Back to Journals » International Medical Case Reports Journal » Volume 15
Rhino-Orbital-Cerebral Mucormycosis in a Young Diabetic Patient with COVID-19 in Ethiopia: A Case Report
Authors Alemayehu FM , Abate HK, Soboka TA, Huluka DK, Worke AB, Abrie MT , Dibaba DK, Asnake YB
Received 1 March 2022
Accepted for publication 10 May 2022
Published 19 May 2022 Volume 2022:15 Pages 251—257
DOI https://doi.org/10.2147/IMCRJ.S364591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Fikremariam Melkeneh Alemayehu,1 Hannibal Kassahun Abate,2 Tariku Assefa Soboka,3 Dawit Kebede Huluka,4 Alemayehu Bedane Worke,5 Mahlet Tsegaye Abrie,6 Dawit Kebebaw Dibaba,7 Yilkal Birhanu Asnake8
1General Practitioner, Intensive Care Unit Trained, Department of Intensive Care Unit, Ministry of Health (MOH) Eka Kotebe General Hospital, Addis Ababa, Ethiopia; 2Internal Medicine Chief Resident (PGY-3), Department of Internal Medicine, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia; 3Anesthesiologist and Intensivist, Department of Intensive Care Unit, MOH Eka Kotebe General Hospital, Addis Ababa, Ethiopia; 4Internist, Pulmonologist and Critical Care Subspecialist, Department of Internal Medicine, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia; 5Radiologist, Subspecialist in Body Imaging and Neuroradiology, Department of Radiology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 6Head and Chief General Ophthalmologist, Department of Ophthalmology, MOH Eka Kotebe General Hospital, Addis Ababa, Ethiopia; 7Otorhinolaryngologist, Department Head of Otorhinolaryngology, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia; 8Ophthalmologist, Department of Ophthalmology, MOH Eka Kotebe General Hospital, Addis Ababa, Ethiopia
Correspondence: Fikremariam Melkeneh Alemayehu, PO Box 100571, Kirkos Sub City, Addis Ababa, Ethiopia, Tel +251921467414, Email [email protected]
Background: There has been a rise in secondary invasive fungal infections reported in COVID-19 patients globally. We report the first published case of COVID-19 associated rhino-orbital-cerebral mucormycosis in Africa in a newly diagnosed diabetic female who presented with diabetic ketoacidosis (DKA) and discuss the prevalence and risk factors of fungal co-infection with the clinical presentation, diagnosis, and management of mucormycosis in COVID-19.
Case Presentation: A 39 years old female patient was admitted to ICU with a diagnosis of severe COVID-19 and newly diagnosed diabetes mellitus (DM) with DKA based on HgbA1c of 13.8% and positive RT-PCR. The patient was treated with dexamethasone in line with evidence in the RECOVERY trial and developed right facial and orbital swelling on her second hospital day. Brain MRI showed characteristic peri-sinonasal invasion with central nervous system (CNS) involvement, features suggestive of invasive fungal infection. Despite all medical and surgical treatments including liposomal amphotericin B and debridement, the patient died within 7 days of symptom onset.
Conclusion: Clinicians should be aware of the potential for Rhino-Orbital-Cerebral Mucormycosis (ROCM) as a complication of COVID-19, especially in steroid taking diabetics who develop periorbital swelling and sinusitis. Timely diagnosis and multidisciplinary treatment are very critical.
