Back to Journals » Infection and Drug Resistance » Volume 15
Risk Factors for Mortality in Hospitalized Patients with Stenotrophomonas maltophilia Bacteremia
Authors Jian J , Xie Z, Chen L
Received 18 April 2022
Accepted for publication 8 July 2022
Published 21 July 2022 Volume 2022:15 Pages 3881—3886
DOI https://doi.org/10.2147/IDR.S371129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jiyong Jian,1– 3 Zeqiang Xie,1– 3 Liang Chen1– 3
1Clinical Laboratory Medicine, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Peking University Ninth School of Clinical Medicine, Beijing, People’s Republic of China; 3Beijing Key Laboratory of Urinary Cellular Molecular Diagnostics, Beijing, People’s Republic of China
Correspondence: Liang Chen, Clinical Laboratory Medicine, Beijing Shijitan Hospital, Capital Medical University, NO. 10, Tie Yi Road, Yang Fang Dian, Haidian District, Beijing, 100038, People’s Republic of China, Email [email protected]
Objective: Stenotrophomonas maltophilia (S. maltophilia) is an opportunistic and nosocomial pathogen with high mortality. And it has intrinsic resistance to a number of antibiotics classes. In this study, we investigated risk factors for death due to S. maltophilia bacteremia.
Methods: A retrospective cohort study was conducted at a tertiary-care hospital in Beijing, China. The patients from the hospital database with S. maltophilia bacteremia between January 2011 and December 2020 were investigated. Univariate and multivariate analyses were performed to identify factors associated with mortality.
Results: 51 patients with S. maltophilia bacteremia were identified. The mortality rate was 37.3%. Based on the univariate analysis, pulmonary disease (P=0.019), chronic kidney disease (P=0.014), shock (P=0.002), foley catheter (P=0.011), the Acute Physiology and Chronic Health Evaluation II (APACHE II) score (P< 0.001), procalcitonin (PCT) (P=0.045) and using antifungal agent (P=0.033) were significantly related to mortality. Based on the multivariate analysis, the APACHE II score (odds ratio [OR] =1.211; 95% confidence interval [CI]: 1.061, 1.382; P=0.005) was independent factor associated with mortality. S. maltophilia was the most susceptible to minocycline (94.7%), followed by trimethoprim and sulfamethoxazole (TMP/SMX, 92.2%).
Conclusion: Our findings suggested that the APACHE II score was a significantly independent predictor in S. maltophilia bacteremia patients. The use of TMP/SMX or minocycline might be the first choice for the treatment of S. maltophilia bacteremia.
Keywords: risk factor, Stenotrophomonas maltophilia, bacteremia, drug susceptibility
Introduction
Stenotrophomonas maltophilia (S. maltophilia) is a multi-drug resistant (MDR) gram-negative bacillus that is widely distributed in the environment.1 S. maltophilia is an opportunistic pathogen for various serious nosocomial infections in humans, such as pneumonia, bacteremia, urinary tract infections, cholangitis, peritonitis, wound infections, eye infections, arthritis, meningitis, and endocarditis.2 Pneumonia and bacteremia are the most common clinical symptoms of S. maltophilia infection.3 And the overall mortality of S. maltophilia bacteremia ranges from 30 to 51% within 30 days.4–6 Intensive care unit (ICU) admission, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, central venous catheter (CVC) and mechanical ventilator were clinical characteristics associated with the mortality of S. maltophilia bacteremia.6,7
Duing the intrinsic resistance of S. maltophilia to a variety of antibiotics, treatment of S. maltophilia infection is very difficult. For S. maltophilia infections, trimethoprim and sulfamethoxazole (TMP/SMX) is considered as the first choice, and fluoroquinolone is the proposed alternative.8,9
The epidemiology of bacterial infections varies greatly, depending on the healthcare institution profile and geographical location. Therefore it is essential to evaluate the local data of bacterial infections to assess trends over time and initiate appropriate treatment. The purpose of this retrospective study was to identify the risk factors for S. maltophilia bacteremia, and the outcomes of S. maltophilia bacteremia in our hospital.
Materials and Methods
Study Population and Design
A retrospective cohort study was conducted to evaluate the risk factors for morality of patients with S. maltophilia bacteremia at the Beijing Shijitan Hospital. We identified S. maltophilia-positive blood cultures from the microbiology laboratory database, and then clinical records were reviewed. Data were collected between January 2011 and December 2020 from digital medical records.
