Back to Journals » Clinical Interventions in Aging » Volume 18
Risk Factors and Dynamic Nomogram Development for Surgical Site Infection Following Open Wedge High Tibial Osteotomy for Varus Knee Osteoarthritis: A Retrospective Cohort Study
Authors Guo H, Song B, Zhou R, Yu J, Chen P, Yang B, Pan N, Li C, Zhu Y, Wang J
Received 16 September 2023
Accepted for publication 13 December 2023
Published 19 December 2023 Volume 2023:18 Pages 2141—2153
DOI https://doi.org/10.2147/CIA.S436816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Haichuan Guo,1,* Bixuan Song,2,* Ruijuan Zhou,3 Jiahao Yu,1 Pengzhao Chen,1 Bin Yang,1 Naihao Pan,1 Chengsi Li,1 Yanbin Zhu,1,4 Juan Wang1,4
1Department of Orthopedic Surgery, The 3rd Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050051, People’s Republic of China; 2Division of Medical Sciences, The Chinese University of Hong Kong, Hong Kong, People’s Republic of China; 3College of Education, Hebei Normal University, Shijiazhuang, Hebei, 050010, People’s Republic of China; 4Orthopedic Research Institute of Hebei Province, Key Laboratory of Biomechanics of Hebei Province, Shijiazhuang, Hebei, 050051, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Juan Wang; Yanbin Zhu, Department of Orthopaedics, the 3rd Hospital, Hebei Medical University, No. 139 Ziqiang Road, Shijiazhuang, 050051, People’s Republic of China, Tel +86-311-88602015, Email [email protected]; [email protected]
Background: As the worldwide population ages, the population receiving open wedge high tibial osteotomy (OWHTO) is growing, and surgical site infection (SSI) is a rare but fatal surgical complication. This study aimed to identify risk factors independently associated with SSI following OWHTO and develop a predictive nomogram.
Methods: Clinical data of patients who received OWHTO and followed up for more than 12 months in our hospital were retrospectively reviewed. Multivariable logistic regression was performed to determine independent risk factors for SSI and to construct predictive nomograms. The study further illustrated the predictive performance of the model by using the receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA).
Results: A total of 1294 eligible patients were included in the study. Multivariate analysis revealed tobacco consumption (OR=3.44, p=0.010), osteotomy size ≥ 12 mm (OR=3.3, p=0.015), the use of allogeneic bone or artificial bone graft substitutes (allogeneic bone vs none, OR=4.08, p=0.037; artificial bone vs none, OR=5.16, p=0.047), Kellgren-Lawrence (K-L) grade IV (OR=2.5, p=0.046), systemic immune-inflammation index (SII) > 423.62 (OR=6.2, p< 0.001), high-sensitivity C-reactive protein (HCRP) > 2.6 mg/L (OR=2.42, p=0.044), and a higher level of fasting blood glucose (FBG) (OR=1.32, p=0.022) were the independent predictors of SSI. The cutoff score of the model was 148, with a sensitivity of 76.0% and specificity of 81.0%. The concordance index (C-index) and Brier score of the nomogram were 0.856 and 0.017, and the corrected values after 1000 bootstrapping validations were 0.820 and 0.018, respectively. Furthermore, the ROC curve, calibration curve, and DCA exhibited excellent predictive accuracy and clinical applicability of the model.
Conclusion: This study developed a dynamic nomogram based on seven predictors, which allowed surgeons to individualize risk stratification of patients and intervene promptly to reduce SSI rates.
Keywords: open wedge high tibial osteotomy, surgical site infection, risk factors, nomogram
Introduction
Open wedge high tibial osteotomy (OWHTO) is a well-established treatment for medial compartment knee osteoarthritis (KOA) with varus deformity.1 However, the patient’s prognosis may be severely compromised by surgical site infection (SSI).2 According to the guidelines, SSI is defined as an infection that affects the incision or deep tissues at the surgical site, usually occurring within 30 days of surgery and up to one year after implantation.3,4 SSI is uncommon, with an incidence of 0.5%- 9.6%,5–8 but when it does occur, it may lead to catastrophic medical consequences, such as incision nonhealing, secondary surgery, and even sepsis and death.9 Furthermore, SSI has been reported to increase patient average hospital stays by 7 to 11 days, contributing approximately $3.5 billion to $10 billion in annual US healthcare expenditures.10 Considering that the population of patients who received OWHTO is growing as the prevalence of KOA increases globally,11 finding appropriate methods to effectively prevent SSI after OWHTO is a challenge that surgeons must address.