Keywords: COVID-19, mucormycosis, zygomycotic, fungal infection, anti-fungal
Background
As of Nov 22, 2021, the number of confirmed coronavirus disease of 2019 (COVID-19) cases in Africa totaled over 8.6 million, representing around 3.35% of infections worldwide.1 Overall, secondary infections in COVID-19 do not appear to be common.2 In a review of nine studies, mainly from China, the reported rate of bacterial or fungal coinfections was 8%; mainly bacteremia but there was widespread use of broad-spectrum antibiotics (72%).4 Overall, studies show candidemia, invasive aspergillosis, and mucormycosis as the three commonest opportunistic fungal infections in COVID-19 patients.5,6,9
Mucormycosis infection frequently begins as acute sinusitis with fever, stuffy nose, purulent nasal discharge, headache, and facial pain. As the infection advances, vascular invasion leads to necrosis with the formation of black eschar over nasal mucosa, palate, or skin overlying the orbit. With Central Nervous System (CNS) involvement, ptosis of the eyelid, proptosis of the eyeball, and multiple cranial nerve palsies also occur.3
Giant cell invasion, thrombosis, and eosinophilic necrosis of tissues due to angioinvasion are the main pathological characteristics of mucormycosis. These mold fungi are found everywhere, especially in soil and decaying flora and contamination occur through the inhalation of fungal spores but most are cleared by the immune system.3
Below we report a case of Rhino-Orbital-Cerebral Mucormycosis in a 39-year-old female patient with COVID-19. We also discuss the diagnosis and treatment challenges faced in critically ill patients in resource-limited settings.
Case Presentation
A 39 years old lady was admitted to a COVID-19 treatment center in July 2021 with a complaint of worsening shortness of breath, easy fatiguability, low-grade fever, chills, frontal headache, and productive cough of yellowish sputum of ten days duration. The patient tested reverse transcription-polymerase chain reaction (RT-PCR) positive for COVID-19 five days before her admission.
She is a known cerebral palsy patient since birth and was on sodium valproate 200mg PO/day for a long time. Two weeks back she was told to have acute angle-closure glaucoma on her right eye and was put on timolol 0.5% eye drop BID, tropicamide 1% eye drop TID, acetazolamide 250 mg PO BID with prednisolone 20mg PO/day.
The patient was referred from a local health center for better ICU care and follow-up after being started on Dexamethasone, Azithromycin, and Ceftriaxone-sulbactam. In the emergency room (ER), her vital signs were BP of 120/80 mmHg, PR of 92 beats/min, RR of 32 breaths/min, T of 36.2 °C, and SpO2 96% on 6L/min facemask oxygen. The only pertinent findings were coarse crepitations over the left lung.
Random blood sugar determined at the ER was recorded high (>600 mg/dL) and urine analysis showed ketone +1 and glucosuria of +3. A chest x-ray from the referral center showed evidence of left-side pneumonia. Laboratory tests on her first day of admission (DOA) showed mild leukocytosis and lymphopenia (14.4 x 103 WBCs with 95% neutrophil and 0.9% lymphocyte), moderate hyponatremia (127 mmol/L) likely due to hyperglycemia, mild hypokalemia (3.2 mmol/L), pre-renal azotemia (serum creatinine of 2.95 mg/dL and BUN of 69 mg/dL) and c-reactive protein of 89.9 mg/L with negative serostatus for HIV. Abdominal ultrasound showed normal-sized kidneys. Arterial blood gas analysis was unfortunately not done because of cartridge stockout at the time.
The patient was admitted to the ICU right away with a problem list of severe COVID-19 with superimposed bacterial pneumonia, prerenal acute kidney injury (AKI), stress-induced hyperglycemia r/o ketosis-prone type 2 diabetes mellitus (T2DM), known cerebral palsy, and right eye glaucoma, and was put on dexamethasone 6mg IV/day, ceftriaxone-sulbactam 1.5 mg IV BID, UFH 17,500 SC BID, vitamin C 200mg PO/day, and Zn 20 mg PO/day, and she was treated for diabetic ketoacidosis (DKA) as per the local protocol.
On the second hospital day, the patient developed right eye swelling and with an added assessment of right orbital cellulitis, vancomycin (renal dose adjusted) was added.
On day three of admission, the patient was started on NPH (Neutral Protamine Hagedorn) insulin 16/8 SC BID with correctional regular insulin since her glycemic control was poor despite being on a modified sliding scale. Her NPH insulin requirement would eventually be escalated to 28/14 until the patient achieved the target glycemic range on the seventh DOA.