Definitions
S. maltophilia bacteremia was defined as a patient having 1 or more positive blood culture, combined with clinical symptoms of systemic inflammatory response syndromes. The source of bacteremia was determined clinically on the basis of the presence of an active site of infection as determined by isolation of the organism from other clinical specimens coincident with the episode of bacteremia. Prior antimicrobial therapy was defined as any antibiotic treatment for more than 24 hours within 1 month before the episode of bacteremia. Specific therapy was regarded as appropriate if the targeted regimen included at least 1 antibiotic agent to which S. maltophilia was susceptible in vitro. The severity of illness was assessed by APACHE II score.
Blood Culture and Drug Sensitivity Test
Bactec-9120 and Bactec-FX200 systems (Becton Dickinson, Sparks, MD) were used for blood cultures. Bacterial identification and antimicrobial susceptibility tests were performed using WalkAway 40 plus system (Beckman Coulter, Inc., Tokyo, Japan) and VITEK-2 Compact system (bioMerieux, Marcyl’Etoile, France). Some drug susceptibility tests were determined by the Kirby–Bauer disk diffusion method (filter paper purchased from Oxoid). The results were interpreted according to the Clinical and Laboratory Standards Institute guidelines.
Statistical Analysis
We conducted univariate analyses of the variables between non-survivors and survivors, using SPSS statistical software (ver. 24.0, SPSS Inc., Chicago, IL, USA). Student t-test was used for continuous variables and the Chi-square test or Fisher exact test was used for categorical variables. Multiple logistic regressions were performed to determine independent risk factors associated with mortality. All statistical tests were two-tailed and P≤0.05 was considered statistically significant.
Results
Clinical Characteristics of S. maltophilia Bacteremia Patients
A total of 51 bacteremia patients were enrolled in this study. The 28-day mortality rate was 37.3% (19/51). The patient baseline characteristics and the outcomes are shown in Table 1. There were 40 males and 11 females, with a mean age of 74.6 years (range, 1–93 years). The mean length of stay in the hospital before the occurrence of S. maltophilia bacteremia was 39.9±49.2 days. For main diagnosis, cardiovascular disease was the most common (44/51, 83.6%), followed by pulmonary disease (41/51, 80.4%) and diabetes mellitus (37/51, 72.5%). Central venous catheter (46/51, 90.2%) was the most common invasive procedure. The main source of bacteremia was in the respiratory tract (23/51, 45.1%). And S. maltophilia could also be identified in CVC (3/51, data not shown in the table), including two patients both in the respiratory tract and CVC. The median APACHE II score was 23.9±6.8.
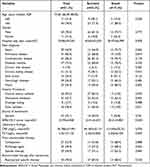 |
Table 1 Overall Characteristics and Univariate Analysis of Mortality in Patients with S. maltophilia Bacteremia |
Risk Factors for Mortality from S. maltophilia Bacteremia
Univariate analysis was performed to screen the risk factors for S. maltophilia bacteremia (Table 1). Pulmonary disease (P=0.019), chronic kidney disease (P=0.014), shock (P=0.002), foley catheter (P=0.011), APACHE II score (P<0.001), PCT (P=0.045) and using antifungal agent (P=0.033) were significantly related to mortality.
The multivariate logistic regressions analysis revealed that high APACHE II score (odds ratio [OR] =1.211; 95% confidence interval [CI]: 1.061, 1.382; P=0.005) was the independent factor associated with mortality (Table 2).
 |
Table 2 Multivariate Analysis of Factors Associated with 28-Day Mortality in Patients with S. maltophilia Bacteremia |
Antimicrobial Susceptibilities
The antibiotic susceptibility of S. maltophilia isolates was summarized in Table 3. The highest antibiotic sensitivity was shown towards minocycline (94.7%), and then TMP/SMX (92.2%). The susceptibility to levofloxacin, ticarcillin-clavulanate and ceftazidime was 78.4%, 29.4% and 22.6%, respectively.
 |
Table 3 Antibiotic Susceptibility of Stenotrophomonas maltophilia Isolates |
Discussion
S. maltophilia is emerging as an opportunistic pathogen among hospitalized patients, causing significant mortality. We conducted this retrospective analysis and analyzed the risk factors for mortality as well as antibiotic susceptibility in patients with S. maltophilia bacteremia.
