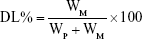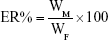Back to Journals » OncoTargets and Therapy » Volume 11
RGD-modified nanoliposomes containing quercetin for lung cancer targeted treatment
Authors Zhou X, Liu H, Zhao H, Wang T
Received 28 March 2018
Accepted for publication 23 June 2018
Published 3 September 2018 Volume 2018:11 Pages 5397—5405
DOI https://doi.org/10.2147/OTT.S169555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Samir Farghaly
Xiao Zhou,1,* Hong-Yan Liu,2,* Hui Zhao,2 Tong Wang3
1Department of General Thoracic Surgery, The Second Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Department of Respiratory, The Second Hospital of Anhui Medical University, Hefei, People’s Republic of China; 3Department of General Medicine, The Second Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Purpose: The aim of this study was to prepare RGD-modified nanoliposomes containing quercetin (QCT) distearoyl-L-a-phosphatidylethanolamine-polyethylene glycol 2000-RGD-liposomes ([DSPE]-PEG2000-RGD-LPs/QCT) for lung cancer targeting treatment.
Methods: The physicochemical parameters of (DSPE)-PEG2000-RGD-LPs/QCT were characterized in terms of the particle size, zeta potential, morphology, entrapment efficiency, drug loading, and in vitro release behavior. In vivo, pharmacokinetics and antitumor studies of prepared formulations were also evaluated.
Results: In this study, QCT was found to be easily dispersed in lipid solution and entrapped by the thin-film hydration method. The encapsulation ratio and drug loading of prepared LPs were 89.2%±7.4% and 9.2%±1.3% and the mean diameter was 93.4±7.2 nm from 3 batches. The results of in vitro experiments showed that the particle size of liposomes was suitable for the fenestrated vasculatures of cancer tissues via the enhanced permeability retention effect. In vitro, a relatively slow QCT release profile was observed in (DSPE)-PEG2000-RGD-LPs, and the release mechanism fit with the Higuchi equation better. In vivo imaging results indicated that RGD-modified LPs had very good tumor targeting ability. (DSPE)-PEG2000-RGD-LPs/QCT showed a significant antitumor activity in mice with A549 tumors.
Conclusion: Through this study, it was found that the RGD-modified LPs loaded with QCT could potentially be a very promising lung-targeted preparation.
Keywords: RGD, liposomes, quercetin, pharmacokinetic, antitumor studies
Introduction
As the second leading cause of death after heart disease,1 cancer includes a group of diseases characterized by irregular cell division and proliferation. Lung cancer is the most deadly type.2 The main reason behind the dismal survival statistics is that most lung cancer patients are terminally ill and cannot be cured with existing therapies.3 As far as we know, there is little information about quercetin (QCT) regulating the ultrastructural changes of lung cancer.
QCT, or 3, 3′, 4′, 5, 7-pentahydroxyflavone, 1 of the 6 subclasses of flavonoids, has a wide range of biological activities.4 The antitumor, antiallergic, and anti-inflammatory effects of QCT have been extensively reviewed.5,6 Their biological activities mainly include electron transfer of free radicals, the activation of antioxidant enzymes, and the ability to inhibit oxidative stress.7 There is evidence that QCT is capable of targeting different types of cancer cells including those of leukemia, breast cancer, esophageal cancer, colon cancer, prostate cancer, nasopharyngeal carcinoma, endometrial cancer, and lung cancer.8,9 The proliferation of these malignant cells may be inhibited by QCT; however, the exact molecular mechanisms underlying the effects of QCT are unknown. For the treatment of these pulmonary diseases, the required therapeutic agents must be taken for extended periods of time. Moreover, the long-term use of QCT may generate many undesirable side effects such as tubule adenoma, renal failure, and liver cancer.10,11 In addition, QCT has low water solubility, poor absorption, and rapid metabolism (bioavailability of about 1%–5%),12 all of which can produce in vivo results that differ from the powerful in vitro efficacy of QCT. Therefore, the loading of QCT in nanoscale droplets can improve its pharmacokinetic profile, and, with the use of ultrasound-triggered rupturing, enable effective drug delivery to provide anticarcinogenic effects.13,14