The National Nosocomial Infection Surveillance (NNIS) score is a widely applicable clinical tool for assessing the risk of SSI,12 whereas its predictive validity is hampered by low discrimination and poor targeting when applied to orthopedic plate internal fixation procedures.13,14 Currently, there are a few publications that have looked at postoperative SSI events following OWHTO.15–17 However, the understanding of its relevant factors is not deep enough. Woodacre et al reported epidemiological data on complications after OWHTO in 115 patients followed for at least 2 years, with a 3.5% incidence of SSI.18 Unfortunately, the study failed to reveal the underlying risk factors. Liu et al found that advanced age and diabetes were significantly associated with a high risk of postoperative SSI based on clinical data from 59 patients with OWHTO,19 but the smaller sample size may have led to a significant bias. Recently, a national database-based retrospective study in Japan noted that artificial bone implantation and long anesthesia duration were independently associated with SSI.20 However, the shorter follow-up period (median of 34 days) and the identification of SSI patients based on code data, rather than clinical assessment, may decrease the accuracy of the conclusions. In addition, all of the above studies ignore the analysis of laboratory biomarkers that have a great predictive value for SSI, and reviewing risk factors in isolation does little to help surgeons get the exact likelihood of a patient developing SSI.
In order to explore more independent risk factors associated with SSI after OWHTO and help surgeons to use them integratively, this study included a relatively large sample size and transformed the combination of the final results into a predictive nomogram to make the risk of SSI in patients visualizable.
Materials and Methods
General Information
This was a retrospective single-center study, and consecutive patients who underwent OWHTO stabilized with a medial locked plate system for the treatment of medial compartment knee osteoarthritis between June 2017 to January 2022 in a tertiary referral and university-affiliated hospital were considered eligible for inclusion. Exclusion criteria were incomplete data, a diagnosis other than primary KOA, failed or less than 12 months follow-up, combined history of knee surgery and/or lower extremity trauma, combined malignancy, or severe psychiatric disorders. This present study was conducted following the consensus of the Declaration of Helsinki and based on the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) guidelines, which were approved by the Institutional Ethics Board (IRB). All patients and/or their family members were informed that their medical data were used for scientific research and have signed an informed consent document. All the data were analyzed with anonymization. According to the requirements for developing a clinical prediction model,21 the required sample size was calculated to be at least 310 cases with a target error of ≤0.05, thus our sample size (1294 cases) was apparently sufficient.
Data Collection
Collected clinical data included demographics, chronic comorbidities, preoperative radiographic images, treatment-related variables, and laboratory biomarkers. Demographic data involved the patient’s gender, age, body mass index (BMI), and place of residence (rural or urban). Chronic comorbidities included hypertension, diabetes, heart disease, tobacco consumption, alcohol consumption, surgical history, and allergy history. The severity of KOA on the preoperative radiological images was graded according to the Kellgren-Lawrence (K-L) grading system.22 Treatment-related variables included pre-operative stay, the overall duration of stay, course of KOA, surgical side, osteotomy size, type of bone graft, ASA score, intraoperative blood loss, and duration of surgery. Laboratory biomarkers included red blood cell count (RBC), hematocrit (HCT), hemoglobin (HGB), white blood cell count (WBC), monocyte-to-lymphocyte ratio (MLR) level, and systemic immune-inflammation index (SII), defined as neutrophil count (NEU)×platelet count (PLT)/ lymphocyte count (LYM);23,24 total protein (TP), albumin (ALB), fasting blood glucose (FBG), and hypersensitive C-reactive protein (HCRP). For patients who underwent multiple hematology tests preoperatively, the study collected data closest to the time of hospitalization.
Surgical Procedures and Clinical Pathways
Intraoperative prophylactic antibiotic treatment was administered intravenously 0.5 hours before making incision and anesthesia was performed by general anesthesia combined with ultrasound-guided femoral nerve block. Arthroscopy was performed routinely and treated as needed, then osteotomy was performed through a longitudinal skin incision inferior to the medial tibial plateau, preserving the pes anserinus and completely releasing the superficial layer of the medial collateral ligament. The horizontal osteotomy was established above the insertion of the pes anserine and parallel to the posterior tibial slope. The ascending coronal osteotomy is at an angle of 110° to the horizontal osteotomy plane. After completing the biplanar osteotomy, the osteotomy gap was slowly opened until the hip-knee-ankle angle reached 180°-182° and finally fixed with T-lock compression plates and screws (Double Medical Technology Inc., Xiamen, Fujian, China). According to the standardized postoperative clinical pathway, continuous negative pressure drainage was retained in all patients and typically removed after 24 hours. Cefazolin sodium Q6H 1g (clindamycin hydrochloride Q12H 0.6g if penicillin-allergic) antibiotic coverage was extended for 24 hours postoperatively. No bracing was utilized post-procedurally, and quadriceps isometric contractions and ankle pump exercises were started on the first day. Postoperatively, the dressing is changed regularly every 3–4 days until the stitches are removed at two weeks. Toes of the affected limb were allowed to touch the ground with the assistance of double crutches for the first 4 weeks, and the transition to full weight-bearing is usually gradual after 8 weeks.