S. maltophilia bacteremia is associated with high mortality rates, the crude mortality estimates range from 21 to 69%.10 In our study, the mortality rate was 37.3%, which was similar to the studies.6,11 Previously published reviews had reported several risk factors for mortality in patients with S. maltophilia bacteremia, including hemodialysis, T-tube, septic shock, sequential organ failure assessment (SOFA) score, removal of the CVC, ICU admission and use of medical devices.6,7,12 Based on the results of our multivariate analysis, S. maltophilia bacteremia patients with high APACHE II scores had higher rates of mortality. In agreement with some studies, patients with severe disease and high APACHE II scores at the time of bloodstream infection had a higher mortality rate.13–15 Since the development of the APACHE II score, the prognostic value of the APACHE II score at admission to the intensive care unit had been demonstrated.16 It seemed that the APACHE II score could also be used to predict mortality in patients with bloodstream infection.
In addition, our results showed that appropriate specific therapy, was not associated with mortality. It was similar with the previous report.6 However, some studies had reported that the appropriate antibacterial therapy was significantly associated with lower mortality.17–19 So further study to evaluate the impact of appropriate antibacterial therapy in mortality should be performed.
In our study, S. maltophilia showed higher susceptibility rates to TMP/SMX, minocycline, and fluoroquinolones than to ceftazidime and Ticarcillin clavulanate. The susceptibility to TMP/SMX was 92.2%, consistent with the reported studies.20,21 Although increasing resistance of S. maltophilia to TMP-SMX had been reported,2 the present study suggested that TMP-SMX might still be suitable as first-line drug for S. maltophilia infection. The susceptibility to minocycline was 94.7%, consistent with the previous studies.22 At the same time, the susceptibility to levofloxacin was 78.4%, similar with 83.4%.23 And the susceptibility to Ticarcillin clavulanate and ceftazidime was 29.4% and 22.6%, respectively, similar with 27.0% in Asian-Pacific region and 25.5%.24,25 The susceptibilities to antimicrobial agents were not consistent with the previous study in china,26 may be due to the frequency of S. maltophilia infections and the region-specific level of antimicrobial consumption. These data suggested that the use of TMP/SMX or minocycline in our hospital might be the best empirical treatment for S. maltophilia bacteremia.
There were some limitations to this study. First, it was a single center study with a small sample size, which may not be generalizable. Second, the design of our study was retrospective, so selection and observational bias might have affected the results. Third, the cause of mortality was not consistently documented and had to be inferred in some cases.
Conclusion
The mortality in patients with S. maltophilia bacteremia was high. High APACHE II score was a significant independent predictor of S. maltophilia mortality in bacteremia patients. The TMP/SMX and minocycline remained high susceptibility rates and these two could be considered as an appropriate antibacterial therapy at our institution. In addition, more elaborate and multi-center study with greater patient numbers may be required in the future.
Abbreviations
S. Maltophilia, Stenotrophomonas maltophilia; APACHE II, Acute Physiology and Chronic Health Evaluation II; PCT, procalcitonin; OR, odds ratio; CI, confidence interval; TMP/SMX, trimethoprim and sulfamethoxazole; CVC, Central venous catheter; ICU, intensive care unit; MDR, multi-drug resistant; CRP, C-reactive protein; SOFA, sequential organ failure assessment.
Ethics Approval and Consent to Participate
The study was approved by the ethics committees of the Beijing Shijitan Hospital. The informed consent was waived, because this study was a retrospective study with review of related data through the electronic medical records.
We declare that this study is in accordance with the Helsinki Declaration and the relevant national laws and policies, and never disclose the information of all patients.
Funding
This work was funded by the Youth Foundation of Beijing Shijitan Hospital (grant no 2018-q05).
Disclosure
The authors declare that there is no conflict of interest.