RGD is a short peptide containing arginine–glycine–aspartic acid and widely exists in the human body. The extracellular matrix and adhesion proteins in blood, including fibrinogen, fibronectin, and collagen, usually contain RGD sequences.15 RGD peptide acts as a recognition site for integrins and their ligands, and allows for adhesion between mediated cells, extracellular matrix, and cells. In recent years, targeting angiogenesis has become an important topic in cancer research. It is generally believed that tumor growth, invasion, and metastasis are caused by angiogenesis. If the blood vessels feeding the tumor were insufficient, the tumor would be necrotic or apoptotic.16 At the same time, molecular biological studies have shown that integrins exist on the cell surface and play an important role in tumor angiogenesis.17 Integrin receptors, especially ανβ3, are highly expressed in some types of tumor cells and vascular endothelial tumor cells, but not in the normal vessels.18 It is also indicated that exogenous RGD peptides can competitively inhibit the ligand binding of integrins, thus inhibiting angiogenesis and migration of tumor cells. At the same time, tumors can be target-marked and anticancer drugs can be target-delivered.19–21 In the present work, we encapsulated QCT in nanoscale liposome using the lipid carriers. The surface morphology and particle size distribution were characterized. The drug loading (DL%), encapsulation ratio (ER%), and in vitro release of the drug were also studied. In addition, pharmacokinetics and antitumor studies of RGD-QCT liposomes were evaluated.
Materials and methods
Materials
QCT was purchased by Natural-Standard Biopharma Co., Ltd (Shanghai, People’s Republic of China). Distearoyl-L-a-phosphatidylethanolamine (DSPE)-PEG2000-RGD was provided by the HuiJia Biopharma Co., Ltd (Xiamen, People’s Republic of China). Soybean phosphatidylcholine, DSPE-PEG2000, and cholesterol were obtained from Sinopharm Chemical Reagent (Shanghai, People’s Republic of China). A549 cell line was purchased from Kobai Biomedical Ltd., Co. (Nanjing, People’s Republic of China). All other reagents were obtained from Sinopharm Chemical Reagent. Methanol and acetonitrile (chromatographic grade) were obtained from Sigma (Aldrich, St Louis, MO, USA). Deionized water used throughout the research was produced using a Milli-Q System (Millipore Corporation, Burlington, MA, USA).
Animals
Experiments were carried out on rats weighing 220±20 g and C57BL/6 mice weighing 20±2 g. The animals were kept in cages in a room at a temperature of 25°C±2°C, with a 12:12 light–dark cycle. There was a free supply of food and water. All experiments were carried out in strict accordance with the rules of experimental animal care used by Chinese national health institutions.
Preparation of liposomes
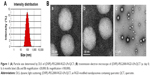
RGD-modified liposomes containing QCT were prepared by the thin-film hydration method as described previously.22 Briefly, QCT, soybean phosphatidylcholine, (DSPE)-PEG2000-RGD, and cholesterol (molar ratio: 2, 55, 5, and 38, respectively) were dissolved in 5 mL of chloroform to form a mixed solution; then the organic solvent was removed under reduced pressure at 40°C by rotary evaporation to form a thin solid film on the inner walls of the round-bottomed flask. This film was then flushed with nitrogen for 30 minutes and stored overnight in a desiccator to remove any traces of chloroform. Glucose and mannitol (1:1, w/w) were dissolved in PBS (pH =7.4). The lipid film was then hydrated with 5 mL of PBS (pH =7.4) at 55°C by rotation (180 rpm ×0.5 hour) to form (DSPE)-PEG2000-RGD-LPs/QCT (Figure 1A, appearance morphology). For the preparation of different QCT LPs distearoyl-L-a-phosphatidylethanolamine-polyethylene glycol 2000-RGD-liposomes ([DSPE]-PEG2000-LPs/QCT; [DSPE]-PEG2000-RGD-LPs/QCT), a similar procedure was carried out.
