Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Rifaximin Alfa and Liver Diseases: More Than a Treatment for Encephalopathy, a Disease Modifier
Authors Torre A , Córdova-Gallardo J , Frati Munari AC
Received 5 July 2023
Accepted for publication 17 September 2023
Published 24 October 2023 Volume 2023:19 Pages 839—851
DOI https://doi.org/10.2147/TCRM.S425292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Aldo Torre,1,2 Jacqueline Córdova-Gallardo,3 Alberto C Frati Munari4
1Guest Research, Metabolic Unit Department, Instituto Nacional de Ciencias Médicas Y Nutrición “Salvador Zubirán”, México City, Mexico; 2Guest Research, Liver Unit Department, Hospital General de México, México City, Mexico; 3Hepatology department, Hospital General Dr. Manuel GEA González, Mexico City, Mexico; 4Internal Medicine Department; Hospital Médica Sur, Mexico City, Mexico
Correspondence: Aldo Torre, Guest Research, Metabolic Unit Department, Instituto Nacional de Ciencias Médicas Y Nutrición “Salvador Zubirán”, México City, Mexico, Email [email protected]
Abstract: RFX, a rifamycin-based antibacterial agent obtained by the culture of the actinomycete Streptomyces mediterranei, has a broad antibacterial spectrum covering gram- positive, gram-negative, aerobic, and anaerobic bacteria. RFX is an antibiotic that elicits its effect by inhibiting bacterial RNA synthesis. When administered orally, its intestinal absorption is extremely low (< 0.4%), restricting antibacterial activity mainly in the intestinal tract, with few systemic side effects. RFX has been recommended by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver guidelines for the treatment of HE. RFX may contribute to restore hepatic function and to decrease the development of liver fibrosis. Its efficacy has been shown in patients with previous hepatic encephalopathy and several complications, such as infections, including spontaneous bacterial peritonitis, ascites and oesophageal variceal bleeding. Thus, RFX has an outstanding role in the therapeutic arsenal in hepatic cirrhosis, under the concept of disease modifier.
Keywords: rifaximin, liver disease, modifier, hepatic cirrhosis therapy
Introduction
Liver diseases are a frequent cause of mortality worldwide. In 2019, liver cirrhosis caused 2.4% of deaths worldwide. Most often due to viral hepatitis, but with a progressive increase in the etiology of non-alcoholic fatty liver and alcohol.1–3 In Mexico, in the year 2020, liver diseases were the sixth cause of death, most of them mainly due to liver cirrhosis. Worldwide, the impact on health systems due to the complications of cirrhosis is and will be a major problem, coupled with the increase in hepatocarcinoma, mostly attributed to hepatic steatosis.
Some epidemiological circumstances can modify this aspect. For example, during the COVID-19 pandemic, mortality from alcoholic disease increased in the United States and the decline due to hepatitis C, hepatitis B and hepatocellular carcinoma stopped. An increasing trend of nonalcoholic fatty liver disease and alcohol consumption was observed.4 Figure 1
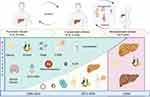 |
Figure 1 Natural history of cirrhosis compensated and decompensated.1–4 After of the initial insult (alcohol, virus, metabolic syndrome, autoimmunity) the progression to compensated cirrhosis can last from 20 to 35 years. Likewise, compensated cirrhosis remains stable between 10 to 15 years before the decompensation phase. The current prevalence is mostly alcohol cirrhosis and hepatic steatosis. Abbreviations: T2DM, type 2 Diabetes Mellitus; HCV, hepatitis C virus; HBV, hepatitis B virus; A1AT, alpha-1 antitrypsin deficiency. |
The increase in the incidence of hepatocarcinoma is worrisome. Seventy percent occur in cirrhotic livers, but the presence in non-cirrhotic liver is becoming alarming. In a multicenter study conducted in India, of 2664 patients with hepatocellular carcinoma, 27.9% occurred in individuals without cirrhosis. The most frequent association in patients with cirrhosis was alcoholism, while in patients without cirrhosis, it was non-alcoholic fatty liver. In the total group, the most frequent causes associated with hepatocellular carcinoma were non-alcoholic fatty liver disease, hepatitis B, hepatitis C and alcoholism, in that order.5
Approximately half of patients with liver cirrhosis in the first five years develop some evidence of decompensation, due to alterations caused by portal hypertension or liver failure, or both, 50% of those who decompensate die in around 36 months and 80% do not reach 10 years of survival.6 The most common complications that affect survival are infections, including spontaneous bacterial peritonitis (SBP), bleeding from esophageal varices, ascites and hepatic encephalopathy (HE).7 Figure 2.
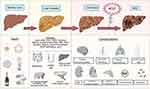 |
Figure 2 Complications of cirrhosis and progression of compensated cirrhosis to ACLF.5–11 A compensated cirrhosis has alteration in intestinal biota, permeability, chronic inflammation, and portal hypertension. During the phase of compensated damage, the liver is subjected to numerous acute insults (alcohol, infections, bleeding, medications, etc) which at some point progress to Acute on Chronic Liver Failure (ACLF), organic failure, and high mortality. Abbreviations: HCV, hepatitis C virus; HBV, hepatitis B Virus; HDV, hepatitis Delta Virus; HAI, Autoimmune Hepatitis; MAFLD/MASLD: Metabolic associated steatosic liver disease, PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; A1AT, alpha-1 antitrypsin deficiency; PAMPS, Pathogen-associated molecular pattern molecules; LPS, Lipopolysaccharides; ACLF, acute-on-chronic liver failure; HCC, hepatocellular carcinoma. |
To reduce mortality due to chronic liver diseases, there are various strategies that complement each other: a) prevent and treat the etiology, b) prevent the progression of chronic liver damage and increase in fibrosis, c) treat the complications.
