Back to Journals » Clinical Ophthalmology » Volume 17
Risk Factors for Meibomian Gland Disease Assessed by Meibography
Authors Kim CK , Carter S, Kim C , Shooshani T, Mehta U, Marshall K, Smith RG, Knezevic A, Rao K, Lee OL, Farid M
Received 1 July 2023
Accepted for publication 16 October 2023
Published 2 November 2023 Volume 2023:17 Pages 3331—3339
DOI https://doi.org/10.2147/OPTH.S428468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr John B Miller
Christine K Kim,1,2 Steven Carter,2,3 Cinthia Kim,2 Tara Shooshani,1,2 Urmi Mehta,2,4 Kailey Marshall,2 Ryan G Smith,1,2,5 Alexander Knezevic,2,6– 8 Kavita Rao,1,2 Olivia L Lee,2 Marjan Farid2
1Department of Ophthalmology, University of California, Irvine, School of Medicine, 1001 Health Sciences Rd, Irvine, CA, 92617, USA; 2Gavin Herbert Eye Institute at University of California, Irvine School of Medicine, 850 Health Sciences Rd, Irvine, CA, 92617, USA; 3Miramar Eye Specialists Medical Group, Ventura, CA, 93003, USA; 4St John’s Episcopal Hospital, Far Rockaway, NY, 11691, USA; 5Pacific Eye Institute, Upland, CA 91786, USA; 6Macy Eye Center, Los Angeles, CA, 90048, USA; 7Cedars-Sinai Medical Center, Los Angeles, CA, 90048, USA; 8Jules Stein Eye Institute at University of California, Los Angeles, CA, 90095, USA
Correspondence: Christine K Kim, Gavin Herbert Eye Institute at University of California, Irvine School of Medicine, 850 Health Sciences Road, Irvine, CA, 92617, USA, Tel +1 818 279 5029, Email [email protected]
Purpose: To elucidate risk factors for meibomian gland disease (MGD) and understand associated changes in meibography and in relation to ocular surface disease.
Patients and Methods: As part of the standard workup for ocular surface disease at a tertiary academic center, 203 patients received an ocular history and lifestyle questionnaire. The questionnaire included detailed inquiries about ocular health and lifestyle, including makeup use, cosmetic eyelid procedures, screen time, and contact lens habits. Subjects also took the standardized patient evaluation of eye dryness (SPEED) II questionnaire. Meibomian gland (MG) dropout and structural changes were evaluated on meibography and scored by three independent graders using meiboscores. Statistical analysis was conducted to identify significant risk factors associated with MG loss.
Results: This retrospective, cross-sectional study included 189 patients (378 eyes) with high-quality images for grading, and the average age was 67 years (77% female). Patients older than 45 years had significantly more dropout than younger patients (p < 0.01). Self-reported eye makeup use did not significantly impact MG loss. Patients with a history of blepharoplasty trended toward higher meiboscores, but the difference was not statistically significant. Self-reported screen time did not affect meiboscores. Contact lens use over 20 years was associated with significant MG loss (p < 0.05). SPEED II scores had no relationship to meiboscores (p = 0.75).
Conclusion: Older age is a significant risk factor for MG loss. Any contact lens use over 20 years also impacted MG dropout. Highlighting the incongruence of symptoms to signs, SPEED II scores showed no relationship to the structural integrity of MGs.
Keywords: dry eye, meibomian gland dropout, meiboscore, ocular surface disease
Introduction
Meibomian Gland Disease (MGD) is a chronic, diffuse abnormality of the meibomian glands, often characterized by terminal duct obstruction and/or changes in glandular secretion. This may lead to alterations in the tear film, eye irritation, inflammation, and ocular surface disease.1 MGD is one of the most common reasons for ophthalmic visits,2 with an estimated 70% of Americans over the age of 60 living with this condition.3
Meibomian glands, found in the upper and lower eyelids, secrete meibum which constitutes the primary lipid component of the outer layer of the tear film.4 These lipids stabilize the tear film and protect against evaporation.5 Thus, any changes in meibum, whether due to deficient secretion or altered compositions of the lipids, have adverse effects on the tear film and lead to evaporative eye disease.4 Alterations in the morphology of meibomian glands, which include shortening, dilation, distortion, and atrophy, are sensitive and early indicators of MGD.6 Various risk factors for MGD have been identified, including age,7 female sex,5 topical medications,8 contact lens wear,9 and refractive surgery.5 Other environmental and social factors may contribute to the development of MGD but are yet to be clinically illustrated. Screen time has increased dramatically with the rise in popularity of smartphones, tablets, and computers. Although increased screen exposure has been linked to evaporative dry eye disease,10 no study has looked at its impact on structural MG changes.