Characteristics
Nanoliposome morphology was observed with an optical microscope. In addition, high-resolution images were obtained of the lyophilized particles using a transmission electron microscope (Philips CM120, Philips, Amsterdam, the Netherlands). In practice, the LPs solution containing 0.1% (w/v) phosphotungstic acid was applied to the copper mesh carbon film and observed by electron microscopy at 80 kV. Particle size distribution and average diameter of (DSPE)-PEG2000-RGD-LPs/QCT were determined by dynamic light scattering using a NICOMP 380 Submicron Particle Sizer (Particle Sizing Systems, Santa Barbara, CA, USA) equipped with a 5 mW heliumneon laser at 632.8 nm. Zeta potential was measured on the same samples prepared for size analysis.
The QCT loading was determined as previously described.23 Briefly, QCT was extracted from the LPs with methanol; then, the QCT concentration was determined at 254 nm using high-performance liquid chromatography method with a QCT calibration curve (1.0–10 μg/mL concentration range). The calibration curve was A =0.0654C +0.0031, r=0.997. Then, DL% and ER% were calculated according to Eq (1) and Eq (2):
|
|
|
|
where WP is the weight of the initial feeding polymer, WM is the weight of drug incorporated in microspheres, and WF is the weight of the initial feeding drug.
Stability
The liposomes were stored for 6 months at 4°C and 20°C, respectively. The morphology and drug content of liposomes were detected regularly.
In vitro release
The in vitro drug release from different formulations was determined in PBS (pH=7.4) containing 10% alcohol at the temperature of 37°C±0.5°C. Hundred milligram QCT-related formulations were filled into a dialysis bag (molecular weight cut-off =12,000) immersed fully in 18 mL of the release medium under magnetic stirring at 75 rpm. At the scheduled time points, 1 mL of the release medium was taken out and replaced with the same amount of fresh release medium. The QCT contents were determined by the high-performance liquid chromatography method. All release experiments were carried out as triplicates. All measurements were used to calculate cumulative drug release. The release behavior of QCT in LPs was analyzed with the different release model.
In vivo evaluation
All the in vivo experimental protocols were approved by the animal care committee of the Second Hospital of Anhui Medical University, and all experiments were conducted in strict accordance with the laboratory animal care and usage guidelines adopted by the National Institutes of Health (Hefei, People’s Republic of China). The pharmacokinetics of QCT preparations was observed in 18 rats after intravenous administration. Rats were randomly divided into 3 groups, given a 5 mg/kg intravenous dose of QCT injection, (DSPE)-PEG2000-LPs/QCT, and (DSPE)-PEG2000-RGD-LPs/QCT via the tail vein. Blood samples (0.5 mL) were collected from the orbital cavity into heparinized tubes at 10 and 30 minutes and 1, 2, 4, 8, 12, 24, and 48 hours after intravenous administration. The blood was immediately processed for plasma by centrifugation at 2,000× g for 10 minutes. Plasma samples were frozen at −70°C until analysis.
In vivo imaging in tumor-bearing mice
The noninvasive optical imaging systems were used to observe the real-time distribution of the functional targeting LPs in A549 cells of xenograft mice. DiR, a lipophilic near-infrared probe, was encapsulated into the LPs as a fluorescence probe at a dose of 0.1 mg/kg. For the preparation of different QCT LPs containing DiR, a similar process was carried out. Tumor-bearing mice models were built according to the previous report. In brief, 1×109 A549 cells were suspended in 200 μL Dulbecco’s Modified Eagle’s Medium and injected into the right forelimb flank of mice. When tumor volume reached ~100–150 mm3, mice were injected with free QCT + DiR, DiR + (DSPE)-PEG2000-LPs/QCT, and DiR + (DSPE)-PEG2000-RGD-LPs/QCT (0.2 mg/kg) via the tail vein (3 each group). After anesthesia, the mice were scanned with the body image system (GelLogic 212 PRO, Carestream Health, Rochester, NY, USA) at 2, 4, 10, and 24 hours, and the systems were equipped with an excitation band-pass filter at 750 nm and an emission at 780 nm. The exposure time of the fluorescence images was 60 seconds.