Of course, prevention and treatment of the causes of cirrhosis is essential. In alcoholic patients, who receive treatment to control their addiction and stop drinking, mortality from cirrhosis is reduced. In a study that included 9121 alcoholics, the 886 that received treatment (naltrexone, acamprosate) has lower mortality than those who did not (RR 0.80, p=0.024).8 Likewise, modern antiviral treatment for hepatitis C obtains a sustained antiviral response, with a decrease in inflammation, the evolution of liver fibrosis, and even the frequency of hepatocellular carcinoma.9,10 Similarly, metabolic control in patients with cirrhosis due to hepatic steatosis impacts on the non-progression of the disease or a decrease in the portal venous gradient. An uncontrolled pilot study included patients with compensated cirrhosis and portal pressure >6 mm Hg, with a body mass index (BMI) >26 kg/m2, who underwent an intense exercise program for 16 weeks, with a personalized normoproteic-hypocaloric diet and 60 weekly minutes of physical activity. Changes in portal venous gradient and IMC were established as significant with decreases >10% and 5%, respectively. Sixty patients were included, 50 completed the study: The etiology of non-alcoholic steatohepatitis was confirmed in 24% of the patients, and it was confirmed a decrease in the portal venous gradient in the patients with the greatest weight loss.11
Given this, rifaximin can contribute to the treatment and prevention of complications.
Rifaximin Alpha (RFX)
Rifaximin is a semisynthetic antibiotic derived from rifamycin, water soluble, with poor gastrointestinal absorption, and excellent bactericidal activity.12 It presents a polymorphism that affects its pharmacokinetics, the polymorph that has been most widely used in the world and with which studies have been carried out in most of the clinical trials is the alpha polymorph.13 Its antimicrobial property is through the union of the β-subunit to RNA polymerase dependent-bacterial DNA, thereby inhibiting bacterial RNA synthesis.14
This antibiotic with intestinal action is very poorly absorbable and has a broad antibacterial action against gram positive, gram negative, and aerobic and anaerobic bacteria, which is why most enteropathogenic bacterial strains that cause acute diarrhea are susceptible to RFX.15–17
The antibacterial action is reinforced because it is capable to modify bacterial virulence and reduce the adherence of bacteria to epithelial cells. Despite the above, research in patients with various characteristics, such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome, has shown that RFX hardly modifies the general composition of the microbiota, and increases the number of bacteria favorable to health, such as Lactobacillus and Faecalibacterium prausnitzii, so has been considered “eubiotic”.18–22
RFX has been shown to decrease bacterial translocation, possibly by increasing tight junction proteins among intestinal epithelial cells, such as occludin and zonula occludens-1.23–25 In addition, rifaximin has been shown to be an agonist of the pregnane X receptor in the intestine, thereby exerting an anti-inflammatory action mediated by NFkB.16,26,27
The effects of rifaximin in the systemic inflammation have not been extensively studied. However, the levels of IL-6, IL-10, and TNFα are decreased in patients with alcohol cirrhosis, NAFLD cirrhosis, and HE cirrhosis which are treated with rifaximin, in treatment periods ranging from 4 to 12 weeks.28 Figure 3
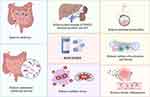 |
Figure 3 Actions sites of Rifaximin in patients with cirrhosis.12–28 The rifaximin in the cirrhotic patients improve dysbiosis, and reduces oxidative stress, inflammation, activation of stellate cells, intestinal permeability, portal passage of PAMPS and lipopolysaccharides, and reduce of ammonia producing bacteria. |
RFX in Hepatic Fibrosis
Alcoholic Hepatic Disease
Alcoholism is one of the major causes of liver fibrosis and hepatic cirrhosis, in many countries of the world. In addition to the direct toxic effect of ethyl alcohol and its derivatives on hepatocytes, there is evidence that involves the translocation of bacterial products to the portal vein and the liver, causing inflammation that would lead to fibrogenesis and hepatic fibrosis. The pharmacodynamics of RFX supports its possible action in this pathology, especially its effect on tight junctions between intestinal cells and bacterial translocation.29
A recent investigation in patients with non-decompensated alcoholic liver disease evaluated the progression of liver fibrosis in 136 individuals, comparing the administration of RFX, 550 mg twice daily, with placebo, for 18 months, 68 with RFX and 68 with placebo. Liver biopsy and non-invasive fibrosis markers (elastography, liver enzymes) were performed in all of them before the end of treatment. The histopathological findings showed that with RFX a lower progression of hepatic fibrosis was observed, as well as a non-significative trend towards reduced inflammation and less progression of steatosis (Figure 4). Non-invasive markers followed similar changes, despite the fact that in the RFX-α group, there was a higher frequency of persistent alcoholism.30
 |
Figure 4 In patients with alcoholic liver disease proven by liver biopsy, treated for 18 months with RFX or placebo, changes observed in the biopsy at the end of treatment compared to the initial biopsy. With data from Israelsen M et al.31 |
Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver includes hepatic steatosis, non-alcoholic steatohepatitis with varying degrees of fibrosis and hepatic cirrhosis and is considered part of the metabolic syndrome. According to various statistics, this is the second cause of liver cirrhosis,31,32 but its prevalence is increasing. Its pathophysiology is complex and includes metabolic alterations, especially linked to insulin resistance, as well as inflammation probably dependent on the passage of bacterial products from the intestine to the liver activating TLR-4 receptors (toll-like receptor 4), inflammation and fibrogenesis.33 Dysbiosis has also been found that could participate in the pathophysiology.34
A pilot studio tested low-dose RFX (400 mg twice daily) in a small group of patients with non-alcoholic steatohepatitis, showing no changes in liver enzymes or insulin sensitivity.35 Meanwhile, in two open investigations, in patients with liver biopsy-proven non-alcoholic steatohepatitis, administering RFX at 1100 and 1200 mg per day, improvements in liver enzymes, circulating endotoxins, various inflammatory markers and metabolic homeostasis were observed at 28 days and after 6 months of treatment (Figures 5 and 6).36,37
 |
