Back to Journals » International Journal of Nanomedicine » Volume 19
Revolutionizing Psoriasis Topical Treatment: Enhanced Efficacy Through Ceramide/Phospholipid Composite Cerosomes Co-Delivery of Cyclosporine and Dithranol: In-Vitro, Ex-Vivo, and in-Vivo Studies
Authors Elhabal SF , Abdelaal N, Al-Zuhairy SAS , Mohamed Elrefai MF , Khalifa MM , Khasawneh MA , Elsaid Hamdan AM , Mohie PM, Gad RA, Kabil SL, El-Ashery MK, Jasti BR , Elzohairy NA, Elfar N, Elnawawy T, Hassan FE, El-Nabarawi MA
Received 28 October 2023
Accepted for publication 28 December 2023
Published 7 February 2024 Volume 2024:19 Pages 1163—1187
DOI https://doi.org/10.2147/IJN.S443812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Sammar Fathy Elhabal,1 Nashwa Abdelaal,2 Saeed AS Al-Zuhairy,3 Mohamed Fathi Mohamed Elrefai,4,5 Mohamed Mansour Khalifa,6 Mohammad Ahmad Khasawneh,7 Ahmed Mohsen Elsaid Hamdan,8 Passant M Mohie,9 Rania A Gad,10 Soad L Kabil,11 Mohamed Kandeel El-Ashery,12,13 Bhaskara R Jasti,14 Nahla A Elzohairy,15,16 Nehal Elfar,17 Tayseer Elnawawy,18 Fatma E Hassan,19,20 Mohamed Ahmed El-Nabarawi21
1Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Modern University for Technology and Information (MTI), Mokattam, Cairo, Egypt; 2Department of Integrative Physiology, Baylor College of Medicine, Houston, TX, USA; 3Department of Pharmacy, Kut University College, Kut, Wasit, Iraq; 4Department of Anatomy, Histology, Physiology, and Biochemistry, Faculty of Medicine, The Hashemite University, Zarqa, Jordan; 5Department of Anatomy and Embryology, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 6Department of Human Physiology, Faculty of Medicine, Cairo University, Cairo, Egypt; 7Department of Chemistry, College of Science, U.A.E. University, Al-Ain, United Arab Emirates; 8Department of Pharmacy Practice, Faculty of Pharmacy, University of Tabuk, Tabuk, Saudi Arabia; 9Department of Clinical Pharmacology, Faculty of Medicine, Alexandria University, Alexandria, Egypt; 10Department of Pharmacology and Toxicology, Faculty of Pharmacy, Nahda University, Beni-Suef, Egypt; 11Department of Clinical Pharmacology, Faculty of Medicine, Zagazig University, Zagazig, Egypt; 12Pharmaceutical Chemistry Department, Faculty of Pharmacy, Cairo University, Cairo, Egypt; 13Medicinal Chemistry Department, Faculty of Pharmacy, King Salman International University, Ras-Sedr, South Sinai, Egypt; 14Department of Pharmaceutics and Medicinal Chemistry, Thomas J. Long School of Pharmacy & Health Sciences, University of the Pacific, Stockton, CA, USA; 15Air Force Specialized Hospital, Cairo, Egypt; 16Department of Microbiology and Immunology, Faculty of Pharmacy, Modern University for Technology and Information (MTI), Mokattam, Cairo, Egypt; 17Department of Pharmaceutical Technology, Faculty of Pharmacy, Horus University, New Demiette, Egypt; 18Department of Pharmaceutics, Egyptian Drug Authority, Cairo, Egypt; 19Medical Physiology Department, Kasr Alainy, Faculty of Medicine, Cairo University, Giza, Egypt; 20General Medicine Practice Program, Department of Physiology, Batterjee Medical College, Jeddah, Saudi Arabia; 21Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
Correspondence: Mohammad Ahmad Khasawneh, Email [email protected]
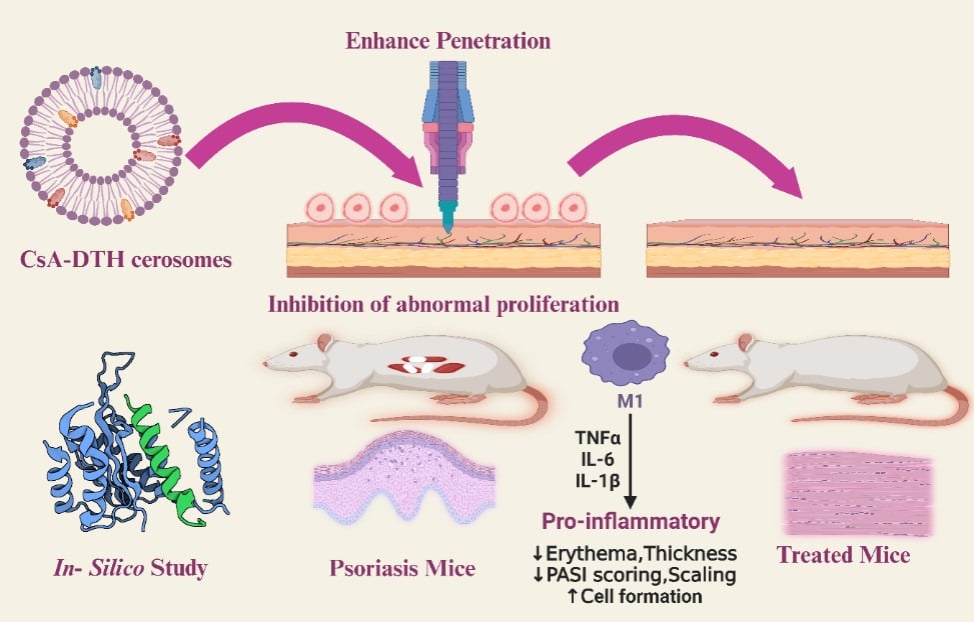
Purpose: Improving the treatment of psoriasis is a serious challenge today. Psoriasis is an immune-mediated skin condition affecting 125 million people worldwide. It is commonly treated with cyclosporine-A (CsA) and dithranol (DTH). CsA suppresses the activation of T-cells, immune cells involved in forming psoriatic lesions. Meanwhile, DTH is a potent anti-inflammatory and anti-proliferative drug that effectively reduces the severity of psoriasis symptoms such as redness, scaling, and skin thickness. CsA and DTH belong to BCS class II with limited oral bioavailability. We aim to develop a drug delivery system for topical co-delivery of CsA and DTH, exploring its therapeutic potential.
