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki.
Patients’ clinical data were collected via medical records. Personal information was anonymized using a personal identification code and only the data collector had access to this information. Anesthesia records, recovery room records, and ward progression records were retrospectively reviewed. Data collected as potential risk factors for RAEs were URI symptom-free period, age, underlying respiratory disease including asthma or allergic rhinitis, abnormalities in preoperative chest images (eg, indicative of pneumonia or bronchiolitis), type of surgery (eg, airway involvement), intubation, and emergency operation. Patients were excluded if their records were incomplete.
Patients were divided into four subgroups based on age: younger than 1 years old, 1–4 years old, 5–8 years old, and 9–13 years old. Additionally, patients were divided into five subgroups based on the duration of the URI symptom-free period prior to the scheduled intervention: symptoms absent for more than 4 weeks, 2- 4 weeks, 1–2 weeks, or less than 1 week, and symptoms still present (0 day, symptomatic at the time of surgery).
Intraoperative and postoperative RAEs were defined as partial or complete airway obstruction due to laryngospasm, excessive respiratory secretions, severe cough, respiratory distress signs such as chest retraction, low oxygen saturation on pulse oximetry (<90%), postoperative diagnosis of pneumonia or bronchiolitis, and abnormalities in postoperative findings on chest imaging indicative of atelectasis, pneumonia, or bronchiolitis.
Statistical Analysis
The normality of continuous data was tested with the Shapiro–Wilk test, Q-Q plot, and histogram evaluation. Values were expressed as mean ± standard deviation (SD), median (interquartile range [IQR]), or numbers of patients (%). The Student’s t-test (two-tailed t-test) or Mann–Whitney U-test was used for numerical values according to the results of normality testing of the distribution to compare variables between the two groups (RAEs vs no RAEs). For categorical values, the chi-square test or Fisher’s exact test was performed when applicable. Statistical significance was considered with p-values <0.05.
To include potential confounders into our multivariable analysis, we set the p-value cut-off of 0.2 instead of 0.05 for univariate analysis. Thus, variables with a significance of <0.20 in the univariate logistic regression analysis were selected as candidates for the multivariate model. To detect independent variables having collinearity, we used the linear regression procedure and found the variance inflation factor (VIF) of all variables. If the VIF was under 10, we considered the variables were not correlated.
Multivariate analysis, using logistic regression, was conducted and variables with a p-value <0.05 were considered to be statistically significant. Risk ratios were displayed as odds ratio (OR) with 95% confidence interval (CI). Variables that were statistically significant in multivariate logistic regression analysis were also analyzed for interaction with other variables. Statistical analysis was performed using SPSS for Windows (version 18.0. SPSS Inc., Chicago, IL, USA).
Results
In total, 1999 children (aged 0 to 13 years old) underwent general anesthesia. Of these, 362 patients were eligible for this study, but 66 and 29 patients were excluded due to the lack of preoperative data related to risk factors and incomplete post-operative records, respectively. Thus, the final analysis included 267 patients (Figure 1).
 |
Figure 1 Recruitment flow chart. |
The comparison of patient characteristics and operative clinical details stratified according to postoperative respiratory adverse events are summarized in Table 1. Of the 267 patients enrolled, 23 (8.6%) experienced perioperative RAEs. Age, sex, URI symptom-free period, and the percentage of patients with a history of underlying respiratory disease did not differ significantly between the two groups. The incidence of RAEs was higher in children younger than 1 year old (17.6%) than in children older than 1 year (8.0%), but the difference was not statistically significant (p = 0.17). Similarly, the incidence of RAEs was higher in children experiencing URI symptoms at the time of surgery (14.8%) than in children who had no symptoms at the time of surgery (7.9%), but this difference was not statistically significant (p = 0.27). Preoperative chest imaging revealed that four patients had pneumonia and 13 had bronchiolitis. The incidence of RAEs was significantly higher in children with abnormal findings on their preoperative chest imaging (35.3%) than in children who presented normal imaging (6.8%; p = 0.001).
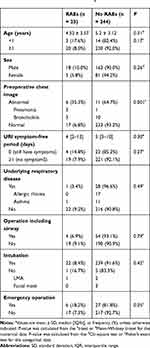 |
Table 1 Comparison of Basic Characteristics by Perioperative Respiratory Adverse Event (RAE) Statusa |
Perioperative RAEs occurred in 23 patients, and the most common RAE (11 patients) was oxygen desaturation (<90%). Eight subjects experienced laryngospasm, four – excessive respiratory secretion, three – persistent cough, two – chest retraction, two – pneumonia, and one – atelectasis. All relevant symptoms were double counted if applicable.