Diagnosis of SSI
The Centers for Disease Control (CDC) definition of SSI was adopted for this study. Superficial SSI was defined as erythema, swelling, fever, and tenderness upon palpation of the skin and subcutaneous tissue at the wound site within 1 month after surgery, which were usually resolved with frequent disinfection and dressing change and empirical oral antibiotics. Deep SSI was defined as infections involving deep soft tissue, muscle, or fascia within 12 months after the surgery, with persistent wound bleeding, dehiscence, visible abscesses or gangrene, which may require surgical debridement, systemic antibiotic application, and implant replacement or removal.25 We reviewed all medical records, and pathogen culture records during the patient’s hospitalization, and routine postoperative telephone follow-ups of the patient for more than 12 months to identify SSI cases. These data were reviewed retrospectively and independently from the electronic medical records by two examiners (HCG and JHY) with expertise in orthopedics and mutually checked for accuracy, with any disagreements resolved by discussion with the senior chief physician.
Statistical Analysis
SPSS version 26.0 (IBM Corporation, Armonk, NY, USA) was applied to determine independent risk factors based on univariate and multivariate analyses. The normality of continuous variables was assessed following the results of the Kolmogorov–Smirnov test, and normally distributed data were expressed as mean ± standard deviation (SD) using the Student’s t-test. Otherwise, the Mann–Whitney test was used and expressed as the median and interquartile range (IQR). Categorical variables were assessed using the chi-square test or Fisher’s exact test.
For inflammatory indicators such as HCRP, MLR, and SII, after the t-test was performed, we further applied the Youden index to determine their optimal cutoff values so as to better guide clinical practice. Potential predictors screened for p <0.1 in univariate analyses were included in multivariate analyses, and backward stepwise logistic regression was used to determine independent predictors of SSI.
The “rms” and “DynNom” packages in R software (R Foundation for Statistical Computing, Vienna, Austria) were used to develop the nomogram. The receiver operating characteristic (ROC) curve, calibration curves, and decision curve analysis (DCA) to visually evaluate the model’s predictive power. The C-index and the area under the curve (AUC) are closer to 1, and the better the model’s discrimination ability. The calibration curve was presented to illustrate the consistency between the predicted probability of the model and the actual probability, and was further evaluated using the Hosmer-Lemeshow goodness-of-fit test. Brier score is an expansion of the Hosmer-Lemeshow test, and the closer it is to 0, the higher the ability of the model calibration. The net clinical benefit of the model was assessed by DCA. Internal validation was performed using the Bootstrap method to obtain the corrected C-index and corrected Brier scores after 1000 replicate samples. p < 0.05 was considered statistically significant.
Results
Clinical Characteristics
Based on the inclusion and exclusion criteria, 1294 eligible patients were finally included in this study (Figure 1), of whom 878 (67.9%) were female, with a mean age of 56.6±7.9 years and mean BMI of 26.9±3.6 kg/m2. Surgical side was left in 609 (47.1%), right in 527 (40.7%), and bilateral in 158 cases (12.2%). The mean duration of surgery was 98.2±37.1 minutes. Postoperative follow-up was at least 12 months, and if a patient experienced the SSI event on multiple occasions, the event was counted only once and by the greatest severity. The results showed that 25 cases (1.93%) were diagnosed with SSI after OWHTO, including 6 cases (0.46%) of deep SSI and 19 cases (1.47%) of superficial SSI.