Methods: Firstly, we developed a niosomal drug delivery system based on ceramide IIIB to form Cerosomes. Cerosomes were prepared from a mixture of Ceramide, hyaluronic acid, and edge activator using a thin-film hydration technique. To co-deliver CsA and DTH topically for the treatment of psoriasis. These two hydrophobic drugs encapsulated into our synthesized positively charged particle cerosomes.
Results: Cerosomes had an average particle size of (222.36 nm± 0.36), polydispersity index of (0.415± 0.04), Entrapment Efficiency of (96.91%± 0.56), and zeta potential of (29.36± 0.38mV) for selected formula. In vitro, In silico, in vivo, permeation, and histopathology experiments have shown that cerosomes enhanced the skin penetration of both hydrophobic drugs by 66.7% compared to the CsA/DTH solution. Imiquimod (IMQ) induced psoriatic mice model was topically treated with our CsA/DTH cerosomes. We found that our formulation enhances the skin penetration of both drugs and reduces psoriasis area and severity index (PASI score) by 2.73 times and 42.85%, respectively, compared to the CsA/DTH solution. Moreover, it reduces the levels of proinflammatory cytokines, TNF-α, IL-10, and IL-6 compared to CsA/DTH solution administration.
Conclusion: The Cerosomes nano-vesicle-containing CsA/DTH represents a more promising topical treatment for psoriasis, giving new hope to individuals with psoriasis, compared to commercial and other conventional alternatives.
Keywords: biopharmaceutics classification system class II, BCS II, niosomes based on ceramide IIIB, cerosomes, psoriasis, psoriasis area and severity index, PASI score, imiquimod, IMQ, proinflammatory cytokines, niosomes
Graphical Abstract:
Introduction
According to the National Psoriasis Foundation, psoriasis is a chronic, immune-mediated skin disorder that affects 125 million people worldwide. It is characterized by red, scaly patches on the skin that can be itchy and painful. Psoriasis occurs when the immune system mistakenly attacks healthy skin cells, causing them to grow and divide more quickly than normal. This results in the formation of thick, scaly patches on the skin.1 Plaque psoriasis, guttate psoriasis, inverse psoriasis, pustular psoriasis, and erythrodermic psoriasis are among the several forms of psoriasis. The severity of psoriasis varies according to the affected skin area, from mild cases that only affect small areas to severe cases that cover large body areas. Psoriasis cannot be cured, yet numerous ways exist to manage the symptoms. Topical treatments that reduce inflammation and slow down skin cell proliferation include corticosteroids, retinoids, and vitamin D analogs that can be applied directly to the affected skin. To lower inflammation and slow down the proliferation of skin cells, phototherapy, also known as light therapy, involves exposing the skin to UV light.2
Systemic treatments, such as methotrexate, cyclosporine, and biological agents, can be taken orally or by injection to suppress the immune system and reduce inflammation. One example of a drug used to manage psoriasis is adalimumab, a biological agent targeting a specific protein in the immune system called tumor necrosis factor-alpha (TNF-α). Through blocking TNF-α, adalimumab can reduce inflammation and slow down skin cell growth, improving psoriasis symptoms. Adalimumab is administered by injection and is typically used for moderate to severe cases of psoriasis that have not responded well to other treatments.3 Current treatments include corticosteroids, keratolytics, phototherapy, calcineurin inhibitors, biologics targeting proinflammatory mediators, vitamin D analogs, and Cyclosporine is an immunosuppressive drug that has been used for many years to prevent rejection in organ transplantation and to treat a variety of immune-mediated conditions. It exerts its effect by suppressing the immune system by suppressing the immune system by inhibiting the activation of T-cells, immune cells that play a role in developing autoimmune diseases and transplant rejection.