On the fourth DOA, rhino-orbital mucormycosis (see Figure 1) was considered and liposomal amphotericin B (5mg/kg/day IV) was started and ceftriaxone-sulbactam changed to meropenem 1g IV TID. On the same day, the ophthalmologist evaluated her and planned to continue amphotericin B and a nasal swab was sent for culture.
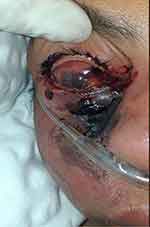 |
Figure 1 Tissue necrosis with the formation of black eschar over the skin overlying orbit associated with proptosis, conjunctival hyperemia, chemosis, and matted eyelashes. |
On the fifth DOA, extensive surgical debridement of necrotic tissue over the rhino-orbital area was done. The next day, the HgA1c result arrived (13.8%) and newly diagnosed T2DM was added to the problem list. Dexamethasone was also held and overall, the patient was improving with resolved fever, decreasing leukocytosis, normalized renal function test, and coagulation profile (PTT of 50.8 and INR of 1.3), and decreasing oxygen support (2 L/min intranasal oxygen).
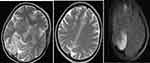
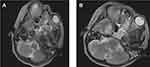
However, on the eighth DOA, the patient developed a new high-grade fever, increased oxygen demand (20L/min facemask oxygen) and meropenem was escalated to 2g IV TID and septic work up sent. Culture from wound site in the face would eventually grow Methicillin-resistant Staphylococcus aureus (MRSA) sensitive to tigecycline and linezolid. At the same time, a brain MRI was done (see Figures 2 and 3), and the finding is shown in the figure caption as reported by a body and neuroradiology subspecialist.
Eventually, patient deteriorated developing lethargy and septic shock of chest focus (new bilateral consolidation on lung ultrasound) with the possibility of fungal invasion into the brain (Rhino-Orbital-Cerebral Mucormycosis). She was started on vasopressors and hydrocortisone; however, intubation was deferred due to likely poor outcome after thorough family counseling. Despite all the above management, the patient eventually died on the ninth day of ICU admission and 20th total duration of illness from symptom onset with the possible immediate cause of death, multi-organ dysfunction syndrome (MODS) secondary to refractory septic shock.
Discussions
Data show worldwide the prevalence of mucormycosis ranges from 0.005 to 1.7 per million population. In a recent estimate, however, it is almost 80 times higher in India (0.14 per 1000). Rhizopus Oryzae is by far the most common species accounting for 60% of mucormycosis cases in humans and almost 90% of the Rhino-Orbital-Cerebral (ROCM) form.7
Besides poorly controlled diabetes with or without DKA, other risk factors consist of hematological malignancies, HIV/AIDS, prolonged neutropenia, immunosuppressive and corticosteroid therapy, severe burns, hemochromatosis, intravenous drug abusers, malnutrition, and open wound following trauma.7,8 One of the largest reviews of 929 cases of mucormycosis that were reported between 1940 and 2003 noted that diabetes mellitus was the most common risk factor (36%), followed by hematologic malignancies (17%) and transplantation (12%).9 Similar risk factors, ie, poorly controlled DM with DKA and steroid therapy, are present in our case and this is congruent with findings from other studies.5,14
Mucormycosis can involve almost any organ. The most common sites were sinus (39%), pulmonary (24%), cutaneous (19%), and dissemination developed in 23% of cases.8,9 The area of involvement varies with an underlying risk factor. For example, ROCM is frequently seen in uncontrolled diabetes and DKA, similar to our case, whereas pulmonary involvement is often observed in patients having neutropenia, transplants, and cancers, and the gastrointestinal tract is involved more in malnourished individuals.7,9 ROCM, the commonest type seen in clinical practice, refers to the entire span ranging from limited sino-nasal disease, progression to orbits (rhino-orbital disease) to CNS involvement (rhino-orbital-cerebral disease).9
There are many explanations given as to why mucormycosis incidence is increasing during the COVID-19 pandemic:7
- DM is associated with increased severity of COVID-19 and it is one of the major risk factors for contracting mucormycosis, which is also seen in our case.