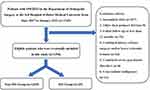 |
Figure 1 Flowchart for screening eligible patients. |
Univariate and Multivariate Analysis
Based on the maximum Youden index, the optimal cutoff value of HCRP for postoperative SSI was 2.6 mg/L. Correspondingly, SII was 423.62, and MLR was 0.24 (Table 1). Backward stepwise logistic regression was performed on the 9 covariates with p <0.1 in the univariate analysis to fit the prediction model. The results showed that tobacco consumption (OR= 3.44, 95% CI 1.35–8.78), osteotomy size ≥12 mm (OR= 3.38, 95% CI 1.27–9.02), the use of allogeneic bone or artificial bone graft substitutes (allogeneic bone vs none, OR= 4.08, 95% CI 1.09–15.30; artificial bone vs none, OR= 5.16, 95% CI 1.03–25.96), Kellgren-Lawrence (K-L) grade IV (OR= 2.57, 95% CI 1.02–6.50), systemic immune-inflammation index (SII) >423.62 (OR= 6.22, 95% CI 2.24–17.30), high-sensitivity C-reactive protein (HCRP) >2.6 mg/L (OR= 2.42, 95% CI 1.03–5.72), and a higher level of fasting blood glucose (FBG) (OR= 1.32, 95% CI 1.04–1.67) with a cut-off value of 5.7 mmol/L were independent risk factors for SSI after OWHTO (Table 2).
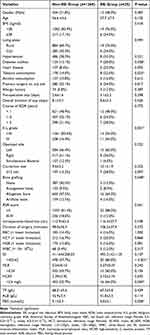 |
Table 1 Univariate Analysis of Variables of Interest Between SSI and Non-SSI Patients |
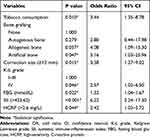 |
Table 2 Multivariate Analyses of the Independent Risk Factors Associated with Postoperative SSI |
Construction and Validation of a Dynamic Nomogram
The seven predictors finally selected by the multivariate analysis were transformed into a simple nomogram and a dynamic nomogram by R software. The use of the simple nomogram (Figure 2a) is not conditioned, it allows the surgeon to draw a vertical line on the score axis of each variable, sum the scores to obtain the total score, and the predicted probability of SSI can be obtained by making a vertical line downward. The dynamic nomogram (Figure 2b) requires surgeons to input the results of each covariate while connected to the network to display the predicted probability of SSI with 95% confidence intervals. The AUC corresponding to the ROC curve was 0.856 (95% CI: 0.783–0.929) (Figure 3), and the cutoff score was 148, with a sensitivity of 76.0% and specificity of 81.0%, which indicates good model discrimination. The C-index and Brier score were 0.856 and 0.017, respectively, and after Bootstrap validation (B= 1000 replicates), the correction values were 0.820 and 0.018, respectively, indicating that the model performed well overall. The Hosmer-Lemeshow χ2 statistic of the calibration curve (Figure 4) was 7.70 (P= 0.464), which illustrated the accuracy of the absolute risk prediction values. DCA shows that the application of the nomogram provides a net positive benefit when the threshold probability is in the range of 0.02~0.58 (Figure 5).
 |
Figure 2 The simple nomogram (a) and dynamic nomogram (b) for predicting the risk of SSI after OWTHO. p values are indicated as one star (*) if p < 0.05, two stars (**) if p < 0.01, and three stars (***) if p < 0.001. (Access to dynamic nomogram: https://dynanomogram.shinyapps.io/dynnomapp/). Abbreviations: HCRP, high-sensitivity C-reactive protein, SII, systemic immune-inflammation index; FBG, fasting blood glucose; K-L grade, Kellgren-Lawrence grade. |
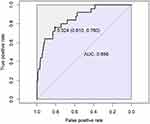 |
Figure 3 Receiver operating characteristic (ROC) curve of the predictive model. The AUC had a positive correlation with the prediction accuracy of the nomogram. |
Discussion
Surgical site infection (SSI) after OWHTO is a rare but serious complication that can lead to nonunion of the osteotomy gap, implant loosening or failure, and even osteomyelitis and death.5 In the present study, tobacco consumption, osteotomy size ≥12 mm, the use of allogeneic bone or artificial bone graft substitutes, K-L grade IV, HCRP >2.6 mg/L, SII >423.62, and elevated FBG were identified as independently associated with the high risk of SSI. Based on these predictors, a nomogram model for predicting SSI after OWHTO was developed for the first time in this study, which has excellent translatability for clinical application.
Diabetes can cause microangiopathy, resulting in local tissue ischemia and thus delaying wound healing and predisposing to infection.26 This conclusion is consistent with the results of the univariate analysis. However, after multifactorial analysis, we found that combined diabetes mellitus was not an independent risk factor for the development of SSI, while preoperative blood glucose level was a significant predictor. This may be because the altered local internal environment caused by hyperglycemia and the suppression of immune cell function are the key factors in SSI,27 not the presence or absence of a history of diabetes. Pennington et al also found that elevated perioperative blood glucose levels are associated with an increased risk of SSI, even when the patient does not have comorbid diabetes mellitus.28 Therefore, surgical teams should tightly control blood glucose levels during the perioperative period, regardless of whether the patient has comorbid diabetes.