Cyclosporine(CsA) can reduce the inflammation and prevent the tissue and organ damage. In addition to its use in Organ transplantation to prevent rejection, including kidney, liver, and heart transplants, psoriasis can reduce the severity of symptoms in psoriasis, including redness, scaling, and skin thickness. CsA is used to treat rheumatoid arthritis by reducing joint inflammation and pain in rheumatoid arthritis, Nephrotic syndrome by reducing proteinuria and improving kidney function in nephrotic syndrome, Atopic dermatitis, Inflammatory bowel disease (IBD), used to treat severe cases of ulcerative colitis or Crohn’s disease and Myasthenia gravis consider as second-line treatment for myasthenia gravis. This autoimmune disorder affects muscle weakness and fatigue. In Myasthenia gravies, CsA is considered a second-line treatment. Besides, CsA is typically administered orally or by injection and is usually used with other medications, such as corticosteroids, to maximize its effectiveness and reduce the risk of side effects.4
Dithranol (DTH)(anthralin) is a synthetic compound derived from the natural compound anthracene. Dithranol has anti-proliferative Action by inhibiting the excessive growth and proliferation of keratinocytes, a hallmark of psoriasis. Psoriasis is characterized by the rapid turnover of skin cells, forming thickened, scaly plaques. Dithranol helps normalize this process by slowing down the abnormal growth of skin cells; it also has anti-inflammatory properties that can help reduce redness and scaling in psoriasis. It is thought to work by inhibiting the production of inflammatory cytokines, immune system proteins that contribute to the development of psoriatic lesions.5 DTH has been used for many years to treat a variety of skin conditions, including psoriasis, through helping in slowing down the excessive growth of skin cells in psoriasis and in reducing the inflammation, leading to an improvement in the appearance of psoriatic lesions, Eczema by reduce inflammation and itching in eczema, a chronic skin condition that causes redness, itching, and dryness. DTH can be used to treat alopecia areata. This autoimmune disorder causes hair loss on the scalp, face, and other body parts. Pityriasis lichenoides by reduces the appearance of skin lesions in pityriasis lichenoides. This rare skin condition causes red, scaly patches. Dithranol can treat early-stage cutaneous T-cell lymphoma, a type of non-Hodgkin’s lymphoma affecting the skin.6
Collectively, CsA and DTH belong to BCS II and have low solubility and high permeability.7 Cerosomes are a novel noisome-based delivery system developed to improve the solubility and bioavailability of poorly soluble drugs, such as CsA and DTH. Niosomes are non-ionic surfactant vesicles that can encapsulate hydrophobic drugs, improving their solubility and stability.
They encapsulate the drug in a niosome coated with a ceramide lipid layer.8 The ceramide layer helps to stabilize the niosomes and improve their permeation through the skin. The niosome core also provides a drug reservoir that can be slowly released over time, improving the drug’s bioavailability and reducing the need for frequent application. In the case of CsA and DTH, cerosomes can improve their solubility by solubilizing the drugs in the niosome core, which is then stabilized by the ceramide layer. This allows the drugs to be delivered through the skin more effectively, improving their efficacy in treating psoriasis. Ceramides, CH, and fatty acids comprise most of the intercellular lipids of the skin’s stratum corneum (SC), which are structurally structured into multilamellar bilayers.9 Sphingolipids, or ceramides, are naturally occurring lipids that comprise most intercellular lipids in the SC (45–50% of all intercellular lipids). Moreover, Ceramides promote epidermal self-renewal, control skin immunological response, and preserve epidermal barrier function to maintain skin homeostasis. Ceramides are most effective as a skin treatment when administrated in a form resembling the lipid bilayer found in the SC lipid matrix.10 According to studies, the psoriasis area severity index (PASI) score is inversely correlated with a considerable decrease in total ceramides in the skin. One of the fundamental medicinal ingredients utilized in psoriasis formulations is ceramide. Ceramides are most effective as a skin treatment when administered in a form resembling the lipid bilayers found in the SC lipid matrix.11 Utilizing the effects of ceramides on the skin, ceramide-based liposomes, coassembled nanovehicles, and ceramide-doped vesicular systems have been developed to deliver medications for topical treatment.12,13 We postulate that creating niosomes based on ceramides (called cerosomes) can aid in the percutaneous administration of a combination of CsA and DTH and psoriasis treatment in light of the properties above of ceramides and niosomes. This study proposes and investigates for the first time the therapeutic effects of CsA and DTH coloaded ceramide niosomes (CsA/ DTH cerosomes) in Imiquimod (IMQ)-induced psoriasis-like mouse models.14
The objective of the present work is to develop. It examines the therapeutic efficacy of ceramide-niosomes loaded with CsA and DTH in mice models with psoriasis-like symptoms produced by IMQ, marking the first instance of such investigation. The prepared particles were evaluated regarding particle size, size distribution, zeta potential, encapsulation efficiency, and microscopic examination, analysis by Fourier transform-infrared (FT-IR) and Differential Scanning Chromatography(DSC). Furthermore, the dialysis membrane was also used to determine the drug release in vitro. Cerosomes exhibit notable improvements in the solubility of CsA and DTH while concurrently decreasing the synthesis of proinflammatory mediators. Permeation tests were conducted both in-vitro, in-silico, and in-vivo to assess the permeability of CsA/DTH cerosomes compared to CsA/DTH solution. The in-vitro investigation examined the antiproliferation and uptake properties of CsA/DTH cerosomes in HaCaT cells. The antipsoriatic effects in the IMQ-induced psoriasis mice model were assessed using PASI scores, histological examination, and measurements of proinflammatory cytokine levels.