Figure 5 In patients with non-alcoholic steatohepatitis, treated with RFX or with placebo, with RFX at 6 months, improvement in inflammation markers was observed. With data from Abdel-Razik A et al38 *p<0.01 **p<0.05 Value compared to the initial 100%. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGTP, gamma glutamyl transpeptidase; Endotox, endotoxin; TLR-4, toll like-4 receptor; IL-10, interleukin 10; TNF-alpha, tumor necrosis factor α; IL-6, interleukin-6; CK-18, cytokeratin-18 serum fragment. |
 |
Figure 6 In patients with non-alcoholic steatohepatitis, treated with RFX or with placebo, with RFX at 6 months, improvement was observed in the metabolic markers of glucose metabolism. HOMA-R= hemostatic model to assess insulin resistance. With data from Abdel-Razik A et al38 Value compared to the initial 100%. |
Several studies on experimental models of non-alcoholic steatohepatitis demonstrate a beneficial effect of RFX to prevent fibrogenesis. In rats and mice with models of steatohepatitis induced with a diet deficient in choline, or choline and methionine, various changes have been found that counteract or delay liver fibrosis (Box 1), with more marked changes if lubiprostone is administered concomitantly, such as recovery of the intestinal microbiota alterations, with an increase in Bacteroides, Lactobacillus and Fecalibacterium, reducing Veillonella, and a decrease in the activity of the sialidase enzyme. In mice, the latter was accompanied by a deoxycholic acid reduction in the ileum. An increase in the tight junction proteins zonula occludens-1 and claudin-1 has been demonstrated, with a decrease in intestinal permeability assessed by dextran-fluorescent isothiocyanate (FITC) method. Intestinal permeability is increased in steatohepatitis models, as result, a decrease in the proteins bound to bacterial lipopolysaccharides (LPS binding protein) in the portal circulation, as well as a reduction in circulating TLR-4 and NFkB, and a decrease in hepatic TGF-β are observed. Likewise, inhibition of liver fibrosis has been observed, an effect that is accentuated by adding losartan or lubiprostone. In mice, the administration of RFX is accompanied by improvement of steatosis, lobular inflammation and fibrogenesis, demonstrated by liver histology. (Box 1).38–40
 |
Box 1 Effects of treatment with rifaximin in experimental fatty liver disease |
Rifaximin on Portal Venous Pressure
Due to the ability to modify the intestinal microbiota and inflammatory cytokines of the intestine-liver axis, RFX can modify the portal venous pressure. Vlachogiannakos showed that rifaximin, 550 mg every 12 hours, decreases the portal venous pressure, associated with decreased plasma levels of endotoxemia, perhaps requiring prolonged treatment between 12 and 24 months.41
Rifaximin in the Liver Cirrhosis Evolution and Its Complications
The evolution of this disease is influenced by the preservation of liver function and the passage of ammonium (ammonia) from intestine into the circulation. Long-term administration of RFX could contribute to improving hepatic reserve. In an observational study in 44 patients with HE, in which RFX was administered for 12 months, in addition to improvement in HE grades at 3 and 12 months, there was also a decrease in circulating ammonia levels and markers of inflammation such as C-reactive protein, neutrophil/lymphocyte, lymphocyte/monocyte and platelet/lymphocyte ratios.42
With the purpose of clarifying the action mechanism of RFX in patients with liver cirrhosis, an investigation was carried out in healthy human and in cirrhotic patients with an intravenous infusion of ammonium chloride solution, to calculate the ammonium clearance rate and ammonium production. It was found that cirrhotic patients had decreased clearance, especially increased ammonium production. In healthy individuals, it was observed that the administration of glycerol polysorbate increased clearance, while in cirrhotic, treatment with RFX administered two weeks before significantly reduced the production.42
Hepatic Encephalopathy
In HE, due to its probable relationship with the intestinal microbiota, therapies aimed to modifying it have been investigated: probiotics, symbiotics, prebiotics, fecal transplantation and RFX, the latter being one of the most successful.43 In addition, in 30 patients with liver cirrhosis who received RFX treatment for 12 weeks, the fecal microbiota was analyzed before and during treatment. RFX was well tolerated, reduced ammonia, improved neuropsychological functions and acted as a microbiota regulator agent as it maintained bacterial diversity, reduced potentially harmful bacteria (Klebsiella, Streptococcus, Clostridium), increased probiotic bacteria (Bifidobacterium, Bacteroides) and reduced the bacteria-mediated metabolic pathways, related to HE.44 It should be considered that some differences in the microbiota could explain the different response to treatment with RFX in HE-patients. For example, in one comparative study, the fecal microbiota of a group of patients with decompensated liver cirrhosis with HE, that of a group of patients with compensated cirrhosis as well as that of patients who did not respond to treatment with RFX and healthy controls was compared. The study found Streptococcus salivarius as the most significant association in HE patients, and Ruminococcus gravus in RFX non-responders.45
The fundamental role of RFX in the treatment of HE has been recognized for several years. The latest publications emphasize that: a) in overt HE, the RFX-lactulose combination is more effective than lactulose alone, lowers blood ammonia (ammonium) concentrations, reduces mortality and frequency of hospitalizations.46–49 b) Patients who are candidates for placement of transjugular intrahepatic portosystemic shunts (TIPS) in order to reduce portal pressure and bleeding from esophageal varices or refractory ascites, have a greater predisposition to suffer HE. In a study with 197 patients who underwent TIPS placement for variceal bleeding or intractable ascites, the administration of RFX reduced new episodes of HE by half (RR 0.48) compared to the control group.50 One review concluded that in patients with TIPS who had previously had HE, the administration of RFX significantly reduces the risk of new episodes of HE.51 c) In concealed or minimal HE, the clinical efficacy of RFX has also been demonstrated, as well as in reducing ammonemia. In these cases, the efficacy of RFX is greater in patients who do not have metabolic syndrome, since metabolic syndrome is associated with greater inflammation. In concealed HE, the administration of RFX with dose of 800 mg/day is similar in efficacy to that of 1200 mg/day.52–54 d) Long-term treatment with RFX and lactulose represents considerable savings for the health system, by reducing the use of hospital resources and mortality, despite the cost of drugs in several countries.