All RAEs were mild and easily managed without any severe life-threatening sequelae. Univariate logistic regression analysis revealed that abnormal preoperative chest imaging and emergency procedures were associated with the occurrence of perioperative RAEs (Table 2). Among the five groups classified by a URI symptom-free period, the 0 day (symptomatic at the time of surgery) group was regarded as the reference. Only the 7-13 day symptom-free group showed a tendency toward reduced incidence of RAEs, compared with the 0-day group. However, there was no trend suggesting a reduction in the incidence of RAEs as the duration of the symptom-free period increased.
 |
Table 2 The Effects of Risk Factors on Perioperative Respiratory Adverse Event |
Patients were divided into four subgroups based on age. The group aged 9-13 years was regarded as the reference, compared with other age groups. We found that children aged younger than 1 year showed a tendency toward an increasing incidence of RAEs compared with children aged 9 to 13 years. The VIF for all variables was under 10, thus none exhibited collinearity.
We selected four variables (age under 1 year, symptom-free period of 7–13 days, abnormalities in preoperative chest imaging, and emergency procedures) with a significance of <0.20 in univariate logistic regression analysis as candidate risk factors for the multivariate model. Among the four variables, abnormal findings on preoperative chest imaging (OR = 7.60; 96% CI 2.28–25.30, p = 0.001) and 7 to 13 days of a URI symptom-free period (OR = 0.13; CI 0.02–0.88, p = 0.04) were associated with RAEs in the multivariate regression analysis (Table 2). These variables were also confirmed to have no interaction with each other.
Discussion
We investigated risk factors associated with perioperative RAEs. It was confirmed that abnormal findings in the preoperative chest images increased RAE incidence and a URI symptom-free period of 7–13 days decreased the incidence of RAEs.
The incidence of perioperative RAEs was higher in symptomatic children (14.8%) than in asymptomatic children (7.9%), but the difference was not statistically significant (Table 1). The retrospective study by Tait and Knight11 reported that the prevalence of intraoperative RAEs was 5.31% in asymptomatic children who had a recent history of URI (within 2 weeks), which was similar to our findings. A prospective study showed that the incidence of perioperative RAEs was 28.3% in children with active URIs,12 which was approximately two times greater than the incidence in our study. This discrepancy was primarily due to the prospective study design.
Epithelial and mucosal damage by URI can lead to the sensitization of pharyngeal receptors, which in turn cause airway hypersensitivity and reactivity that may persist for 6–8 weeks.7,8,13,14 Anesthesia in patients with hyperreactive airways is associated with perioperative laryngospasm, bronchospasm, breath holding, and hypoxia.15 Children experience 2–6 episodes of URI per year;16 thus, if anesthesia is delayed for 4–6 weeks after each episode, there may be only a short period in which patients are asymptomatic.13 It is therefore challenging for anesthesiologists to determine whether or not to proceed with the procedure as well as to determine the appropriate time frame for postponement. Because airway hyperreactivity decreases over time,7,8,14 the risk for perioperative RAEs is high with an active URI or URI within 2 weeks, reduced after 2 weeks, and further reduced after 4 weeks.10,17–19 Postponement of surgery has been recommended for more than 4 weeks,10 or at least 2 weeks in patients with complicated URI.20,21 However, Berry suggested that a delay of 1–2 weeks may be sufficient for children with uncomplicated URI.10,22 To date, no evidence-based guideline has been established.
We found that an asymptomatic period of 7–13 days could reduce the incidence of RAEs. This result is supported by previous studies, where a 1–2 week delay was sufficient for uncomplicated URIs10,22 and at least 2 weeks for a complicated URI.20,21 Considering that the URI symptoms persist for an average of 1 week, the symptom-free period of 7–13 days in this study corresponds to approximately 2–3 weeks after the occurrence of the URI. Unfortunately, it was not possible to confirm statistical significance for the decrease in the odds ratio of RAE occurrence for symptom-free periods of 14 days or longer. Statistically significant results can be expected with larger sample sizes.
Children younger than 5 years of age, and particularly those in their infancy have been considered a risk factor for perioperative RAEs in children with URI.6,8,20 In addition, the risk of perioperative RAEs decreases by 8% with each additional year of age, irrespective of the presence of an URI.23 Although the difference was not statistically significant, the incidence of RAEs in children younger than 1 year old, tended to be higher than that in children aged 1 year or older. This result can be explained by the well-known increased susceptibility to airway obstruction in infants, due to their unique anatomic features of a relatively large tongue volume in the mouth, preferential nose breathing, small absolute airway size and highly compliant chest wall, which results in relatively low transpulmonary pressures at end expiration and with an increased tendency for closure of small peripheral airways during tidal breathing.24,25
Active URI symptoms (eg, copious secretions and nasal congestion) or parental smoking have also been reported to be independent risk factors for RAEs.8,12 Surgeons at our hospital mostly delay surgical procedures in children with active URI symptoms such as rhinorrhea, sore or scratchy throat, sneezing, nasal congestion, malaise, cough, or fever >38°C, unless the operation is emergent. This precautionary measure may have contributed to the lack of active URI symptoms as a risk factor for perioperative RAEs in this study. In addition, we could not assess whether parental smoking was a risk factor for RAEs because of the lack of records.