Materials and Methods
Drugs, Chemicals, and Reagent Kits
Cyclosporine-A (CsA) and Dithranol(DTH) were bought from HiMedia Laboratories in Secunderabad, India, and Shanghai Aladdin Biochemical Technology Co., Ltd. in Shanghai, respectively. Both hyaluronic acid and chinL-a phosphatidylcholine from egg yolk were bought from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Stearylamine (SA) was purchased. Evonic Co. (Germany) graciously donated Ceramide IIIB. The BASF Co. (New Jersey, NY, USA) donated the kolliphor RH40 and TPGS. At El-Nasr Pharmaceutical Chemicals Co., Cairo, Egypt, methanol and chloroform were purchased. Market Aldara (Imiquimod (IMQ) cream (5%, w/w)) was purchased from Meda Pharmaceuticals, a global healthcare. Acetonitrile (HPLC grade, > 99.9%) and ethanol were acquired from Fischer Scientific in New Jersey, USA, and were the bag dialysis method of HPLC grade. A typical molecular weight threshold for a dialysis membrane is 14000 Da (source: Sigma-Aldrich Co.). Analytical-grade solvents and chemicals were used throughout the study.
Animals
The Experimental Cairo University’s Faculty of Pharmacy’s Animal House in Giza, Egypt, sold thirty mice (male, 29–35 g) and C57BL/6 mice (male, 7–10 weeks old). Divided into five groups, each group containing six mice were employed. The animals were kept in specialized pathogen-free environments with constant humidity, temperature, and 12-hour light/dark cycles, and they were given access to food and water at will. All research techniques were approved by the National Research Center’s Animal Care Ethics Committee (PI 3219). Animal experiments were conducted according to the guidelines set forth by the Faculty of Pharmacy at Cairo University’s Study Ethics Committee (REC). In this study, mice were handled and used in compliance with the European Union Directive on Animal Experimentation.
Method
Design and Optimization of Formulation
Surface response design was used to examine the effects of various formulation parameters on the properties of cyclosporine-A (CsA) and dithranol(DTH)(CsA-DTH)-cerosomes using Design Expert (Version 10.0.3.0, Stat-Ease Inc., Minneapolis, MN, USA). Table 1 illustrates (A): The independent variables were the amount of ceramide (mg), the amount of HA (mg), which was employed in two separate amounts (10 and 20 mg), and the kind of Edge activator (EA) (Kolliphor RH40 and TPGS). The dependent variables in all formulations were drug release percentage, polydispersity index, zeta potential, and particle size (PS). Analysis of variance (ANOVA) was used to determine the importance of the components and the model; significance is indicated by probability values (P<0.05). Thirteen different formulas were used in the testing runs. 3D surface graphs are displayed using the R software’s plot 3D package. The optimization was set to have the smallest size and smallest polydispersity index and a big zeta-potential and Drug release to obtain the best formula. The data were reported as mean and standard deviation after three iterations of each formulation.
 |
Table 1 Independent Variable Levels are Designated by the Categorical Multilevel Factorial Design, the Measured Dependent Responses, and Their Desirability Constraints |
Preparation of CsA and DTH – Cerosomes
Cerosomes were created using the thin-film hydration method. To summarize, 10 mL of chloroform: methanol (2:1; V/V) was used to dissolve ceramide IIIB and Edge activator (EA) type (Kolliphor RH40 or TPGS) in different amounts, along with egg yolk phospholipid (10 mg), stearyl amine (SA) as a positive charge inducer (5 mg), and Cyclopsorine-A (CsA) and DTH, as indicated in Figure 1. The organic phase was progressively evaporated at 60°C using a rotatory evaporator (Rotavapor, Heidolph VV 2000, Burladingen, Germany) at 90 rpm for 30 minutes to produce a thin, transparent layer of PCs. Using 10 mL of distilled water with HA at 60°C, above the lipid phase transition temperature (Tc), the film was fully hydrated in 45 minutes.8 The vesicle dispersion was kept at four °C for the entire night to create mature vesicles, as shown in Table 1.