Pharmacoeconomic estimates also support the use of the RFX and lactulose instead of lactulose alone for the treatment and prevention of HE.55,56 e) The importance of long-term RFX treatment is also reinforced by real-life observations in outpatient treatment. In an observational study in patients admitted for HE after discharge from a hospital in the United States, only 58% of the patients had access to RFX, in these patients was observed that the number of subsequent hospitalizations was half that of those patients without antibiotic treatment.57
Infections
In a Belgian study with 66 HE patients who received RFX for six months, they were compared with the previous six months who had not received the antimicrobial. It was clearly demonstrated that in the period with the antibiotic there were fewer infections, mainly respiratory infections, as well as a decrease in hospital admissions, both those related to HE and those for all causes. The number of days spent in hospital, the length of stay in the Intensive Care Unit, and visits to the Emergency Department also decreased.58
A characteristic infection of individuals with liver cirrhosis, especially severe, is spontaneous bacterial peritonitis (SBP). RFX is effective in preventing new episodes of this infection in patients who have previously had it. In a randomized study, the administration of norfloxacin was compared with RFX for the prophylaxis of SBP. The results showed that for primary prophylaxis (those who had not previously had peritonitis) RFX and norfloxacin were equally effective but in the subgroup with previous episodes of peritonitis (secondary prophylaxis) RFX was superior, as there were significantly fewer new episodes of peritonitis (Figure 7).59 A recent systematic review indicated that the most widely used antimicrobial in this prophylaxis is norfloxacin, but in secondary prophylaxis RFX is more effective and with fewer adverse effects than norfloxacin and other antimicrobials.60 In a meta-analysis in this field, 13 randomized controlled clinical trials including 1742 patients were accepted to compare different antibiotics and placebo. RFX was superior to ciprofloxacin, trimethoprim-sulfamethoxazole, norfloxacin and placebo; more over, with RFX there was less mortality and liver transplants.61
 |
Figure 7 Proportion (%) of patients who developed spontaneous bacterial peritonitis (SBP) during antibiotic prophylaxis for 6 months. Primary prophylaxis = patients without previous episodes of SBP. Secondary prophylaxis = patients with previous episodes of SBP. All had risk factors for the development of SBP. Using data from Praharaj et al.60 Abbreviations: RFX, rifaximin; NFC, norfloxacin. |
Ascites
In 75 patients with refractory ascites, RFX administration for 6 months (n=50) was compared with patients without antibiotics (n=35). The comparative showed that RFX treatment was able to mitigate ascites and reduce mortality. There was improvement in ascites in 112 patients with overt HE treated with RFX for 36 months, in whom improvement in liver function parameters62,63 also was observed.
Bleeding from Esophageal Varices
One of the main causes of mortality in cirrhotic patients is bleeding from esophageal varices.64 An investigation carried out in Mexico found that patients who received RFX after discharge had fewer episodes of variceal bleeding, and also fewer HE recurrences than those who did not continue with RFX.65
In general, RFX has the potential to decrease the occurrence of complications from liver cirrhosis, as demonstrated in a study that included 299 subjects with cirrhosis and HE, who received RFX 550 mg twice daily or placebo for 6 months. At the end of the period, it was observed that in patients with severe hepatic impairment (MELD>12) there was a lower risk of any complication in the group receiving RFX (p<0.001), including spontaneous bacterial peritonitis, variceal bleeding, and marginally acute kidney disease.66 In another study with 200 patients with decompensated cirrhosis, the frequency of complications was evaluated, with RFX 400 mg twice daily compared with usual treatment without RFX. It was observed that the group that received RFX had a significantly lower number of HE episodes and bleeding from esophageal varices, ascites, and non-significant decrease (p=0.058) in infections.67 Together with non-selective beta-adrenergic blockers, RFX has been considered among the drugs that can favorably modify the evolution of patients with liver cirrhosis.68 (Figure 8)
 |
Figure 8 Patients with cirrhosis and HE, with prophylactic treatment with RFX or without RFX (control) for 6 months. Proportion of patients without complications (p<0.001 between groups). With data from Zeng et al.68 |
It has been suggested that an additional benefit could be the reduction of colon and rectal cancer in patients with cirrhosis who received long-term RFX, as observed in 5244 patients who received lactulose, 3502 with rifaximin and 3502 with RFX-lactulose.69 Rifaximin reduced the risk of colon cancer and esophageal cancer by 59.42% and 70.37%, respectively, compared to patients taking lactulose only.69,70 This observation is consistent with investigations in animals with experimental colon cancer, in which activation of pregnane X by RFX may protect against neoplasia.29
Rifaximin as a Disease Modifier
Each year, 5 to 7% of patients with compensated cirrhosis develop major complications. This transition to decompensated cirrhosis is often characterized by an abrupt course, directly impacting survival and life expectancy if a transplant is not achieved.69
Patients with decompensated cirrhosis are subject to numerous treatments to reduce complications. In this regard, disease-modifying agents are defined as those which interventions demonstrate a benefit in the course of the disease regardless of their original treatment indication. At present, numerous clinical studies, doses, administration time, treatments based on etiology, cost-effectiveness, global access, and guidelines have to be established.