Methods to visualize the meibomian glands effectively and with minimal patient discomfort have been developed over the past decades.11 Meibography, an imaging technique that has been used for over 40 years, allows observation of morphologic changes in the Meibomian glands in vivo.12 Several noninvasive meibography (NIM) devices are now available commercially. NIM allows quantitative analysis of meibomian gland morphology, which can serve as a marker for severity or progression of disease.11 A score of meibomian gland changes, or meiboscore, was created to quantify the partial or complete loss of meibomian glands. The meiboscore has demonstrated good within reader and between reader reliability.13 Many prior studies on MGD risk factors relied on clinical exam findings without using infrared meibography.7,8
In this study, we aimed to use meibography to visualize changes in meibomian glands associated with common and likely risk factors. A statistical analysis ran potential risk factors including age, contact lens usage, eye makeup usage, cosmetic procedures, screen time, and SPEED II scores against the meiboscore values.
Materials and Methods
Study Design
This retrospective, cross-sectional, observational study was conducted on 203 patients at a tertiary academic center. This study followed the tenets of the Declaration of Helsinki, was approved by the institutional review board (IRB) at the University of California Irvine, and adhered to the Health Insurance Portability and Accountability Act. All subjects provided written informed consent after receiving an explanation of the nature of the study.
Subject Recruitment
Healthy individuals over 18 years of age and with the ability to consent were considered for the study. Consecutive patients with dry eyes, determined by subjective symptoms including ocular discomfort, tearing, pain, redness, or fluctuating vision were included in the study. Exclusion criteria included previous corneal disease (ie, herpetic eye disease or neurotrophic keratitis) or corneal surgery. Subjects with a history of eye surgery, including cataract surgery within the past three months, were also excluded from the study.
Survey Design
Subjects received an ocular history and lifestyle questionnaire detailing various factors contributing to MGD. The survey included detailed questions about makeup use, cosmetic eyelid procedures, screen time, and contact lens habits (attached as Supplementary Data). All subjects also completed the Standardized Patient Evaluation of Eye Dryness (SPEED) II questionnaire.
Meibography and Grading
Meibomian gland dropout and structural changes were evaluated on meibography with Lipiview II (Johnson & Johnson Vision, Inc.) and scored using meiboscores by three independent graders based on a standardized scoring scale of 0 to 3 (Figure 1). Changes in meibomian glands were scored using the following grades in each eyelid: grade 0, no loss of meibomian glands; grade 1, area loss was less than one-third of the total meibomian gland area; grade 2, area loss was between one-third and two-thirds; grade 3, area loss was more than two-thirds.13 For each subject, an average of the three graders’ scores were calculated for each eye. Then, the average score of both eyes was calculated to yield one final meiboscore for each patient.
 |
Figure 1 Representative meiboscore images for grades 0–3. Notes: (A) Grade 0, no gland loss. (B) Grade 1, <33% gland loss. (C) Grade 2, 33–66% gland loss. (D) Grade 3, >66% gland loss. |
Statistical Analysis
All statistical analysis was conducted using the statistical package for the social sciences (SPSS) version 29 (IBM, Corp., Armonk, NY). Average meiboscores of each independent variable were compared using a students’ t-test or ANOVA (analysis of variance). Independent variables were defined as risk factors included in the questionnaire. An independent samples t-test was used to compare the mean meiboscores between variables that had two groups (age: <45 or ≥45, length of contact lens wear: 0–20 or >20 years, eye makeup use: yes or no, blepharoplasty: yes or no). ANOVA was used to compare means for variables with three or more groups (contact lens type, eye makeup type, screen time, SPEED II scores). Using a confidence interval of 95%, a p-value of <0.05 was considered statistically significant.