 |
Figure 1 Graphic representation of the method employed for preparing CsA/DTH cerosomes using the thin-film hydration technique. |
In vitro Characterization of the Prepared CsA and DTH-Loaded Cerosomes
Determination of Entrapment Efficiency (EE%)
The percentage of entrapment efficiency (EE%) of Cerosomes NPs was determined by indirectly measuring free CsA and DTH (unentrapped CsA and DTH). The produced formula (1 mL) was centrifuged in a cooling centrifuge (Beckman Optima, Ultracentrifuge, California, USA) for 60 minutes at 4 °C.15 After the clear supernatant was extracted, the quantities of unentrapped CsA and DTH were measured using HPLC (C18 column 250×4.6 mm, 5 m particle size) (Shimadzu, Kyoto, Japan). This was done with a guard column (45 x 4.6 mm). The mobile phase was injected at a flow rate of 1.5 mL/min and a volume of 20 mL. It contained acetonitrile, acetic acid (1%), and water (5:50:45 v/v) at room temperature. An SPD-M10Avp diode array UV detector was used for detection at 405 (Shimadzu, Kyoto, Japan). The retention periods for DTH and CsA were determined to be 33 and 25 minutes, respectively. The calibration curve (concentration range 0.4871–0.8211 g mL−1, n = 3, R2= 0.9998) was used to find the unknown values. The EE% was determined by using the following formula16–18
Measurements of Particle Size, Distribution, and Zeta Potential
CsA-DTH cerosome nanoparticles were diluted in bidistilled water (1 mL to a total volume of 10 mL), and the Zetasizer 3600 (Malvern Instruments, Malvern, UK) was used to measure the particle size (PS) at 25 °C using the light scattering method (DLS). The range of laser obscuration was 10%–20%, while the angle of light scattering was fixed at 90°. Three cycles were performed on each sample. The relationship between the formula’s particle size and zeta potential was tested statistically using a one-way ANOVA.19
In-Vitro Release Studies
The in-vitro release of CsA-DTH from cerosome nanoparticles was determined using a Bag dialysis technique. For the dialysis membrane (typical molecular weight threshold of 14,000 Da; Sigma-Aldrich Co.), the release medium (phosphate buffered saline solution, pH 7.4) with 25% ethanol to maintain the sink state was soaked in the membrane overnight.15 The dialysis bag with 2 mL (or 0.5 mg of CsA-DTH) of each formula or CsA-DTH suspension was then put into amber bottles with a 25 mL release medium. After that, the bottles were put into a shaker set to run at 100 rpm and 37±0.5 °C. The 3 mL aliquots were taken and then replaced with an equivalent volume of fresh release medium at predefined intervals (0, 0.5, 1, 2, 4, 6, 8, 10, 12, and 48 h) to maintain the sink condition. For CsA-DTH, the estimated retention times were 25 and 33 minutes, respectively. The calibration curve was compared to 405 nm HPLC data (R2= 0.9997) to calculate the percentage liberated. The zero, initial, Higuchi diffusion and Korsmeyer-Peppas models were fitted to the release profiles. The most suitable model is the one with the highest coefficient (R2).18
Selection of the Optimum Formula
Design-Expert software version 10 (Stat-Ease, Inc., Minneapolis, Minnesota, USA) was used to apply numerical optimization to discover desired factors and eliminate non-significant ones. The selection criteria were as follows: ZP (absolute value), lowest PS, highest EE%, and in-vitro release (Q48h) > 80% (Table 2). The optimal formula (almost 1) was employed for more examination.13
In-Vitro Characterization of the Optimized Formula (C-D12)
Drug Polymer Interaction Using Fourier Transform Infrared (FT-IR) Spectroscopy
FTIR spectroscopy (Shimadzu 43,000 spectrophotometer, Kyoto, Japan) examined the chemical interactions between drugs and polymers. In a nutshell, CsA and DTH-loaded cerosomes samples were made into a CD using KBr. Spectra were captured with a 4000–400 cm−1 scanning range, and a mean spectrum of 32 scans with a 2 cm−1 resolution was acquired.15,19
Differential Scanning Calorimetry (DSC) Analysis
A Perkin-Elmer instrument was used to perform the Differential Scanning Calorimetry (DSC) analysis. Samples (6.5–10 mg) were heated on an aluminum pan at 100 °C/min in a nitrogen environment, with temperatures ranging from 0 to 400 °C. A nitrogen gas flow rate of 20 lb/in2 for the DSC experiment was utilized.11
Transmission Electron Microscopy (TEM)