- Since the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, corticosteroids have become a routine part of COVID-19 management. However, steroids often lead to poor control of hyperglycemia and even precipitate DKA. This acidosis is an ideal medium for these fungi spores to grow. Moreover, steroid use also reduces bronchoalveolar macrophage’s migration, ingestion, and fusion. All of these predispose to fungal infection as evident in our patient.
- COVID-19 often causes lymphopenia and reduction in CD4+ and CD8+ T-cell levels, predisposing to secondary fungal infection.
- The hyperglycemia and acidosis discussed above along with COVID-19 cytokine storm (esp. IL-6) increases ferritin level leading to increased free iron which is a good resource for mucormycosis.
- Lastly, antibiotic abuse, a common scenario in COVID-19 treatment centers, suppresses the normal bacterial flora and facilitates the establishment and growth of fungi.
Data from a literature review of 208 cases between 1970 and 1993 of rhino-orbital-cerebral mucormycosis and the Smith and Kirchner 1950 criteria found the following frequency of symptoms and signs:3 fever (44%), nasal ulceration or necrosis (38%), periorbital or facial swelling (34%), decreased vision (30%), ophthalmoplegia (29%), sinusitis (26%), and headache (25%). As mentioned above, all these clinical features are also present in our patient.
The diagnosis of mucormycosis depends on clinical presentation, imaging (CT and/or MRI), and histopathological study with culture confirmation. Microbiological identification of the hyphae (irregular, broad, non-septate hyphae with right-angle branching) helps it differentiate from other fungal infections.7 While serum tests, such as the 1,3-beta-D-glucan assay and the aspergillus galactomannan assay, are useful in other invasive fungal infections, the fungi of mucormycosis lack these cell wall components and are thus not helpful in mucormycosis diagnosis.3 MRI is more sensitive than CT for detecting ROCM, with perisinus invasion being the most diagnostic finding.10 This is also true in our patient and case reports from other countries.5,14 Unfortunately, histopathological examination and fungal culture were not done in our case due to resource limitations.
A timely combination of surgery and antifungal therapy with possible correction of the underlying immunosuppression has improved survival in mucormycosis. Surgery is sino-nasal debridement in most cases, which can often be disfiguring.7 In the literature review done by Roden et al that analyzed 929 cases, survival was 3% for cases that were not treated, 57% for cases treated with surgery alone, 61% for cases treated with conventional amphotericin B alone, and 70% for cases treated with antifungals and surgery.9
Early diagnosis and initiation of antifungal therapy have a significant impact on mortality. This was shown in a retrospective study of 70 patients with hematologic malignancy who had mucormycosis in which delayed amphotericin B therapy (starting treatment ≥6 days after diagnosis) resulted in an almost two-fold increase in mortality at 12 weeks after diagnosis (83 versus 49%).11 When we come to our patient, even though a presumptive diagnosis of mucormycosis was made early and empirical antifungal treatment started within 48hrs of symptom onset despite having significant laboratory, imaging, and drug supply constraints, it was still not possible to salvage the patient. This indicates the high mortality associated with this disease, esp. in low- and middle-income countries, and the need for emphasis on prevention.
IV amphotericin B (liposomal) is the drug of choice for initial therapy, with a dose of 5–10mg/kg/day12 as suggested by the “One World One Guideline” initiative of the European Confederation of Medical Mycology (ECMM). The azole group of antifungals (posaconazole or isavuconazole only) is mainly used in two scenarios. One is as oral step-down therapy and the second as salvage therapy for those who cannot tolerate or respond to amphotericin B.3 Other azole antifungals are not effective against the Mucorales. Therapy is usually continued until there is a clinical and radiologic resolution of active disease.3
Overall, mortality varied with the site of infection ranging from 25 to 62%, 76%, 85%, and 96% for ROCM, pulmonary, GI, and disseminated infections in respective order.9
Lastly, we discuss the three case definitions for mucormycosis cases in COVID-19 according to the recently published guideline of “Code Mucor: Guidelines for the Diagnosis, Staging, and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19”.13 This guideline describes rhino-orbital-cerebral mucormycosis. For those with sites other than ROCM, the term “probable” was used to include imaging and endoscopic findings compatible with mucormycosis. In short, “possible” refers to the presence of typical clinical mucormycosis findings; “probable” is used when MRI/CT imaging and/or nasal/lung/GI endoscopic findings are present in addition to typical clinical findings and in addition to these if mucormycosis is confirmed by microbiologic, histologic, and molecular means, it is designated as “proven”.13,14
A cumulative prednisone dose of greater than 600 mg in the month before, ie, even a brief course (5–14 days), has been linked with mucormycosis.15 Similarly, our patient took an equivalent cumulative dose of 1720 mg of prednisolone including the two weeks of prednisolone given before her admission.