Antitumor efficacy in tumor-bearing mice
C57BL/6 mice were inoculated with A549 cells as described. The treatments began on the day when the tumor volume reached 100–150 mm3, which was the eighth day of the whole experiment. On day 8, the mice were randomly divided into four groups (blank LPs, QCT injection, [DSPE]-PEG2000-LPs/QCT, and [DSPE]-PEG2000-RGD-LPs/QCT) (n=10). A total of 10 mg/kg samples were injected intravenously via the tail vein every 3 days. Four administrations were carried out in total. During the study, the tumor size and body weight were measured 3 times a week. On the 30th day, the mice were sacrificed and the resected tumor weighed. The test drug administration procedure was as follows: weighing and first injection on day 0, 3, 9, and 12 and euthanasia on day 30. The diameter of tumor was measured using a digital caliper. The tumor volume (mm3) was calculated with the following formula: tumor volume = length × width2 ×0.5. In the course of the study, rats were regularly weighed to monitor potential toxicity.
Results and discussion
Characteristics
As shown in Figure 1Ba and Bc, the surface morphology of (DSPE)-PEG2000-RGD-LPs/QCT was observed by transmission electron microscopy. The LPs were spherical, smooth and uniform in size, and suitable for intravenous administration. Various strategies, such as polymeric nanoparticles, microspheres, liposomes, and solid lipid nanoparticles, were assessed for the sustained delivery of activated agents to the lungs. Peptide-modified target preparation has become a popular drug carrier system in recent years. In this study, water-insoluble reagents, such as QCT, were found to be easily dispersed in lipid solution and entrapped by the thin-film hydration method. The method is simple and reproducible with high entrapment efficiency and ideal DL. The ER% and DL% of prepared LPs were 89.2%±7.4% and 9.2%±1.3% (n=6), respectively, and the mean diameter was 93.4±7.2 nm for 3 batches. The polydispersity index was 0.14±0.02, and the zeta potential was −20.4±0.6 mV (Table 1). The results of in vitro experiments showed that the particle size of liposomes was suitable for the fenestrated vasculatures of cancer tissues through the enhanced permeability retention effect. In addition, compared with normal liposomes, the size of (DSPE)-PEG2000-RGD-LPs/QCT was not significantly affected by RGD modification. The zeta potentials of (DSPE)-PEG2000-RGD-LPs/QCT were negative due to the addition of DSPE-PEG2000. High ER% and DL% of drugs in LPs would help prevent the rapid leakage during the process of drug delivery and the accumulation of drug in tumor tissues.
Stability
The stability data of (DSPE)-PEG2000-RGD-LPs/QCT showed that after storage at 4°C or room temperature 20°C for 6 months, the surface morphology and other physicochemical properties of QCT had no notable changes (Table 1 and Figure 1Bb).
In vitro release
The release behavior of QCT in different formulations in vitro was studied by the dialysis bag method. The release profiles of free QCT, (DSPE)-PEG2000-LPs/QCT, and (DSPE)-PEG2000-RGD-LPs/QCT were shown in Figure 2. We observed that the release of free QCT was rapid, while the cumulative release rates of the other 2 QCT liposomes were much slower, followed by a sustained release. In the free QCT group, 90% of QCT was released in the first 2 hours. In contrast, only 30% or 65% of QCT were released from DSPE-PEG2000-RGD-LPs/QCT within the initial 2 or 48 hours (P<0.01).
The kinetics of in vitro release was analyzed according to the zero-order, first-order, and diffusion controlled release mechanisms. As shown in Table 2, by analyzing the amount of drug released vs the square root of time, a relatively high correlation coefficient was obtained, indicating that the release followed the Higuchi kinetic model. The results showed that (DSPE)-PEG2000-RGD-LPs/QCT had good sustained release effect.
  | Table 2 Dissolution kinetic parameters of QCT from (DSPE)-PEG2000-RGD-LPs/QCT (n=3) |
In vivo evaluation
Pharmacokinetic studies were carried out in rats using different QCT formulations. The time course of the plasma concentrations of QCT injection, (DSPE)-PEG2000-LPs/QCT, and (DSPE)-PEG2000-RGD-LPs/QCT are summarized in Figure 3. Table 3 lists the pharmacokinetic parameters calculated from the plasma drug concentration vs time profiles.