The finding that an emergency surgical procedure was associated with the occurrence of RAEs by univariate regression analysis was consistent with the findings of a previous study.26 However, in the present study, it showed borderline significance on multivariate analysis and the effect size of the odds ratio was not small. It may prove to be an independent risk factor for RAEs in a larger sample size study.
An abnormal finding on a chest image may indicate progression to lower respiratory tract infection, as a complication of URI. In this study, abnormal findings on chest images were indicative of pneumonia and bronchiolitis. An abnormality in the chest image had a large odds ratio of 7.60, which has great significance as a risk factor although the confidence interval was wide and it might be insufficient to interpret the clinical significance.
Of four patients with evidence of pneumonia on the chest image, three patients experienced perioperative RAEs; two of which necessitated surgery because of emergency and one was treated by antibiotics. For the one patient who underwent surgery after treatment, perioperative RAEs did not occur. Although it is difficult to draw definitive conclusions because of the small number of patients, symptoms and signs combined with chest imaging reflecting lower respiratory tract infections may be considered a reason to delay surgical procedures.
Our study had several limitations. First, it was a retrospective, single-center study with a small sample size. We had to collect data based on written information in medical records retrospectively, which might have affected the results of this study. Therefore, to validate and confirm the significance of borderline risk factors, additional prospective multi-center studies with larger sample sizes are needed. Second, we could not classify the severity of URI based on clinical manifestations. It is possible that, more severe symptoms would likely elicit greater side effects, but this assumption could not be verified. Third, although several authors have reported that tracheal intubation is a risk factor for perioperative RAEs,6,8,9 we could not confirm its effects due to the limited number of non-intubated patients [n = 6, 3 used Laryngeal mask airway (LMA) and 3 used a facial mask]. Fourth, we could not obtain results of culture tests for causative organisms in children with pneumonia or bronchiolitis. Fifth, we did not retrieve any details on the type of operation performed or on the duration of the surgical procedure. At our hospital, most of the procedures involving children are usually minor surgeries, for example, orthopedic surgery such as open or closed bone reduction, otorhinolaryngologic surgery such as tonsillectomy with or without adenoidectomy and general surgery such as appendectomy or herniorrhaphy, and most procedures are performed within 2 hours. Although it has been reported that a prolonged surgery duration, >2 hours27 or 3–4 hours,28 was an independent risk factor for postoperative pulmonary complications in adults, there is a lack of evidence supporting the duration of surgery as an independent risk factor for adverse respiratory events in children with URI.8,12,18 Thus, it is unlikely that the lack of data on the duration of surgery affected our results or conclusions despite.
The results of this study cautiously suggest the necessity for evaluating preoperative chest imaging in addition to at least 1–2 weeks of an URI symptom-free period to prevent and manage perioperative RAEs in children when complications by lower respiratory tract infections due to a recent URI are suspected. Although preoperative chest imaging is costly, time consuming, and radiation itself is harmful, we propose that it would be optimal to delay anesthesia and surgery for children with abnormal chest findings for at least 2 weeks, whenever possible. In addition, anesthesiologists should implement meticulous anesthesia protocols to reduce RAEs if surgery cannot be delayed in such patients.
Conclusion
Performing surgery following at least 1–2 weeks of URI symptom-free period and confirming that there is no abnormal preoperative chest image can reduce the incidence of perioperative RAEs in children with recent URI.
Data Sharing Statement
Deidentified participant data are available for sharing by the authors upon request, please contact Hyun Jung Lee (Email address: [email protected]).