Results
Demographics
Of the 203 patients who completed the survey, 189 patients (N = 378 eyes) had images of high enough quality to grade. One hundred and fifty-seven (77.3%) were female and 46 (22.6%) were male and had a mean age of 67 ± 15.6 years (age range: 17–89). This information is summarized in Table 1.
 |
Table 1 Properties and Distribution of Patients Included in Our Study |
Age
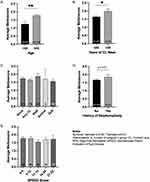
Patients older than 45 years (n = 165, 136 female) demonstrated significantly more meibomian gland dropout than patients younger than 45 (n = 24, 20 female), with average meiboscores 1.78 ± 0.81 and 1.24 ± 0.76, respectively (p < 0.01) (Figure 2A).
Contact Lens Usage
Patients with contact lens (CL) use over 20 years (n = 30, 26 female, mean age 68.33 ± 10.97) showed greater meibomian gland loss than those who used CL for 0–20 years (n = 157, 123 female, mean age 64.99 ± 17.15), with average meiboscores 1.98 ± 0.75 and 1.65 ± 0.82, respectively (p < 0.05) (Figure 2B). The difference in mean age between these two groups was not significant (p = 0.31). Patients who used CL for less than 20 years had average meiboscores 1.46 ± 0.81 (n = 42, 37 female, mean age 53.91 ± 21.33), and those who never wore CL had average meiboscores 1.73 ± 0.83 (n = 115, 86 female, mean age 69.26 ± 13.01). The difference in mean age between these two groups was significant (p < 0.05). Among CL wearers, those who used them for more than 20 years (n = 30, 26 female, mean age 68.33 ± 10.97) had higher scores than those who used them for 1–20 years (n = 42, 37 female, mean age 53.91 ± 21.33) (p < 0.05). The difference in mean age between these two groups was significant (p < 0.05).
Meiboscores of those who never used CL were higher than those who used CL for 1–20 years (p < 0.05).
Among subjects ≤55 years old, those who used CL for >20 years (n = 4) had higher meiboscores than those who used CL for 0–20 years (n = 36), with mean scores 1.958 ± 0.89 and 1.484 ± 0.90, respectively. However, this difference was not statistically significant (p = 0.32).
There were no significant differences between the meiboscores of patients using different CL types, including Rigid Gas Permeable (n = 24), scleral (n = 4), and soft lenses (n = 46) (Figure 2C).
Eye Makeup
Eye makeup use (self-reported) did not have any statistically significant correlation with meibomian gland loss. When broken down by type of makeup, there was also no significant correlation in patients using eyeliner, mascara, or eyeshadow (Table 2).
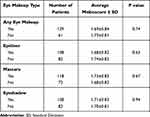 |
Table 2 Summary of Average Meiboscores Based on Eye Makeup Use |
Blepharoplasty
Patients with a history of blepharoplasty (n = 40) trended toward higher meiboscores, but the difference was not significant (p = 0.21) (Figure 2D).
Screen Time
Screen time, assessed by self-reported number of hours using a screen and the nature of the participant’s occupation, had no impact on meiboscores (Table 3). Screen time was determined as the average time spent across the following devices: cell phones, tablets, TV, and computers. Individual responses for time spent on each of these devices were collected. None of the individual categories was statistically significant for hours spent per day.
 |
Table 3 Summary of Average Meiboscores Based on Screen Time, Assessed by Nature of Occupation and Hours per Day |
SPEED II Scores
Standardized Patient Evaluation of Eye Dryness (SPEED) II scores had no relationship to meiboscores (p = 0.75) (Figure 2E).