As a novel indicator of systemic inflammation, elevated SII values, mostly caused by elevated neutrophil and platelet levels and decreased lymphocyte levels, indicate that the patient’s organism is in a state of increased inflammatory response and diminished immune response.29 In the present study, we found that SII >423.62 was significantly associated with a 6.2-fold increased risk of SSI after OWHTO. In addition, numerous studies have shown that SII has a good diagnostic and predictive value for different types of infections.30–32 HCRP is an acute-phase protein whose levels increase with the progression of inflammation. However, in addition to infection, numerous confounding factors such as advanced age and trauma can affect the level of HCRP,33 so the study applied the Youden index to redefine the threshold (2.6 mg/L). Compared to the traditional threshold (6.0 mg/L), this will undoubtedly significantly improve the diagnostic sensitivity and facilitate the detection of early as well as minor infections. In conclusion, SII and HCRP, as low-cost and readily available predictors of SSI, can significantly improve clinicians’ diagnostic accuracy for SSI when combined with other risk factors.
To our knowledge, this is the first study to associate K-L grade IV with the risk of SSI after OWHTO. It is likely due to the following facts: first, severe KOA often means a greater degree of intraoperative orthopedic and tissue trauma, as well as a longer postoperative healing period,34 which tends to increase the likelihood of exposure to bacteria. Second, prolonged and significant knee pain in patients with severe KOA results in decreased local sensory sensitivity,35 which is not conducive to early detection and treatment of infection. Furthermore, altered local vascular status, such as stenosis or sclerosis, exacerbates microcirculatory abnormalities and impairs recovery in the surgical area.36 In addition, severe KOA was accompanied by significantly elevated levels of systemic inflammatory factors, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and a significant association between these factors and infection was recognized.37,38 Therefore, the role of the K-L grade in predicting SSI events should not be ignored.
This study found that intraoperative osteotomy size ≥1.2 mm significantly increased the risk of SSI after OWHTO. This is associated with greater intraoperative trauma, bleeding, and poor circulation due to increased local tissue tension. Therefore, special attention should be paid to patients with an osteotomy size ≥1.2 mm in the perioperative period, while other risk factors should be more strictly controlled. Furthermore, after adjusting for confounders in the present study, the use of allogeneic bone or artificial bone graft substitutes was significantly associated with SSI, which was also the conclusion of a large national database analysis involving 12,853 patients.20 This may be because bone graft substitutes cause soft tissue irritation and may even derange osseous consolidation.39 Therefore, we recommend that surgeons should fully weigh the pros and cons of different bone grafting modalities and use allogeneic bone or artificial bone implants with caution, especially in patients with a support height ≥12 mm or combined with other high-risk factors, in order to reduce the risk of SSI.
In addition, tobacco consumption is a recognized risk factor for postoperative SSI in surgical patients.40 Harmful substances such as nicotine and tar in tobacco cause peripheral vasoconstriction decreased elasticity, and increased platelet aggregation, predisposing to micro thrombosis and leading to local hypoxia.41 These factors adversely affect wound healing and tissue regeneration. In the present study, smokers had a 3.55-fold increased risk of SSI compared to non-smokers, which is consistent with the report by John et al.42 These results suggest that patients may benefit from strict preoperative smoking cessation interventions.
We did not find a significant association of age with the risk of SSI after OWHTO, inconsistent with some studies focused on other orthopedic procedures,43–45 but in line with a more recent study with the same context as ours, that is, focus on SSI after OWHTO for varus KOA.46 We think there are several possible explanations. Firstly, it is related to the aggregation of operative age in KOA patients. More than 80% of the patients in our study were located in the age spectrum of 50~60 years, and the narrow age interval makes it difficult to accentuate the cumulative effect of increased risk. Secondly, the physiological age of the patient is more likely to have a meaningful effect than the biological age,47 as the increased age reflects more of a substitute or proxy indicator for factors such as decreased immune function, combined underlying diseases, poor nutritional status, and adverse lifestyle.48,49 Therefore, the effect of age is no longer statistically significant in a multifactorial model after adjustment for these factors. Finally, the lower prevalence of SSI prevented a statistically significant risk effect from being obtained with a relatively limited sample size, ie, our results may be biased by statistical type II error.50 A further increase in sample size might yield statistically significant results.
The main advantages of this study are the large sample size and the transformation of independent risk factors into a predictive nomogram model, which allows surgeons to make individualized risk assessments of patients with OWHTO. All seven predictors can be quickly obtained from the patient’s routine postoperative laboratory results and admission report. On this basis, the predicted probability of SSI can be obtained in a few minutes by keying in the results on a web page or by drawing vertical lines on the corresponding axes.