To keep cirrhosis compensated for longer, we must have effective support interventions by virtue of the etiology. Emerging evidence shows that diet and exercise improve elements related to frailty and quality of life in patients with chronic liver damage, so these maneuvers should start in the initial phases of the disease.71 In addition to this, alcohol withdrawal, optimization of metabolic syndrome, treatment of virus C, B, or control of immunity are actions that must be carried out, since their impact on the portal venous gradient, stages of cirrhosis, MELD (Model for Stage Liver Disease), and even the stabilization and reversibility of fibrosis have been observed.72–74
Rifaximin as a Modifier of Chronic Liver Disease
RFX is known to have beneficial effects on the intestine-liver axis improving hemostasis at the level of intestinal membranes, decreasing inflammatory pathways, decreasing bacterial adhesiveness to enterocytes, and modulating the intestinal microbiome.75
In this regard, the therapeutic options of RFX beyond the treatment of HE are extremely attractive. Several controlled clinical studies have associated the use of RFX with better control of refractory ascites, reduction in the incidence of decompensation, causes of hospitalization and readmissions, together with a lower incidence of SBP, variceal bleeding, acute kidney injury-hepatorenal syndrome (AKI-HRS), with decreased need for replacement therapy,76–78 some studies even suggesting a positive impact on survival.79
Refractory Ascites
The prevalence of refractory ascites is close to 17% of the patients with decompensated cirrhosis, with extreme splanchnic vasodilatation, as well as activation of vasoconstrictor system (renin-angiotensin-aldosterone, and non-osmotic secretion of antidiuretic hormone) being the main causes of refractoriness.
A recent study of decompensated cirrhosis with ascites evaluated 600 patients, who were divided in 2 groups: (200 control group vs 400 under treatment with midodrine plus rifaximin). Body weight, blood pressure, glomerular filtration, and serum renin and aldosterone measurement were evaluated over 2-year period.
Mean arterial pressure increased significatively in the treatment group, with significant weight loss evident after 12 weeks of treatment (average 12.5 kg). At the biochemical level, the serum sodium level, urinary output, urinary sodium excretion, and creatinine clearance increased. Total response determined by creatinine levels was achieved in 78% of the patients with treatment vs 15% of the control group. Lastly, the need for evacuating paracentesis was markedly less in patients receiving RFX treatment (18 vs 75 patients).80
Side Effects and Real-World Use
Side effects of rifaximin are rare: headache, constipation, abdominal pain, diarrhea, flatulence, nausea, rectal urgency, transient hyperthermia, vomiting, and dizziness have been reported in 1% of the patients under treatment. There are anecdotal reports of C. difficile infection, in which cases the patients had predisposing factors for it.81 Lastly, it has been suggested that rifaximin in conjunction with statins may enhance muscle toxicity since isolated cases of rhabdomyolysis have been reported at a dose of 20 mg of simvastatin in conjunction with RFX.82
Other Complications of Cirrhosis
It would be convenient to perform large multicenter double-blind, randomized studies around RFX, alone or in combination with other disease-modifying agents (albumin, simvastatin) to assess its impact on survival and development of Acute on Chronic Liver Failure (ACLF) in patients with decompensated cirrhosis.
Conclusions
In patients with HE, long-term treatment achieves various benefits: less recurrence of HE, less frequency of hospitalizations, less frequency of various acute complications including infections, SBP, refractory ascites and bleeding from esophageal varices, which affects mortality, and cause the need of liver transplantation. In addition to the above, several data suggest that it could decrease the progression of liver fibrosis in patients with alcoholic liver disease and in non-alcoholic steatohepatitis. Lastly, its role as a disease-modifying agent, in an attempt to reduce the decompensation of patients with compensated cirrhosis more quickly or more severely, makes RFX a complete and attractive molecule in the management of cirrhotic patients.
Consent for Publication
Figures and tables are original and no copyright consent is needed.
Funding
There is no funding to report.
Disclosure
AT and JCG have no conflicts of interests related to this publication. ACFM is medical advisor of Alfasigma Laboratories Mexico.
References
1. Younunossi ZH, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver Diseases in USA in the past three decades. Gut. 2020;69:203–209.
2. Escorcia Charriz EJ, et al. INEGI Comunicado de prensa núm 592/21. Bioc. 2018;13:17–30.
3. Huang DQ, Tarrault NA, Tacke F, et al. Global epidemiology of cirrhosis- aetiology, trends and predictions. Nature Rev Gastroenterol Hepatol. 2023;28:1–11. doi:10.1038/s41575-023-00750-2
4. Yeo YH, He X, Lu F, et al. Trends of cirrhosis-related mortality in USA during the COVID-19 pandemic. J Clin Transl Hepatol. 2023;11(3):751–756. doi:10.14218/JCTH.2022.00313
5. Prabhakar T, Kaushal K, Prasad H, et al. Etiologic fractions in patients of hepatocellular carcinoma in India with and without a background of cirrhosis: s multicentric study. Hepatol Int. 2023. doi:10.1007/s12072-023-10498-16
6. D’Amico G, García-Tsao G, Pagliaoro L. Natural history and prognostic indicators of survival in cirrhosis. A systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi:10.1016/j.jhep.2005.10.013
7. Zubieta-Rodríguez R, Gómez-Correa J, Rodríguez-Amaya R, et al. Mortalidad hospitalaria en pacientes cirróticos en un hospital de tercer nivel. Rev Gastroenterol Mx. 2017;82:203–209.