Using a transmission electron microscope (TEM) (JEM-1230, Joel, Tokyo, Japan), the morphology of the optimized formula of cerosomes loaded with DTH and CsA was investigated. We diluted the optimized formula on a carbon-coated grid, stained it with 1% phosphotungstic acid solution, and let it dry until visible at room temperature.13
Short-Term Stability of Cerosomes
The improved formula cerosome nanoparticles were kept at four °C and 25 °C for three months, respectively. Using a Zeta-sizer Nano ZS from Malvern Instruments Ltd., Malvern, UK, the mean PS, ZP, drug release%, EE%, PDI, and Q48h values were assessed following sample collection. The tests were run in triplicate, and t–tests were used to find any significant statistical differences using GraphPad Instate version 3 software (GraphPad Instate Software, Inc. USA).2
Ex-Vivo Study
Ex-Vivo Skin Permeation Study
By the University of Cairo’s Ethics Committee for Animal Experimentation and under the direction of a veterinarian, the skin of male New Zealand mice (29–35g) was utilized to evaluate ex-vivo CsA and DTH permeation from CsA-DTH solution and CsA-DTH-cerosomes. The mice were given unrestricted access to ordinary food and water, which they may take whenever desired. The animals were administered sodium pentobarbital (100 mg/kg) overdoses through the marginal ear vein while under deep anesthesia, and the mice were slaughtered after receiving intramuscular ketamine hydrochloride (35 mg/kg) and xylazine (5 mg/kg) anesthesia. Each mice had the hair from its abdomen surgically removed. The skin was then submerged in 60 °C warm water for 45 seconds to check for fat adhesions. Extracted skin was soaked in distilled water, cleaned at −20 °C, and put away until needed. This investigation utilized a Franz diffusion apparatus to measure the drug’s penetration through the mice’s skin (CsA-DTH solution and CsA-DTH-cerosomes). Excised skin stored at 20 °C was moisturized for at least an hour before the experiment. The skin was then positioned in between the donor and receptor compartments. The donor compartment of the Franz cell apparatus was facing the stratum corneum (SC) side of the skin. The mice’s skin was then covered with a 1 cm2 piece of the prepared cerosomes. The designed cerosomes drug-releasing surface was directed toward the SC of the mice skin. Phosphate buffer (pH 7.4) was applied in the receptor compartment. Water circulated in the water jackets surrounding the receptor compartment to keep the temperature of the receptor fluid at 37± 0.5 °C. Magnetic beads were used to agitate the receptor fluids. A 2 mL sample of the receptor fluid was obtained at specified intervals of 0.5, 1, 1.5, 2, 4, 8, 12, 16, 20 and 24 hours. Fresh receptor fluid of an identical amount was introduced to the receptor compartment to maintain sink conditions. These samples underwent analysis to determine the amount of drugs they contained. The samples were examined using RP-HPLC.8,20 The results were presented as the mean and standard deviation of triplicates. To calculate the parameters and determine the X–intercept using linear regression analysis, the total CsA and DTH data were plotted against time. The permeability coefficient (Kp) (cm/h) (Eq. 2), steady-state flux (J) (µg/cm2/h), and amount penetrated after 48 hours (Q48) (g) were calculated.
Kp = J / C0 (Eq. 2)
where C0 is the starting drug concentration (µg/cm3), and J is the steady state flux considered as the slope of the linear part (µg/cm2/h).
The amount of drugs retained inside the tissue (QR) was determined after the recovery of the skin sample. The tissue was weighed, rinsed in distilled water, and sonicated for 1 hour in MQ water in an ultrasonic bath. The results were presented as the mean and standard deviation of triplicates.15
In silico Study
AutoDock Vina 1.1.221,22 the The free docking software was used to perform the in silico study. The protein data bank (https://www.rcsb.org/)23 was the source of protein files which were downloaded in pdb format, the code 2AZ5 for tumor necrosis factor-alpha “TNF-α”, 6Y8M for interleukin-1β “IL-1β” and 7XJ0 for Transient receptor potential vanilloid-3 (TRPV3). MGL tools 1.5.7. was utilized for the preparation of the protein and ligand files saved as pdbqt, “the required format for AutoDock V”. a. BIOVIA Discovery Studio Visualizer v21.1.0.20298 was used to visualize the formed protein-ligand interactions.