However, there are still several limitations of this study. First, the retrospective study inherited an unavoidable selection bias. Second, the acquisition of some of the covariates (eg, medical comorbidities, duration of disease) mainly relies on patients’ self-reports, and the accuracy of this information depended on how well patients knew their condition. Third, this retrospective data failed to obtain specific information about surgeons, such as years in practice, and these details may potentially affect the results. Fourth, to improve the predictive accuracy of the model, we did not include patients with several special conditions (eg, combined trauma, malignancy, or severe mental disorders), so the nomogram may not apply to this population. Fifth, despite good internal validation results, this single-center study still requires a large prospective study with multi-center data to confirm the external applicability of the model.
Conclusion
In conclusion, the incidence of SSI after OWHTO in patients with KOA is relatively low (1.93%), but it should not be ignored. Tobacco consumption, osteotomy size ≥12 mm, the use of allogeneic bone or artificial bone graft substitutes, K-L grade IV, HCRP >2.6 mg/L, SII >423.62, and elevated FBG were identified as independent risk factors for SSI after OWHTO. We developed a combination of seven predictors as a dynamic nomogram model with a cutoff score of 148. Surgical teams should recognize the importance of actively optimizing risk factors, such as encouraging smoking cessation, tightly controlling glycemic, and using artificial bone implants cautiously, to decrease SSI rates and preclude catastrophic medical outcomes whenever possible.
Data Sharing Statement
All data in this study can be obtained from the corresponding author (Juan Wang) based on reasonable demand.
Acknowledgments
We sincerely thank all the patients in this study.
Author Contributions
All authors made significant contributions to the work reported, whether in concept and design, implementation, data acquisition, analysis, and interpretation, or all of these areas; participated in the drafting of the article or the critical revision of important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to take responsibility for all aspects of the work.
Funding
This research was supported by the National Natural Science Foundation of China (No. 91949203) and the Hebei Provincial Key Research and Development Program (No. 192777113D) and the Hebei Department of Science and Technology High-Level Talent Team Construction Project (No. 225A7703D).
Disclosure
All authors declare no competing interests.
References
1. Duivenvoorden T, Brouwer RW, Baan A, et al. Comparison of closing-wedge and opening-wedge high tibial osteotomy for medial compartment osteoarthritis of the knee: a randomized controlled trial with a six-year follow-up. J Bone Joint Surg Am. 2014;96(17):1425–1432. doi:10.2106/JBJS.M.00786
2. Karatosun V, Demir T, Unver B, Gunal I. A brief report on managing infected nonunion of a high tibial osteotomy in two stages: a case series involving seven knees. J Bone Joint Surg Am. 2011;93(7):904–906. doi:10.1302/0301-620X.93B7.25833
3. Allegranzi B, Bischoff P, de Jonge S, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e276–e287. doi:10.1016/S1473-3099(16)30398-X
4. Ma X, Hu Y, Wang K. Chinese clinical practice guidelines in treating knee osteoarthritis by periarticular knee osteotomy. Orthop Surg. 2022;14(5):789–806. doi:10.1111/os.13281
5. Anagnostakos K, Mosser P, Kohn D. Infections after high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2013;21(1):161–169. doi:10.1007/s00167-012-2084-5
6. Han SB, In Y, Oh KJ, Song KY, Yun ST, Jang KM. Complications associated with medial opening-wedge high tibial osteotomy using a locking plate: a multicenter study. J Arthroplasty. 2019;34(3):439–445. doi:10.1016/j.arth.2018.11.009
7. Schenke M, Dickschas J, Simon M, Strecker W. Corrective osteotomies of the lower limb show a low intra- and perioperative complication rate-an analysis of 1003 patients. Knee Surg Sports Traumatol Arthrosc. 2018;26(6):1867–1872. doi:10.1007/s00167-017-4566-y
8. Miltenberg B, Puzzitiello RN, Ruelos VCB, et al. Incidence of complications and revision surgery after high tibial osteotomy: a systematic review. Am J Sports Med. 2023;3635465221142868. doi:10.1177/03635465221142868