Discussion
To the best of our knowledge, this is the first study to evaluate multiple potential risk factors for meibomian gland disease using meibography as an objective tool. Our present study measured the effect of six different variables on MGD using meiboscores. These variables included age, contact lens usage, eye makeup, cosmetic procedures, screen time, and SPEED II scores. Of these factors, age and years of contact lens wear demonstrated a significant relationship to meibomian gland loss. Interestingly, dry eye symptom severity as assessed by SPEED II scores was not associated with the structural integrity of the meibomian glands. Other variables, including contact lens type, eye makeup, and cosmetic procedures, demonstrated trends that may be consistent with current literature.
Among previous research on precipitating factors for MGD, age is a commonly accepted risk factor, with the severity of MGD increasing with age.7,8 Changes in the lid margin and meibomian gland anatomy are related to age, demonstrated through notable abnormalities in patients ≥50 years of age.14 MGD is an underlying cause of tear dysfunction in many patients over the age of 40, especially in those with an unstable tear film but normal tear production and tear volume.15 Our results are consistent with these prior findings. Patients older than 45 years demonstrated greater meibomian gland dropout than those younger than 45 (p < 0.01). This is attributed to age-related processes, including decreased meibomian gland density and diameter and increased gland dropout and obstruction rate.16 The pathogenesis of MGD in aging may involve mechanisms of PPARγ, a fatty acid-activated nuclear transcription factor expressed mainly in adipocytes and sebocytes, that regulate meibocyte differentiation and directly impact meibum quality, lipid synthesis, and acinar atrophy.17,18 In conjunction with molecular changes, these factors result in MGD and tear film instability in older patients.16
The Standardized Patient Evaluation of Eye Dryness (SPEED) questionnaire is a survey that asks patients to rate the frequency and severity of their symptoms on eight different categories, resulting in a total score out of 28. The SPEED questionnaire is a repeatable and standardized tool for assessing dry eye symptoms.19 Our study utilized the SPEED II questionnaire, which is an updated version with the same purpose and format. Interestingly, our study showed no correlation between meiboscores and SPEED II scores. This is in concordance with studies that displayed no correlation between SPEED II scores and meibomian gland morphology.20 More broadly, this is consistent with the lack of correlation between objective dry eye findings and subjective symptoms of dry eye disease.21 Previous studies have demonstrated a poor correlation between the signs and symptoms of dry eye disease, especially when screening patients for cataract surgery.22,23 Our findings suggest that subjective patient-reported symptoms may not be reliable in assessing the severity of MGD. The measurable clinical signs of dry eye frequently do not correlate with reported patient symptoms, which makes dry eye challenging to approach.19 Additionally, questionnaires are subjective by nature, and there is no current method to account for differences in perceptions of ocular dryness.19 Thus, it is crucial to incorporate objective methods like meibography in conjunction with patients’ symptoms to determine the progression of MGD and guide its treatment.
Present research on contact lens (CL) usage and MGD remains controversial. One study used a noncontact meibographic technique to demonstrate that CL wearers have a significantly greater degree of meibomian gland loss than non-wearers, suggesting that meibomian gland loss is one mechanism underlying CL-related dry eye.24 Another study demonstrated that 30% of lens wearers develop MGD after six months of wear, while only 20% of non-wearers develop MGD.25 These results provide compelling data to support the relationship between CL usage and MGD. However, considerable evidence also suggests a lack of an association between CL wear and MGD.26 Two distinct studies demonstrated no significant difference between CL wearers and non-wearers in MGD prevalence27 and MG secretion,28 respectively. Our study demonstrated greater meibomian gland loss in patients with CL use over 20 years than those who used them for 0–20 years (p < 0.05). The mean difference in age between the two groups was not significant, reducing the impact of age as a confounding variable.