Cell Culture Assays, Determination of Proinflammatory Cytokine
Cytotoxicity Assays
In-vitro cytotoxicity testing was done on human skin epithelial cells (HSE-2). The cell line was purchased commercially from American Type Culture Collection ATCC Manassas, VA, USA),(ATCC PCS-201–012) from Vacsera Egypt For the examination of CsA-DTH solution and CsA-DTH-cerosomes for the aim of skin delivery, HSE-2 cells show potential appropriateness as a model. The HSE-2 cells were raised in a keratinocyte medium devoid of serum. The solution contained penicillin at a concentration of 100 ng/mL, streptomycin at a concentration of 100 mg/mL, hydrocortisone at a concentration of 500 ng/mL, insulin at a concentration of 0.005 mg/mL, and bovine serum at a volume-to-volume ratio of 10%. 5 ng/mL of epidermal growth factor and 0.005 mg/mL of bovine pituitary extract were added to the solution. According to the technique, cells were to be grown in a culture flask under precise guidelines until they reached 80% confluence. These settings included a humidified setting with 10% CO2 content, kept at a 37 °C temperature. MTT, especially the bromide of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazole, was used as a cell viability indicator in the viability experiments on the HSE-2 skin cell line. The cells were then moved to a 96-well plate after a 24-hour incubation at 37 °C. The cells were subsequently treated with a solution comprising CsA-DTH and CsA-DTH-cerosomes at doses ranging from 0.05 to 0.1 mg/mL of the drug. After being exposed to a fresh medium containing 0.25% MTT for two hours, the cells were rinsed with phosphate-buffered saline (PBS) and given another 24 hours. After removing the media, dimethyl sulfoxide (DMSO) was used to lyse the cells. Turner BioSystems, based in California, USA, produces the automated Modulus microplate reader that was used to measure the absorbance of cells at a wavelength of 560 nm. Compared to untreated (control) cells, the MTT experiment finding was presented as a percentage decrease of.15,18,24
Enzyme-Linked Immunosorbent Assay (ELISA)
To detect the levels of interleukin (IL)-6, interleukin (IL)-1β, and tumor necrosis factor (TNF-α), an enzyme-linked immunosorbent assay (ELISA) was utilized. ELISA kits from B.D. Biosciences, CA, USA, were utilized for this, following the guidelines provided by the manufacturer. The measurements were done using the treatment groups. Skin samples were kept at −80°C until they were used for the enzyme-linked immunosorbent assay (ELISA). These samples were taken during the psoriatic efficacy trial. Using a tissue homogenizer (Remi Electrokinetic, Ltd., Mumbai, India) at a speed of 3000 rpm for five minutes, the tissue samples were homogenized in a double quantity of an extraction buffer consisting of 10 mM Tris pH 7.4, 150 mM NaCl, and 1% Triton X-100. The homogenate samples were centrifuged for 15 minutes at 10,000 revolutions per minute at 4 degrees Celsius. The resulting supernatant was collected and stored at −80°C until it was time for analysis.14
In-Vivo Study
Imiquimod-Induced Psoriatic Plaque Model
For the experiment, 30 male C57BL/6 mice, aged 7 to 10 weeks, weighing 29 to 35 grams, were randomly divided into five equal groups in separate cages. The animal was chosen because research suggests it more clearly mimics human psoriasis than other species. A 12-hour light/dark cycle, room temperature (25–28°C), air humidity of 50–10%, normal laboratory animal food, and unrestricted access to water were all provided for the mice in their housing. The experimental investigation received clearance from Cairo University’s research ethics committee (approval no. PI 3219). An electric razor was used to shave a 4 cm2 region of the dorsal skin. Subjects receiving topical administration of 62.5 mg of 5% imiquimod (IMQ) Aldara cream on their shaved dorsal backs and 3.25 mg on their right ear pinna were administered daily for seven days in all groups except the healthy control group.8 An hour after applying the 5% IMQ cream, the prepared CsA-DTH loaded Cerosomes and the commercial ointment were applied. The division of the five groups was as follows:
Group A: Stable negative control mice that got Vaseline just applied to the inside of their right ear pinna and dorsal back.
Group B: untreated animals with IMQ-induced psoriasis as a negative control.
Group C: positive control (animals with psoriasis produced and treated with BetnovateVR).
Group D: Their dorsal back and the inside of their right ear pinna were treated with 5% topical IMQ following the administration of the Cyclosporin A (CsA) and dithranol (DTH) solution.
Group E: Their dorsal back and within their right ear pinna with 5% topical IMQ, and they subsequently got the ready-made 1% Cyclosporin A-dithranol (CsA-DTH)cerosomes.
Ear Thickness
The thickness of the right ear pinna that had been treated with 5% IMQ cream was measured using a Vernier caliper manufactured by Mitutoyo in the USA (model 541–874) to assess skin inflammation. This measurement of ear thickness was used to determine how severe the skin inflammation was.
Psoriasis Area Severity Index (PASI) Scoring
The degree of inflammation, skin thickening, and scaling that affected the dorsal region of the animal groups was rated using the PASI method. The scoring was recorded on the first, second, fourth, and sixth treatment days. On a scale of 0 to 4, the degree of erythema (redness), scaling, and skin thickening were rated; 0 indicated no symptoms, 1 mild, two moderate, three severe, and four very severe. Plotting these scores resulted in the time points on the x-axis and the PASI score on the y-axis.2
In-Vivo Skin Histopathological Examination
After the study, formalin-fixed skin samples were provided for histological examination. This study aimed to identify any pathological changes during treatment, including acanthosis, inflammatory infiltrates, hyperkeratosis, and parakeratosis. After the obtained skin samples were paraffin-embedded and stained with hematoxylin and eosin (H and E), standard slide mounting techniques were carried out.13 We examined the produced slices using a Nikon microscope (Melville, NY).