9. Shiferaw WS, Aynalem YA, Akalu TY, Petrucka PM. Surgical site infection and its associated factors in Ethiopia: a systematic review and meta-analysis. BMC Surg. 2020;20(1):107. doi:10.1186/s12893-020-00764-1
10. Seidelman JL, Mantyh CR, Anderson DJ. Surgical site infection prevention: a review. JAMA. 2023;329(3):244–252. doi:10.1001/jama.2022.24075
11. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi:10.1016/S0140-6736(19)30417-9
12. System Arft N. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. doi:10.1016/S0196655304005425
13. Ercole FF, Chianca TCM, Duarte D, Starling CEF, Carneiro M. Surgical site infection in patients submitted to orthopedic surgery: the NNIS risk index and risk prediction. Rev Lat Am Enfermagem. 2011;19(2):269–276. doi:10.1590/s0104-11692011000200007
14. Clements ACA, Tong ENC, Morton AP, Whitby M. Risk stratification for surgical site infections in Australia: evaluation of the US National Nosocomial Infection Surveillance risk index. J Hosp Infect. 2007;66(2):148–155. doi:10.1016/j.jhin.2007.02.019
15. Berk AN, Gachigi KK, Trofa DP, Piasecki DP, Fleischli JE, Saltzman BM. Early postoperative complications and associated variables after high tibial osteotomy and distal femoral osteotomy: a 15-year experience from a single academic institution. Am J Sports Med. 2023;51(10):2574–2582. doi:10.1177/03635465231183092
16. Ruangsomboon P, Chareancholvanich K, Harnroongroj T, Pornrattanamaneewong C. Survivorship of medial opening wedge high tibial osteotomy in the elderly: two to ten years of follow up. Int Orthop. 2017;41(10):2045–2052. doi:10.1007/s00264-017-3517-z
17. Seki K, Sakka A, Tokushige A, Imagama T, Mutou M, Taguchi T. Treatment for Staphylococcus aureus infection following open wedge high tibial osteotomy using antibiotic-impregnated calcium phosphate cement. Knee Surg Sports Traumatol Arthrosc. 2013;22(11):2614–2617. doi:10.1007/s00167-013-2460-9
18. Woodacre T, Ricketts M, Evans JT, et al. Complications associated with opening wedge high tibial osteotomy--A review of the literature and of 15 years of experience. Knee. 2016;23(2):276–282. doi:10.1016/j.knee.2015.09.018
19. Liu TW, Chiu CH, Chen AC, Chang SS, Chan YS. Risk factor analysis for infection after medial open wedge high tibial osteotomy. J Clin Med. 2021;10(8). doi:10.3390/jcm10081727
20. Kawata M, Jo T, Taketomi S, et al. Type of bone graft and primary diagnosis were associated with nosocomial surgical site infection after high tibial osteotomy: analysis of a national database. Knee Surg Sports Traumatol Arthrosc. 2021;29(2):429–436. doi:10.1007/s00167-020-05943-4
21. Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020. doi:10.1136/bmj.m441
22. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi:10.1136/ard.16.4.494
23. Weng Y, Zeng T, Huang H, et al. Systemic immune-inflammation index predicts 3-month functional outcome in acute ischemic stroke patients treated with intravenous thrombolysis. Clin Interventions Aging. 2021;16:877–886. doi:10.2147/CIA.S311047
24. Li J, He D, Yu J, et al. Dynamic status of SII and SIRI alters the risk of cardiovascular diseases: evidence from kailuan cohort study. J Inflamm Res. 2022;15:5945–5957. doi:10.2147/JIR.S378309
25. Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–791. doi:10.1001/jamasurg.2017.0904
26. Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–539. doi:10.1038/s41574-022-00690-7
27. Reaven PD, Emanuele NV, Wiitala WL, et al. Intensive glucose control in patients with type 2 diabetes - 15-year follow-up. N Engl J Med. 2019;380(23):2215–2224. doi:10.1056/NEJMoa1806802
28. Pennington Z, Lubelski D, Westbroek EM, Ahmed AK, Passias PG, Sciubba DM. Persistent postoperative hyperglycemia as a risk factor for operative treatment of deep wound infection after spine surgery. Neurosurgery. 2020;87(2):211–219. doi:10.1093/neuros/nyz405
29. Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273(3):532–541. doi:10.1097/SLA.0000000000003370
30. Wang RH, Wen WX, Jiang ZP, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. 2023;14:1115031. doi:10.3389/fimmu.2023.1115031