8. Rabiee A, Mahmud N, Falker C, et al. Medication for alcohol use disorder improve survival in patients with hazardous drinking and alcohol associated cirrhosis. Hepatol Commun. 2023;7(4):20093. doi:10.1097/HC9.0000000000000093.eCollection
9. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma. Ann Int Med. 2013;158(5_Part_1):329–337. doi:10.7326/0003-4819-158-5-201303050-00005
10. Aiza-Haddad I, Ballesteros-Amozurrutia A, Borjas-Almaguer OD, et al. Consenso mexicano para el tratamiento de la hepatitis C. Rev Mex Gastroenterol. 2018;83:295–324.
11. Berzigotti A, Albillos A, Villanueva C, et al. Effects of an intensive intervention program on portal hypertension in patients with cirrhosis and obesity. The SportDiet study. Hepatology. 2017;65(84):1293–1305. doi:10.1002/hep.28992
12. Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotheraphy. 2005;51(Suppl 1):36–66.
13. Viscomi GC, Campana M, Barbanti M, et al. Crystal form of rifaximin and their effect on pharmaceutical properties. Cryst Eng Comm. 2008;10(8):1074–1108. doi:10.1039/b717887e
14. Ojetti V, Lauritano EC, Barbaro F, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol. 2009;5(6):675–682. doi:10.1517/17425250902973695
15. Novoa-Farías O, Frati-Munari AC, Peredo MA, et al. Susceptibility of bacteria isolated from acute gastrointestinal infections to rifaximin and other antimicrobial agents in Mexico. Rev Gastroenterol Mex. 2016;81(1):3–10. doi:10.1016/j.rgmx.2015.07.003
16. Novoa-Farias O, Frati-Munari AC, Peredo MA, et al. Susceptibility to rifaximin and other antimicrobials of bacteria isolated in patients with acute gastrointestinal infections in Southeast Mexico. Rev Gastroenterol Mex. 2017;82(3):226–233. doi:10.1016/j.rgmx.2016.10.006
17. Solórzano-Santos F, Piña-Flores LI, Priego-Hernández G, et al. Actividad antibacteriana de la rifaximina y otros siete antibióticos contra las bacterias enteropatógenas aisladas de niños con diarrea aguda. Rev Mex Pediat. 2018;85:45–52.
18. Calanni F, Renzulli C, Barbanti M, Viacomi GC. Rifaximin: beyond the traditional antibiotic activity. J Antibiot. 2014;67(9):667–670. doi:10.1038/ja.2014.106
19. Brigidi P, Swennen E, Rizzello F, et al. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J Chemother. 2002;14(3):290–295. doi:10.1179/joc.2002.14.3.290
20. Maccaferri S, Vitali B, Klinder A, et al. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65(12):2556–2565. doi:10.1093/jac/dkq345
21. Soldi S, Vasileiadis S, Uggeri F, et al. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol. 2015;8:309–325. doi:10.2147/CEG.S89999
22. Ponziani FR, Scaldaferri F, Petito V, et al. The Role of Antibiotics in Gut Microbiota Modulation: the Eubiotic Effects of Rifaximin. Dig Dis. 2016;34(3):269–278)SE. doi:10.1159/000443361
23. Fiorucci S, Distrutti E, Mencarelli A, et al. Inhibition of intestinal bacterial translocation with Rifaximin modulates lamina propia monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion. 2002;66(4):246–256. doi:10.1159/000068362
24. Xu D, Gao J, Gilliland M, et al. Rifaximin alters intestinal bacteria and prevent stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146(2):484–496. doi:10.1053/j.gastro.2013.10.026
25. Jin Y, Ren X, Li G, et al. Rifaximin exerts beneficial effects in PI-IBS mouse model beyond gut microbiota. J Gastroenterol Hepatol. 2018;33(2):443–452. doi:10.1111/jgh.13841
26. Mencarelli A, Migliorati M, Barbanti M, et al. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol. 2010;80(11):1700–1707. doi:10.1016/j.bcp.2010.08.022
27. Cheng J, Shah YM, Ma X, et al. Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Ex Ther. 2010;335(1):32–41. doi:10.1124/jpet.110.170225
28. Patel VC, Shawcross DL, McPhail MJL Scientific report: a placebo controlled single center double blind randomized trial to investigate the efficacy of rifaximin versus placebo in improving systemic inflammation and neutrophil malfunction in patients with cirrhosis and chronic hepatic encephalopathy. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-004708-20/results.
29. Cheng J, Ze-Fang Z, Nagaoka K, et al. Activation of intestinal human pregnane X receptor protects against azoxymethane/dextran sulfate sodium induce colon cancer. J Pharmacol Exper Ther. 2014;351(3):559–567. doi:10.1124/jpet.114.215913
30. Xie C, Singal AK. Rifaximin-α in alcohol-associated liver disease. Lancet Gastroenterol Hepatol. 2023;8(6):495–497. doi:10.1016/s2468-1253(23)00033-x)
31. Israelsen M, Madsen BS, Torp N, et al. Rifaximin-αfor liver fibrosis in patients with alcohol-related liver disease (GALA-RIF): a randomized. Double-blind, placebo-controlled, phase-2 trial. Lancet Gastroenterol/Hepatol. 2023. doi:10.1016/s2468-1253(23)00010-9
32. Bernal-Reyes R, Castro-Narro G, Malé-Velázquez R, et al. Consenso mexicano de la enfermedad por hígado graso no alcohólico. Rev Gastroenterol Mex. 2019;84:69–99.