References
1. Gulcan H, Kuzucu C, Durmaz R. Nosocomial Stenotrophomonas maltophilia cross-infection: three cases in newborns. Am J Infect Control. 2004;32(6):365–368. doi:10.1016/j.ajic.2004.07.003
2. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012;25(1):2–41. doi:10.1128/CMR.00019-11
3. Gales AC, Jones RN, Forward KR, et al. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999). Clin Infect Dis. 2001;32 Suppl 2:S104–13. doi:10.1086/320183
4. Sumida K, Chong Y, Miyake N, et al. Risk factors associated with Stenotrophomonas maltophilia bacteremia: a matched case-control study. PLoS One. 2015;10(7):e0133731. doi:10.1371/journal.pone.0133731
5. Araoka H, Baba M, Yoneyama A. Risk factors for mortality among patients with Stenotrophomonas maltophilia bacteremia in Tokyo, Japan, 1996–2009. Eur J Clin Microbiol Infect Dis. 2010;29(5):605–608. doi:10.1007/s10096-010-0882-6
6. Jeon YD, Jeong WY, Kim MH, et al. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. Medicine. 2016;95(31):e4375. doi:10.1097/MD.0000000000004375
7. Osawa K, Shigemura K, Kitagawa K, et al. Risk factors for death from Stenotrophomonas maltophilia bacteremia. J Infect Chemother. 2018;24:632–636. doi:10.1016/j.jiac.2018.03.011
8. Chung HS, Hong SG, Kim YR, et al. Antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from Korea, and the activity of antimicrobial combinations against the isolates. J Korean Med Sci. 2013;28:62–66. doi:10.3346/jkms.2013.28.1.62
9. Cho SY, Kang CI, Kim J, et al. Can levofloxacin be a useful alternative to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob Agents Chemother. 2014;58:581–583. doi:10.1128/AAC.01682-13
10. Paez JI, Costa SF. Risk factors associated with mortality of infections caused by Stenotrophomonas maltophilia: a systematic review. J Hosp Infect. 2008;70(2):101–108. doi:10.1016/j.jhin.2008.05.020
11. Ebara H, Hagiya H, Haruki Y, et al. Clinical characteristics of Stenotrophomonas maltophilia bacteremia: a regional report and a review of a Japanese case series. Intern Med. 2017;56(2):137–142. doi:10.2169/internalmedicine.56.6141
12. Chen Y, Suo J, Du M, et al. Clinical features, outcomes, and risk factors of bloodstream infections due to Stenotrophomonas maltophilia in a tertiary-care hospital of China: a retrospective analysis. Biomed Res Int. 2019;2019:4931501. doi:10.1155/2019/4931501
13. Marra AR, Bearman GM, Wenzel RP, Edmond MB. Comparison of the systemic inflammatory response syndrome between monomicrobial and polymicrobial Pseudomonas aeruginosa nosocomial bloodstream infections. BMC Infect Dis. 2005;5(1):94. doi:10.1186/1471-2334-5-94
14. Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–1803. doi:10.1111/j.1469-0691.2011.03514.x
15. Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766. doi:10.1128/AAC.49.2.760-766.2005
16. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
17. Senol E, DesJardin J, Stark PC, et al. Attributable mortality of Stenotrophomonas maltophilia bacteremia. Clin Infect Dis. 2002;34:1653–1656. doi:10.1086/340707
18. Friedman ND, Korman TM, Fairley CK, et al. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect. 2002;45(1):47–53. doi:10.1053/jinf.2002.0978
19. Metan G, Uzun O. Impact of initial antimicrobial therapy in patients with bloodstream infections caused by Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2005;49:3980–3981. doi:10.1128/AAC.49.9.3980-3981.2005
20. Alsuhaibani M, Aljarbou A, Althawadi S, et al. Stenotrophomonas maltophilia bacteremia in children: risk factors and mortality rate. Antimicrob Resist Infect Control. 2021;10(1):19. doi:10.1186/s13756-021-00888-w
21. Duan Z, Qin J, Liu Y, et al. Molecular epidemiology and risk factors of Stenotrophomonas maltophilia infections in a Chinese teaching hospital. BMC Microbiol. 2020;20(1):294. doi:10.1186/s12866-020-01985-3
22. Safdar A, Rolston KV. Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clin Infect Dis. 2007;45(12):1602–1609. doi:10.1086/522998
23. Chang YT, Lin CY, Lu PL, et al. Stenotrophomonas maltophilia bloodstream infection: comparison between community-onset and hospital-acquired infections. J Microbiol Immunol Infect. 2014;47(1):28–35. doi:10.1016/j.jmii.2012.08.014
24. Farrell DJ, Sader HS, Jones RN. Antimicrobial susceptibilities of a worldwide collection of Stenotrophomonas maltophilia isolates tested against tigecycline and agents commonly used for S. maltophilia infections. Antimicrob Agents Chemother. 2010;54:2735–2737. doi:10.1128/AAC.01774-09
25. Kaur P, Gautam V, Tewari R. Distribution of class 1 integrons, sul1 and sul2 genes among clinical isolates of Stenotrophomonas maltophilia from a tertiary care hospital in North India. Microb Drug Resist. 2015;21(4):380–385. doi:10.1089/mdr.2014.0176
26. Hu LF, Xu XH, Li HR, et al. Surveillance of antimicrobial susceptibility patterns among Stenotrophomonas maltophilia isolated in China during the 10-year period of 2005–2014. J Chemother. 2018;30(1):25–30. doi:10.1080/1120009X.2017.1378834
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