31. Pricop M, Ancusa O, Talpos S, Urechescu H, Bumbu BA. The predictive value of systemic immune-inflammation index and symptom severity score for sepsis and systemic inflammatory response syndrome in odontogenic infections. J Pers Med. 2022;12(12). doi:10.3390/jpm12122026
32. Gu W, Huang W, Zhang J, Qian S, Cao H, Ge L. Evaluation of urinary inflammatory index in rapid screening of urinary tract infection. Sci Rep. 2020;10(1):19306. doi:10.1038/s41598-020-76352-3
33. von Dach E, Albrich WC, Brunel AS, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA. 2020;323(21):2160–2169. doi:10.1001/jama.2020.6348
34. Kuwashima U, Iwasaki K, Kurakazu I, et al. Effect of osteoarthritis severity on survival and clinical outcomes after high tibial osteotomy. Knee. 2021;29:441–447. doi:10.1016/j.knee.2021.02.031
35. Sharma L. Osteoarthritis of the Knee. N Engl J Med. 2021;384(1):51–59. doi:10.1056/NEJMcp1903768
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of cardiology foundation/American heart association task force on practice guidelines: developed in collaboration with the Society for cardiovascular angiography and interventions Society of interventional radiology, Society for vascular medicine, and Society for vascular surgery. Catheter Cardiovasc Interv. 2012;79(4):501–531. doi:10.1016/j.jvs.2011.09.001
37. Runhaar J, Beavers DP, Miller GD, et al. Inflammatory cytokines mediate the effects of diet and exercise on pain and function in knee osteoarthritis independent of BMI. Osteoarthritis Cartilage. 2019;27(8):1118–1123. doi:10.1016/j.joca.2019.04.009
38. Rossi JF, Lu ZY, Massart C, Levon K. Dynamic immune/inflammation precision medicine: the good and the bad inflammation in infection and cancer. Front Immunol. 2021;12:595722. doi:10.3389/fimmu.2021.595722
39. Spahn G. Complications in high tibial (medial opening wedge) osteotomy. Arch Orthop Trauma Surg. 2004;124(10):649–653. doi:10.1007/s00402-003-0588-7
40. Li J, Zhu Y, Liu B, Dong T, Chen W, Zhang Y. Incidence and risk factors for surgical site infection following open reduction and internal fixation of adult tibial plateau fractures. Int Orthop. 2018;42(6):1397–1403. doi:10.1007/s00264-017-3729-2
41. Park HJ, Kang SB, Chang MJ, Chang CB, Jung WH, Jin H. Association of Gap healing with angle of correction after opening-wedge high tibial osteotomy without bone grafting. Orthop J Sports Med. 2021;9(5):23259671211002289.
42. Scolaro JA, Schenker ML, Yannascoli S, Baldwin K, Mehta S, Ahn J. Cigarette smoking increases complications following fracture: a systematic review. J Bone Joint Surg Am. 2014;96(8):674–681. doi:10.2106/JBJS.M.00081
43. Li H, Li Y, Wang D, Huang Q, Liu D. Evaluation of risk factors for surgical site infections in osteoarthritis patients undergoing total knee arthroplasty. Int Wound J. 2023. doi:10.1111/iwj.14521
44. Bergström J, Möller Rydberg E, Wennergren D, Svensson Malchau K. Incidence and risk factors for surgical site infection in ankle fractures: an observational study of 480 patients in Sweden. J Clin Med. 2023;12(20). doi:10.3390/jcm12206464
45. Sung S, Kwon J-W, Lee S-B, Lee H-M, Moon S-H, Lee BH. Risk factors of clostridium difficile infection after spinal surgery. Nat Health Insur Database Sci Rep. 2020;10(1). doi:10.1038/s41598-020-61327-1
46. Jia G, Sun C, Xie J, et al. Incidence and risk factors for surgical site infection after medial opening‐wedge high tibial osteotomy using a locking T‐shape plate. Int Wound J. 2023;20(7):2563–2570. doi:10.1111/iwj.14124
47. Muertizha M, Cai X, Ji B, Aimaiti A, Cao L. Factors contributing to 1-year dissatisfaction after total knee arthroplasty: a nomogram prediction model. J Orthopaedic Surg Res. 2022;17(1). doi:10.1186/s13018-022-03205-2
48. Imtiaz R, Hawken S, McCormick B, Leung S, Hiremath S, Zimmerman D. Diabetes mellitus and younger age are risk factors for hyperphosphatemia in peritoneal dialysis patients. Nutrients. 2017;9(2). doi:10.3390/nu9020152
49. Wen S-R, Yang Z-H, Dong T-X, et al. Deep fungal infections among general hospital inpatients in southwestern china: a 5-year retrospective study. Front Public Health. 2022;10. doi:10.3389/fpubh.2022.842434
50. Kim H-Y. Statistical notes for clinical researchers: type I and type II errors in statistical decision. Restor Dent Endod. 2015;40(3). doi:10.5395/rde.2015.40.3.249
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