Funding
No external funding declared.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581–595. doi:10.7326/0003-4819-144-8-200604180-00009
2. Fisher BW, Majumdar SR, McAlister FA. Predicting pulmonary complications after nonthoracic surgery: a systematic review of blinded studies. Am J Med. 2002;112(3):219–225. doi:10.1016/S0002-9343(01)01082-8
3. Flick RP, Wilder RT, Pieper SF, et al. Risk factors for laryngospasm in children during general anesthesia. Paediatr Anaesth. 2008;18(4):289–296. doi:10.1111/j.1460-9592.2008.02447.x
4. Bathla S, Mohta A, Gupta A, Kamal G. Cancellation of elective cases in pediatric surgery: an audit. J Indian Assoc Pediatr Surg. 2010;15(3):90–92. doi:10.4103/0971-9261.71748
5. Green RJ. Symptomatic treatment of upper respiratory tract symptoms in children. S Afri Fam Pract. 2006;48(4):38–42. doi:10.1080/20786204.2006.10873374
6. Cohen MM, Cameron CB. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth Analg. 1991;72(3):282–288. doi:10.1213/00000539-199103000-00002
7. Aquilina AT, Hall WJ, Douglas RG, Utell MJ. Airway reactivity in subjects with viral upper respiratory tract infections: the effects of exercise and cold air. Am Rev Respir Dis. 1980;122(1):3–10.
8. Tait AR, Malviya S, Voepel-Lewis T, Munro HM, Seiwert M, Pandit UA. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95(2):299–306. doi:10.1097/00000542-200108000-00008
9. Parnis S, Barker D, Van Der Walt J. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11(1):29–40. doi:10.1046/j.1460-9592.2001.00607.x
10. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory tract infection: still a dilemma? Anesth Analg. 2005;100(1):59–65. doi:10.1213/01.ANE.0000139653.53618.91
11. Tait AR, Knight PR. Intraoperative respiratory complications in patients with upper respiratory tract infections. Can J Anaesth. 1987;34(3):300–303. doi:10.1007/BF03015170
12. Kim SY, Kim JM, Lee JH, Kang YR, Jeong SH, Koo BN. Perioperative respiratory adverse events in children with active upper respiratory tract infection who received general anesthesia through an orotracheal tube and inhalation agents. Korean J Anesthesiol. 2013;65(2):136–141. doi:10.4097/kjae.2013.65.2.136
13. Bösenberg A. The child with a runny nose! Upper respiratory tract infection in children: impact on anaesthesia. S Afri J Anaesth Analg. 2007;13(2):33–35. doi:10.1080/22201173.2007.10872472
14. Dicpinigaitis PV. Effect of viral upper respiratory tract infection on cough reflex sensitivity. J Thorac Dis. 2014;6(Suppl 7):S708.
15. Parameswara G. Anesthetic concerns in patients with hyper‑reactive airways. Karnataka Anaesth J. 2015;1(1):8–16. doi:10.4103/2394-6954.149714
16. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164–169. doi:10.1001/jama.1974.03230150016004
17. von Ungern-sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet. 2010;376(9743):773–783. doi:10.1016/S0140-6736(10)61193-2
18. Rachel Homer J, Elwood T, Peterson D, Rampersad S. Risk factors for adverse events in children with colds emerging from anesthesia: a logistic regression. Paediatr Anaesth. 2007;17(2):154–161. doi:10.1111/j.1460-9592.2006.02059.x
19. Lee BJ, August DA. COLDS: a heuristic preanesthetic risk score for children with upper respiratory tract infection. Paediatr Anaesth. 2014;24(3):349–350. doi:10.1111/pan.12337
20. Becke K. Anesthesia in children with a cold. Curr Opin Anaesthesiol. 2012;25(3):333–339. doi:10.1097/ACO.0b013e3283534e80
21. Regli A, Becke K, von Ungern-sternberg BS. An update on the perioperative management of children with upper respiratory tract infections. Curr Opin Anaesthesiol. 2017;30(3):362–367. doi:10.1097/ACO.0000000000000460
22. Berry FA. Preexisting medical conditions of pediatric patients. Semin Anesth. 1984;3:24–31.
23. Mamie C, Habre W, Delhumeau C, Argiroffo CB, Morabia A. Incidence and risk factors of perioperative respiratory adverse events in children undergoing elective surgery. Paediatr Anaesth. 2004;14(3):218–224. doi:10.1111/j.1460-9592.2004.01169.x
24. Stocks J. Respiratory physiology during early life. Monaldi Arch Chest Dis. 1999;54(4):358–364.
25. Lermann J. Pediatric anesthesia. In: Barash PG, Cullen BE, Stoelting RK, editors. Clinical Anesthesia. 8th ed. Philadelphia, PA: Wolters Kluwer; 2017:1219–1221.
26. Budić I, Simić D. Risk factors for respiratory adverse events during general anesthesia in children. Emergency. 2004;158(14):8–9.
27. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–1350. doi:10.1097/ALN.0b013e3181fc6e0a
28. Qaseem A, Snow V, Fitterman N, et al. Risk assessment for strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144(8):575–580. doi:10.7326/0003-4819-144-8-200604180-00008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