33. Escorcia Charris EJ, Marrugo-Balcero WR. Caracterización biológica y clínica de la cirrosis hepática en un centro regional del caribe colombiano: clínica General del Norte. Enero 2012 a marzo 2017. Biociencias. 2018;13(1):17–30. doi:10.18041/2390-0512/bioc.1.2242
34. Oseinim AM, Sanjal AJ. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017;37(suppl 1):97–103. doi:10.1111/liv.13302
35. Perumpail BJ, Li AA, John V, et al. The therapeutic implications of the gut microbiome and probiotics in patients with NAFLD. Diseases. 2019;7(27):1–12. doi:10.3390/diseases7010027
36. Cobbold JFL, Atkinson S, Marchesi JR, et al. Rifaximin in non-alcoholic steatohepatitis: an open-label pilot study. Hepatol Res. 2018;48(1):60–77. doi:10.1111/hepr.12904
37. Gangarapu V, Ince AT, Baysal B, et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with non-alcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27(7):840–845. doi:10.1097/MEG.0000000000000348
38. Abdel-Razik A, Mousa N, Shebana W, et al. Rifaximin in nonalcoholic fatty liver disease: hit multiple targets with a single shot. Eur J Gastroenterol Hepatol. 2018;30(10):1237–1246. doi:10.1097/MEG.0000000000001232
39. Fujinaga Y, Kawaratani KI, Kawa D, et al. Effective combination therapy of angiotensin-II receptor blocker and rifaximin for hepatic fibrosis in rat model of nonalcoholic steatohepatitis. Int J Mol Sci. 2020;21(15):5589. doi:10.3390/ijms21155589
40. Enomoto M, Kaji K, Nishimura N, et al. Rifaximin and lubiprostone mitigate liver fibrosis development by repairing gut barrier function in diet-induced rat steatohepatitis. Dig Liver Dis. 2022;54(10):1392–1402. doi:10.1016/jdid2022.04.012
41. Vlachogiannakos J, Viazis N, Vasianopoulou P, et al. Long term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28(3):450–455. doi:10.1111/jgh.12070
42. Jian J, Nie MT, Xiang B, et al. Rifaximin ameliorates non-alcoholic steatohepatitis in mice through regulating gut microbiome-related bile acids. Front Pharmacol. 2022;13. doi:10.3389/fpharm2022.841132
43. Eriksen PL, Djernes L, Vilstrup H, Ott P. Clearance and production of ammonia quantified in humans by constant ammonia infusion- The effect of cirrhosis and ammonia targeting treatments. J Hepatol. 2023;s0168-8278:220.
44. Won SM, Oh KK, Gupta H. The link between gut microbiota and hepatic encephalopathy. Int J Mol Sci. 2022;21(16):8999. doi:10.3390/ijms23168999
45. Yu X, Jin Y, Zhou W, et al. Rifaximin moderates the gut microbiota to prevent hepatic encephalopathy in liver cirrhosis without impacting the resistant. Front Cell Infect Microbiol. 2022.
46. Yukawa-Muto Y, Kamlya T, Fujii H, et al. Distinct responsiveness to rifaximin in patients with hepatic encephalopathy depends on functional gut microbial species. Hepatol Comm. 2022;1:1–15.
47. Fu J, Gao Y, Shi L. Combination therapy with rifaximin and lactulose in hepatic encephalopathy: a systematic review and meta-analysis. PLOSone. 2022;17:e8267647. doi:10.1371/journal.pone.0267647
48. Chang C, Huang CH, Tseng H, et al. Real-world experience of one-year efficacy of rifaximin add-on to lactulose is superior to lactulose alone in patients with cirrhosis complicated with recurrent hepatic encephalopathy in Taiwan. J Pers Med. 2021;11:478. doi:10.3390/jpm11060478
49. Volk ML, Burne R, Guerin A, et al. Hospitalizations and health care costs associated with rifaximin versus lactulose treatment among commercially insured patients with hepatic encephalopathy in the United States. J Med Econ Doi. 2021;24:202.
50. Hayakawa Y, Tawaki N, Nakanishi H, et al. Add-on therapeutic effects of rifaximin on treatment – resistant hepatic encephalopathy. Internal Med. 2022. doi:10.2169/internalmedicine0212
51. Bureau C, Thabut D, Jezequel C, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled trial. Ann Intern Med. 2021;174(5):533–640. doi:10.7326/M20-0202
52. Garring J, Müller L, Kloeckner R, et al. Review article: post- TIPSS hepatic encephalopathy—current knowledge and future perspectives. Aliment Pharmacol Ther. 2022;55(10):1265–1276.
53. Nakai N, Suda G, Ogawa K, et al. Efficacy of rifaximin against covert hepatic encephalopathy and hyperammonemia in Japanese patients. PLoS One. 2022;17(7):20270786. doi:10.1371/journal.pone.0270786
54. Ballester MP, Gallego JJ, Fiorillo A, et al. Metabolic syndrome is associated with poor response to rifaximin in minimal hepatic encephalopathy. Scientific Rep. 2022;12(1):2463. doi:10.1038/s41598-022-06416-z
55. Tau W, Wang J, Shi PM, et al. Effect of low-dose and high-dose rifaximin in the treatment of covert hepatic encephalopathy. J Clin Transl Hepatol. 2022;10(6):1099–1106. doi:10.14218/JCTH.2021.00457
56. De Jong LA, van Schoonhoven AV, Hofstra HS, et al. Budget impact of optimizing rifaximin-α use for prevention of recurrent hepatic encephalopathy in the Netherlands. J Med Econ. 2021;24(1):1149–1163. doi:10.1080/13696998.2021.1983291
57. Frati-Munari AC, Galindo-Sanchez RM. Rifaximina-α en la encefalopatía hepática. Consideraciones farmacoeconómicas para México. Med Int Mex. 2020;31:621–663.