Among CL wearers, those who used them for more than 20 years (n = 31) had higher scores than those who used them for 1–20 years (n = 42) (p < 0.05). This corroborates the relationship between the duration of contact lens wear and resulting meibomian gland loss. The mechanism is unknown but may include decreased blink rates or chronic subclinical inflammation in CL wearers. Interestingly, the meiboscores of those who never used CL were higher than those who used CL for 1–20 years (p < 0.05). This may be attributed to the higher sample size in the no CL wear group (n = 115) compared to the 1–20 years group (n = 42). Confounding variables, like age, may have resulted in higher meiboscores in the first group despite no history of CL wear. Among subjects ≤55 years old, there was no statistically significant difference among those who used CL for >20 years and those who used CL for 0–20 years. This may be attributed to the small sample size. A larger sample is needed to determine if contact lens wear is a true risk factor without age as a confounding variable. The type of CL wear may also affect these factors. However, our study revealed that the type of CL, including Rigid Gas Permeable (RGP), scleral, and soft lenses, did not affect meibomian gland dropout. In this study, the numbers of patients in these sub-categories were low and differences may be seen in a larger population of contact lens users.
Blepharoplasty has been associated with changes related to dry eye markers, including a significant decrease in Schirmer test results at six months, decreased tear break-up time (TBUT) values, and changes in corneal topography.29 Tear inflammatory cytokines and tear film instability were demonstrated to increase and contribute to the development of postoperative dry eye.30 The impact on dry eye disease is usually attributed to post-operative lagophthalmos that can exacerbate ocular surface exposure. However, there is a paucity in current literature on the impact of blepharoplasty on MGD dropout. Our study revealed that patients with a history of blepharoplasty trended toward higher meiboscores, suggesting more gland dropout, but the difference was not significant. In this study however, the numbers of patients with a history of blepharoplasty were low and a larger study in this population may reveal a stronger correlation.
Eye cosmetics are widely used and have the potential to migrate onto the ocular surface and contaminate the tear film.31 Eyeliner and mascara specifically may be agents of destabilizing the lipid layer.32 One study demonstrated that regular use of eyeliner induces MG dysfunction, as demonstrated by higher meiboscores.33 Another study revealed that patients using eyeliner, mascara, or both eyeliner and mascara had significantly higher meiboscores than those without eye makeup.34 The hypothesis is that chronic inflammation from varying makeup ingredients or physical obstruction of MG orifices by the makeup can cause long-term dysfunction and structural changes in the MGs. In our present study, self-reported eye makeup use did not significantly impact meibomian gland loss. This may be due to the subjective nature of the survey; detailed and accurate data regarding makeup usage may be difficult to elicit with a questionnaire. Furthermore, varying brands and chemical compositions in makeup products make studying the long-term relationship with MGD a challenge. It is important to note that both females and males were given the opportunity to respond to questions regarding eye makeup use. In our study, all male subjects responded with “no”, excluding them from this parameter. This may have reduced bias by isolating the effects of this variable to the female population. Despite this, our study demonstrated no significant differences among eye makeup users.
Increased digital screen exposure positively correlates with dry eye.35 The mechanism is understood to be due to a reduction in blink rate and completeness.36 A study on screen time and meibomian gland morphology in children demonstrated a weak and positive correlation between meiboscore for gland atrophy and screen time. The study also demonstrated a weak but significantly positive correlation between meibomian gland tortuosity and screen time.37 However, a different study looking at screen time during the COVID-19 pandemic found that the prevalence of diagnosed dry eyes did not increase among first-visit patients, despite increased screen time.38 Current research on screen time and MGD in adult patients is lacking. Our present study demonstrates a lack of relationship between self-reported screen time and meiboscores. The effect of screen size was also investigated by requiring subjects to specify the use of cell phones, tablets, TV, and/or computers. However, screen size showed no significant relationship to meiboscores.
Study limitations include the use of a self-reported survey, which may have introduced response bias. Additionally, age may be a confounding variable for other factors measured, including contact-lens usage, makeup use, and screen time. The effects of age on these factors make it challenging to isolate our results into a single variable. Further studies focusing on assessing these factors in the younger population may better demonstrate the impact of lifestyle variables on MGD.
Conclusion
In conclusion, meibography is an effective instrument to visualize and quantify changes in meibomian glands associated with various risk factors. Older age is a significant risk factor for meibomian gland loss. Contact lens usage for greater than 20 years is another risk factor. Self-reported lifestyle habits including makeup usage and screen time, as well as symptom severity, assessed by SPEED II scores, showed no correlation to the structural integrity of the meibomian glands.