There were significant differences in half-life (t1/2), area under the curve (AUC0–t), AUC0–∞, mean residence time, and plasma clearance between the QCT injection and (DSPE)-PEG2000-RGD-LPs/QCT (P<0.05). As shown, after the single QCT injection, the plasma drug concentration quickly reached the maximum level (4,563.2±398.5 ng/mL) in 10 minutes, before it decreased rapidly, leaving around 10% of the maximum concentration value 2 hours later. This implied that a rapid in vivo elimination of QCT existed in rats. In the case of intravenous administration, the in vivo profile of (DSPE)-PEG2000-LPs/QCT was smoother than the QCT injection group. The t1/2 and AUC0–∞ of (DSPE)-PEG2000-LPs were 2.72-fold and 3.62-fold higher, respectively, compared with free drug. Therefore, it is reasonable to conclude that LPs could significantly extend the role of QCT in vivo (provide higher bioavailability). Meanwhile, (DSPE)-PEG2000-RGD-LPs/QCT provided higher AUC0–∞, mean residence time, and t1/2 when compared with (DSPE)-PEG2000-LPs/QCT. However, (DSPE)-PEG2000-RGD-LPs/QCT showed decreased clearance when compared with the (DSPE)-PEG2000-LPs/QCT. There was no significant difference in pharmacokinetic parameters between the 2 liposomes.
In vivo imaging in tumor-bearing mice
The biodistribution of (DSPE)-PEG2000-RGD-LPs/QCT was evaluated by in vivo fluorescence imaging, and Figure 4 shows the real-time imaging observation after intravenous administration of varying DiR formulations. Results showed that strong fluorescent signals were observed in blood circulation at the tumor site after administration of (DSPE)-PEG2000-RGD-LPs/QCT + DiR, and fluorescent signals remained at the tumor site for 24 hours. In contrast, the fluorescent signal of the (DSPE)-PEG2000-LPs/QCT + DiR group could not be detected at the tumor site at 24 hours. Free DiR was mainly distributed in the liver without any signal in the tumor site, suggesting that the free DiR was not specific to the tumor tissue. According to the experimental results, the high tumor targeting ability of (DSPE)-PEG2000-RGD-LPs/QCT + DiR might be related to the combination of enhanced permeability retention effect and receptor-mediated cell uptake. Although in vitro data provided an important preliminary background to the promotion of the potential use of RGD-modified QCT LPs, these results should be further confirmed and verified in vivo.
Antitumor efficacy in tumor-bearing mice
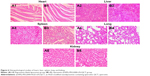
Figure 5 summarizes body weight changes and tumor volume changes after treatments with varying QCT formulations. Figure 5A shows tumor volume changes. (DSPE)-PEG2000-RGD-LPs/QCT-treated mice showed a significantly suppressed tumor growth and there was no recurrence after 30 days of treatment, while free QCT- and (DSPE)-PEG2000-LPs/QCT-treated groups, similar to the control group, showed ever-increasing tumor growth. As can be seen in Figure 5B, the body weight of mice in treatment groups did not decline significantly compared with blank LPs groups. Figure 6 exhibits the hematoxylin and eosin staining assay of hearts, livers, spleens, lungs, and kidneys in tumor-bearing mice. Compared with the control group, there was no obvious abnormality or organ damage after treatments with RGD-modified preparations. The sustained release of QCT from DSPE-PEG2000-LPs revealed its applicability as a drug delivery system that minimizes the exposure of healthy tissues and increases the accumulation of therapeutic drug in tumor sites. In lung cancers, the overexpression of cell surface receptors is often exploited for targeted delivery of therapeutics with ligand-/antibody-modified nano-drug delivery vehicles. The integrin (ανβ3) receptor is of particular interest, since its expression is high in tumor endothelium and tumor cells. Using RGD peptide to target integrin in tumor vascular endothelium is a well-known strategy to suppress angiogenesis and metastasis. The expression of integrins is relatively weak in normal cells. Overall, the results indicated that the RGD-modified QCT LPs could more significantly increase tumor inhibition ability compared with ordinary liposomes.