58. Stoll AM, Guido M, Pence A, Gentene AJ. Lack of access to rifaximin upon hospital discharge is frequent and results in increased hospitalizations for hepatic encephalopathy. Ann Pharmacoither. 2022. doi:10.1177/10600280221100537)
59. De Graeve J, Vanderstracten E, Dlvaeye T, et al. The impact of rifaximin in the hospital burden and infections in patients with hepatic encephalopathy: a retrospective observational study. Acta Gastroenterol Belg. 2022;85(1):1–5. doi:10.51821/85.1.9365
60. Praharaj DL, Premikuwar H, Roy A, et al. Rifaximin Vs norfloxacin for spontaneous bacterial peritonitis prophylaxis: a randomized controlled trial. J Clin Exper Hepatol. 2022;12(2):336–342. doi:10.1016/j.jceh.2021.08.010
61. Pimentel R, Gregorio C, Figuereido V. Antibiotic prophylaxis for prevention of spontaneous bacterial peritonitis: a systematic review. Acta Gastroenterool Belg. 2021;84(2):333–341. doi:10.51821/84.2.333
62. Faust N, Yamada A, Haider H, et al. Systematic review and network meta-analysis. Prophylactic antibiotic therapy for spontaneous bacterial peritonitis. World J Hepatol. 2020;18(5):239–252. doi:10.4254/wjh.v12.i5.239
63. Lu XY, Ding HG, Zheng JF, et al. Rifaximin improves survival in cirrhotic patients with refractory ascites: a real world study. World J Gastroenterol. 2020;26(2):199–218. doi:10.3748/wjg.v26.i2.199
64. Yokohama K, Fukuda J, Yamauchi R, et al. Long-term effects of rifaximin on patients with hepatic encephalopathy: its possible effects on the improvement in the blood ammonia concentration levels, hepatic spare ability and refractory ascites. Medicine. 2022;58:1276.
65. Zubieta-Rodeíguez S, Gómez-Correa J, Rodríguez-Amaya R, et al. Mortalidad hospitalaria en pacientes cirróticos en un hospital de tercer nivel. Rev Gastroenterol Mex. 2017;82(3):203–209. doi:10.1016/j.rgmx.2016.10.002
66. Higuera-De la Tijera F, Soto-Martinez K, Funez-Madrid VM, et al. Long-term rifaximin is safe and its related to less frequency of complications like variceal bleeding in cirrhotic patients. Ann Hepatol. 2021;24:100366. doi:10.1016/j.aohep.2021.100518
67. Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Ther Adv Gastroenterol. 2018;11:1–10. doi:10.1177/1756284818800307
68. Zeng X, Sheng X, Wang PQ, et al. Low-dose rifaximin prevents complications and improves survival in patients with decompensated cirrhosis. Hepatol Int. 2021;15(1):155–165. doi:10.1007/s12072-020-10117-y)
69. Lee S, Saffo S. Evolution of care in cirrhosis. Preventing hepatic decompensation through pharmacotherapy. World J Gastroenterl. 2023;29(1):61–74. doi:10.3748/wjg.v29.i1.61
70. Patel AH, Li Y, Minacapoll CD, et al. Reduction in gastrointestinal cancers in cirrhotic patients receiving rifaximin vs lactulose only therapy for hepatic encephalopathy. Cureus. 2023;15(2):e35259. doi:10.7759/cureus.35259
71. D´Amico M, Pasta L, Malizia G, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68(3):563–576. doi:10.1016/j.jhep.2017.10.020
72. Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. 2018;69: (5(5):1164–1177. doi:10.1016/j.jhep.2018.06.017
73. Degré D, Stauber RE, Englebert G, et al. Long-term outcomes in patients with decompensated alcohol-related liver disease, steatohepatitis and Maddrey’s discriminant function <32. J Hepatol. 2020;72(4):636–642. doi:10.1016/j.jhep.2019.12.023
74. El Sherif O, Dhaliwai A, Newsome PN, et al. Sarcopenia in nonalcoholic fatty liver disease: new challenges for clinical practice. Expert Rev Gastroenterol Hepatol. 2020;14(3):197–205. doi:10.1080/17474124.2020.1731303
75. Lens S, Baiges A, Alvarado Tapias E, et al. Clinical outcome and hemodynamics changes following HCV eradication with oral antiviral therapy in patients with clinical significant portal hypertension. J Hepatol. 2020;73(6):1415–1424. doi:10.1016/j.jhep.2020.05.050
76. Bajaj JS. Potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther. 2016;43(Suppl1):11–26. doi:10.1111/apt.13435
77. Lv X-Y, Ding H-G, Zheng J-F, Fan C-L, Li L. Rifaximin improves survival in cirrhotic patients with refractory ascites: a real world study. World J Gastroenterol. 2020;26(2):199–218.
78. Flamm SL, Mullen KD, Heimanson Z. Rifaximin has the potential to prevent complications of cirrhosis. Ther Adv Gastroenterol. 2018;11:1756284818800307.
79. Dong T, Aronsohn A, Gautham Reddy K, et al. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig Dis Sci. 2016;61(12):3621–3626. doi:10.1007/s10620-016-4313-0
80. Hanafy AS, Hassanenn AM. Rifaximin and midodrine improve clinical outcome in refractory ascites including renal function, weight loss, and short term survival. Eur J Gastroenterol Hepatol. 2016;28(12):1455–1461. doi:10.1097/MEG.0000000000000743
81. Prantera C, Lochs H, Giochetti P. Rifaximin-EIR (Extended Intestinal Release) 400 mg tablets (investigator´s brochure) Covington, LA. Alfasigma. Gut. 2016:59.
82. Mullen KD, Sanyal AJ, Bass NM, et al. Rifaximin is safe and well tolerated for long term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12(8):1390–1397 e.2−. doi:10.1016/j.cgh.2013.12.021
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