Acknowledgments
The authors acknowledge departmental support from an RPB unrestricted grant.
Disclosure
Dr. Farid, Dr. Smith, and Dr. Knezevic are consultants for Johnson & Johnson Vision. Dr. Lee reports personal fees from Cloudbreak Therapeutics, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–1937. doi:10.1167/iovs.10-6997b
2. Hashemi H, Asharlous A, Aghamirsalim M, et al. Meibomian gland dysfunction in geriatric population: Tehran geriatric eye study. Int Ophthalmol. 2021;41(7):2539–2546. doi:10.1007/s10792-021-01812-2
3. Chader GJ, Taylor A. Preface: the Aging Eye: normal Changes, Age-Related Diseases, and Sight-Saving Approaches. Investig Opthalmology Vis Sci. 2013;54(14):ORSF1. doi:10.1167/iovs.13-12993
4. Adil MY, Xiao J, Olafsson J, et al. Meibomian Gland Morphology Is a Sensitive Early Indicator of Meibomian Gland Dysfunction. Am J Ophthalmol. 2019;200:16–25. doi:10.1016/j.ajo.2018.12.006
5. Chan TCY, Chow SSW, Wan KHN, Yuen HKL. Update on the association between dry eye disease and meibomian gland dysfunction. Hong Kong Med J Xianggang Yi Xue Za Zhi. 2019;25(1):38–47. doi:10.12809/hkmj187331
6. Chang P, Qian S, Xu Z, et al. Meibomian Gland Morphology Changes After Cataract Surgery: a Contra-Lateral Eye Study. Front Med. 2021;8:766393. doi:10.3389/fmed.2021.766393
7. Tulsyan N, Gupta N, Agrawal N. Risk Factors Associated with Meibomian Gland Dysfunction: a Hospital Based Study. Nepal J Ophthalmol Biannu Peer-Rev Acad J Nepal Ophthalmic Soc NEPJOPH. 2021;13(25):59–64. doi:10.3126/nepjoph.v13i1.30605
8. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423. doi:10.1136/bjo.86.4.418
9. Arita R, Fukuoka S, Morishige N. Meibomian Gland Dysfunction and Contact Lens Discomfort. Eye Contact Lens. 2017;43(1):17–22. doi:10.1097/ICL.0000000000000351
10. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al. Demographic and lifestyle risk factors of dry eye disease subtypes: a cross-sectional study. Ocul Surf. 2021;21:58–63. doi:10.1016/j.jtos.2021.05.001
11. Arita R, Fukuoka S, Morishige N. New insights into the morphology and function of meibomian glands. Exp Eye Res. 2017;163:64–71. doi:10.1016/j.exer.2017.06.010
12. Swiderska K, Read ML, Blackie CA, Maldonado-Codina C, Morgan PB. Latest developments in meibography: a review. Ocul Surf. 2022;25:119–128. doi:10.1016/j.jtos.2022.06.002
13. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115(5):911–915. doi:10.1016/j.ophtha.2007.06.031
14. Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association Between Meibomian Gland Changes and Aging, Sex, or Tear Function. Cornea. 2006;25(6):651. doi:10.1097/01.ico.0000227889.11500.6f
15. de Paiva CS. Effects of Aging in Dry Eye. Int Ophthalmol Clin. 2017;57(2):47–64. doi:10.1097/IIO.0000000000000170
16. Kitazawa K, Inomata T, Shih K, et al. Impact of aging on the pathophysiology of dry eye disease: a systematic review and meta-analysis. Ocul Surf. 2022;25:108–118. doi:10.1016/j.jtos.2022.06.004
17. Nien CJ, Massei S, Lin G, et al. Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol. 2011;129(4):462–469. doi:10.1001/archophthalmol.2011.69
18. Hwang HS, Parfitt GJ, Brown DJ, Jester JV. Meibocyte differentiation and renewal: insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp Eye Res. 2017;163:37–45. doi:10.1016/j.exer.2017.02.008
19. Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric Properties and Validation of the Standard Patient Evaluation of Eye Dryness Questionnaire. Cornea. 2013;32(9):1204. doi:10.1097/ICO.0b013e318294b0c0