Disclosure
The authors report no conflicts of interest in this work.
References
He Z, Xia Y, Tang S, Chen Y, Chen L. Detection of occult tumor cells in regional lymph nodes is associated with poor survival in pN0 non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2016;8(3):375–385. | ||
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. | ||
Yamamoto S, Huang D, du L, et al. Radiogenomic analysis demonstrates associations between (18)F-fluoro-2-deoxyglucose PET, prognosis, and epithelial-mesenchymal transition in non-small cell lung cancer. Radiology. 2016;280(1):261–270. | ||
Kelly GS. Quercetin. Monograph. Altern Med Rev. 2011;16(2):172–194. | ||
Guazelli CF, Fattori V, Colombo BB, et al. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J Nat Prod. 2013;76(2):200–208. | ||
Liu H, Xue JX, Li X, Ao R, Lu Y. Quercetin liposomes protect against radiation-induced pulmonary injury in a murine model. Oncol Lett. 2013;6(2):453–459. | ||
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–584. | ||
Linsalata M, Orlando A, Messa C, Refolo MG, Russo F. Quercetin inhibits human DLD-1 colon cancer cell growth and polyamine biosynthesis. Anticancer Res. 2010;30(9):3501–3507. | ||
Chuang-Xin L, Wen-Yu W, Yao C, Xiao-Yan L, Yun Z. Quercetin enhances the effects of 5-fluorouracil-mediated growth inhibition and apoptosis of esophageal cancer cells by inhibiting NF-κB. Oncol Lett. 2012;4(4):775–778. | ||
Wang JB, Ma YG, Zhang P, et al. [Effect of processing on the chemical contents and hepatic and renal toxicity of rhubarb studied by canonical correlation analysis]. Yao Xue Xue Bao. 2009;44(8):885–890. Chinese. | ||
National Toxicology Program. NTP toxicology and carcinogenesis studies of EMODIN (CAS NO. 518-82-1) feed studies in F344/N rats and B6C3F1 mice. Natl Toxicol Program Tech Rep Ser. 2001;493:1–278. | ||
Reinboth M, Wolffram S, Abraham G, Ungemach FR, Cermak R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br J Nutr. 2010;104(2):198–203. | ||
Wang P, Heber D, Henning SM. Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells. Nutr Cancer. 2012;64(4):580–587. | ||
Ghosh A, Ghosh D, Sarkar S, Mandal AK, Thakur Choudhury S, Das N. Anticarcinogenic activity of nanoencapsulated quercetin in combating diethylnitrosamine-induced hepatocarcinoma in rats. Eur J Cancer Prev. 2012;21(1):32–41. | ||
Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. | ||
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. | ||
Pasqualini R, Arap W, McDonald DM. Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med. 2002;8(12):563–571. | ||
Zetter BR. On target with tumor blood vessel markers. Nat Biotechnol. 1997;15(12):1243–1244. | ||
Danhier F, Le Breton A, Préat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9(11):2961–2973. | ||
Garanger E, Boturyn D, Dumy P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med Chem. 2007;7(5):552–558. | ||
Zitzmann S, Ehemann V, Schwab M. Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res. 2002;62(18):5139–5143. | ||
Daeihamed M, Dadashzadeh S, Haeri A, Akhlaghi MF. Potential of liposomes for enhancement of oral drug absorption. Curr Drug Deliv. 2016;14:289–303. | ||
Doshi GM, Une HD. Quantification of quercetin and rutin from Benincasa hispida seeds and Carissa congesta roots by high-performance thin layer chromatography and high-performance liquid chromatography. Pharmacognosy Res. 2016;8(1):37–42. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.