As discussed above, this was a “probable” case of ROCM, since it was not possible to do culture due to the lack of fungal media in the country at the time. However, the clinical presentation, the presence of mentioned risk factors, and the MRI findings are in direct correlation with reports by others.14,16
To the best of our knowledge, this is the first published report of COVID-19 associated mucormycosis in the African continent. Since the establishment of the treatment center, there were two “probable” case fatalities due to COVID-19 associated Mucormycosis so far (including this patient) out of 5783 hospitalized patients in Eka Kotebe General hospital, the largest COVID-19 treatment center in the country (ie, an incidence of around 0.03%).
Conclusions
Cautious evidence-based use of corticosteroids and rational use of antibiotics should be applied in patients with COVID-19, in addition to maintaining optimal hyperglycemia.
Finally, we emphasize a high index of suspicion for secondary fungal infections like mucormycosis in the COVID-19 pandemic, and urgent diagnosis and multidisciplinary approach to treatment could be life-saving. However, in resource-limited countries like Ethiopia, this would be challenging since it requires building up our ICU capacities significantly in terms of medications, laboratory and imaging modalities as well as trained specialists.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of EKA KOTEBE GENERAL HOSPITAL, Addis Ababa (IRB reference number Eka/150/5/50 on Dec 13, 2021).
Informed Consent Statement
Informed written consent was obtained from the family members of the patient for publication of this case report and accompanying images.
Acknowledgments
The authors would like to thank the ICU team, especially Hiwote Abebe (M.D, ICU coordinator) at Eka Kotebe General Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no conflicts of interest.
References
1. COVID-19 cases in the African continent database. Hamburg, Germany: Statista data platform; 2021. Available from: https://www.statista.com/statistics/1170463/coronavirus-cases-in-africa.
2. McIntosh K, Hirsch MS, Bloom A, et al. COVID-19: clinical features. UpToDate®. (Accessed on Dec 1,2021).
3. Cox GM, Kauffman CA, Hall KK, et al. Mucormycosis (zygomycosis). UpToDate®. (Accessed on Nov 28, 2021).
4. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459. doi:10.1093/cid/ciaa530
5. Patel A, Agarwal R, Rudramurthy SM, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–2359. doi:10.3201/eid2709.210934
6. White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1298
7. Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes & metabolic syndrome. Clin Res Rev. 2021;15:102146. doi:10.1016/j.dsx.2021.05.019
8. Sugar AM. Mucormycosis. Clin Infect Dis. 1992;14:S126–S129. doi:10.1093/clinids/14.supplement_1.s126
9. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634. doi:10.1086/432579
10. Groppo ER, El-Sayed IH, Aiken AH, Glastonbury CM. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2011;137:1005–1010. doi:10.1001/archoto.2011.170
11. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503. doi:10.1086/590004
12. Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):405. doi:10.1016/S1473-3099(19)30312-3
13. Honavar SG. Code mucor: guidelines for the diagnosis, staging, and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69:1361–1365. doi:10.4103/ijo.IJO_1165_21
14. Dilek A, Ozaras R, Ozkaya S, Sunbul M, Sen EI, Leblebiciogluh H. COVID-19-associated mucormycosis: case report and systematic review. Travel Med Infect Dis. 2021;44:102148. doi:10.1016/j.tmaid.2021.102148
15. Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi:10.1016/s0140-6736(03)
16. Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9):10726.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.