20. Chan AY, Chuang JC, Wong VW. Evaluation of Meibomian Gland Dysfunction Among Ophthalmic Healthcare Workers. Clin Ophthalmol. 2021;15:1201–1206. doi:10.2147/OPTH.S299338
21. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762–770. doi:10.1097/01.ico.0000133997.07144.9e
22. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald M, Stonecipher KC, Goldberg D. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423–1430. doi:10.2147/OPTH.S120159
23. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669–684. doi:10.1016/j.jcrs.2019.03.023
24. Arita R, Itoh K, Inoue K, Kuchiba A, Yamaguchi T, Amano S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116(3):379–384. doi:10.1016/j.ophtha.2008.10.012
25. Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt J Br Coll Ophthalmic Opt Optom. 1990;10(2):144–148. doi:10.1111/j.1475-1313.1990.tb00968.x
26. Machalinska A, Zakrzewska A, Adamek B, et al. Comparison of Morphological and Functional Meibomian Gland Characteristics Between Daily Contact Lens Wearers and Nonwearers. Cornea. 2015;34(9):1098. doi:10.1097/ICO.0000000000000511
27. Ong BL. Relation between contact lens wear and Meibomian gland dysfunction. Optom Vis Sci off Publ Am Acad Optom. 1996;73(3):208–210. doi:10.1097/00006324-199603000-00015
28. Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of Meibomian gland dysfunction. Optom Vis Sci off Publ Am Acad Optom. 1990;67(9):710–712. doi:10.1097/00006324-199009000-00010
29. Aksu Ceylan N, Yeniad B. Effects of Upper Eyelid Surgery on the Ocular Surface and Corneal Topography. Turk J Ophthalmol. 2022;52(1):50–56. doi:10.4274/tjo.galenos.2021.63255
30. Zhao S, Song N, Gong L. Changes of Dry Eye Related Markers and Tear Inflammatory Cytokines After Upper Blepharoplasty. Front Med. 2021;8:763611. doi:10.3389/fmed.2021.763611
31. Ng A, Evans K, North RV, Purslow C. Migration of Cosmetic Products into the Tear Film. Eye Contact Lens. 2015;41(5):304–309. doi:10.1097/ICL.0000000000000124
32. Franck C. Fatty layer of the precorneal film in the “office eye syndrome”. Acta Ophthalmol. 1991;69(6):737–743. doi:10.1111/j.1755-3768.1991.tb02052.x
33. Prabhasawat P, Chirapapaisan C, Chitkornkijsin C, Pinitpuwadol W, Saiman M, Veeraburinon A. Eyeliner Induces Tear Film Instability and Meibomian Gland Dysfunction. Cornea. 2020;39(4):473–478. doi:10.1097/ICO.0000000000002198
34. Ercan ZE. Effect of eyeliner and mascara use on tear film and meibomian glands. Saudi J Ophthalmol off J Saudi Ophthalmol Soc. 2022;36(1):113–116. doi:10.4103/sjopt.sjopt_170_21
35. Wang MTM, Muntz A, Mamidi B, Wolffsohn JS, Craig JP. Modifiable lifestyle risk factors for dry eye disease. Contact Lens Anterior Eye. 2021;44(6):101409. doi:10.1016/j.clae.2021.01.004
36. Golebiowski B, Long J, Harrison K, Lee A, Chidi-Egboka N, Asper L. Smartphone Use and Effects on Tear Film, Blinking and Binocular Vision. Curr Eye Res. 2020;45(4):428–434. doi:10.1080/02713683.2019.1663542
37. Kocamiş Ö, Temel E, Aşikgarip N, Örnek K. Electronic Device Screen Time and Meibomian Gland Morphology in Children. J Ophthalmic Vis Res. 2021;16(4):531–537. doi:10.18502/jovr.v16i4.9741
38. Ayaki M, Negishi K. The ocular symptoms and signs during the COVID-19 pandemic. PLoS One. 2022;17(10):e0276435. doi:10.1371/journal.pone.0276435
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.