Results and Discussion
Statistical Evaluation of the Factorial Design
Design-expert Using virtual reality software, the various formulation factors in a full factorial design were examined to prepare the best CsA and DTH-loaded cerosomes. The designed design suggested 13 experimental runs corresponding to all possible combinations of the different amounts of the studied components (Table 1). The dependent variables were analyzed using the two-factor interaction (2FI) model with the highest prediction R2 value. The acceptable precision value for the space.15 As seen in Table 2, the desired ratio is greater than four for each dependent variable. The adjusted and expected R2 should be within 0.20 of each other to demonstrate a fair agreement. Table 3 shows that the modified R2 values and anticipated R2 values for each dependent variable agreed well.
 |
Table 3 Kinetics of CsA-DTH in-vitro Release from Cerosomes and Drug Solution |
In-Vitro Evaluation of Particles Size (PS), Polydispersity Index(PDI), Zeta Potential (ZP), Entrapment Efficieny (EE%), and Realase % of CsA-DTH- Cerosomes Formulas
The entrapment of a sufficient amount of drug by the generated vesicles is a crucial factor that determines their potential for use as a topical drug delivery method. In our investigation, the strong integration of the water-insoluble Cyclosporin (CsA) and Dithranol (DTH) molecules into the prepared Cerosomes was supported by the presence of the hydrophobic ceramide and phosphatidylcholine, according to Table 2.
Effect of (EE%) on the Formulation
The calculated cerosomes loaded with CsA-DTH had an EE% that ranged from 63.25±0.35 to 96.91±0.56%. All of the investigated variables had a statistically significant impact on the EE%, as shown in (Figure 2), according to the results of the data’s ANOVA statistical analysis. The amount of hyaluronic acid (X2) and the amount of ceramide (X1) were increased, and this had a substantial favorable impact on the EE% (p 0.0001). It has been previously noted that adding more ceramide and hyaluronic acid makes the formed colloidal dispersion viscous. Higher EE% values are guaranteed by the highly viscous formulations, which will prevent drug diffusion to the exterior aqueous phase.25 Also, the type of EA (X3) showed a significant effect on the EE% of the formulated Cerosomes (p < 0.0001). It was found that cerosomes formulated using TPGS showed higher CsA-DTH entrapment when compared to Kolliphor RH40. This finding might be related to the HLB values of both surfactants (14.9 and 13.5 for Kolliphor RH40 and TPGS, respectively).26 Higher EE% was reported with vesicles that utilized TPGS rather than Kolliphor RH40 since surfactants with low HLB values exhibit stronger lipophilicity and are more suited for integrating hydrophobic medicines like CsA and DTH. As opposed to Kolliphor RH40, which has a shorter and less hydrophobic alkyl chain, TPGS has a longer and more hydrophobic alkyl chain. This can aid in maintaining the lipid bilayer of the nanoparticles and stop the leaking of the medicine or other active ingredients. This might result in better trapping effectiveness. The molecular weight of TPGS is larger than that of Kolliphor RH40, which can aid in steric stabilization and keep the nanoparticles from aggregating or fusing. This may also result in a greater degree of trapping effectiveness. It has been noted that TPGS and Kolliphor RH40 have different drug affinities. This may enhance the amount of drug integrated into the nanoparticles and boost the effectiveness of trapping. This impact was predicted because of the increase in the membrane’s fluidity brought on by more surfactant molecules and the inclusion of more porous holes in the vesicle bilayer, which permits drug leakage and lower EE% values.27
Effect of Particle Size, Distribution, and Zeta Potential
PS effects of the formulation elements The small particle size of the nanodispersion is essential to forming a system that withstands sedimentation and particle aggregation. Furthermore, the particle size of the nanosystem may affect the degree of skin permeability and medicine retention.28 According to Table 2, every cerosome that was created had a narrow range that spanned from 222.36±0.36 to 524.31±0.32 nm. ANOVA statistical examination of the PS data revealed that, as shown in
Figure 2 shows that all factors under investigation substantially impact the PS. Vesicles with greater PS were produced as a result of the formulation’s incorporation of more ceramide (A) (p <0.0001). Increasing ceramide levels are known to cause aggregation formation with eventual PS hypertrophy. Ceramide’s restricted capacity to cross membrane leaflets was the basis of one theory proposed to explain ceramide-induced structural alterations. Increased ceramide levels in the leaflet would promote changes to the membrane’s curvature and consequent PS expansion.11 Increasing the amount of hyaluronic acid (B) (p < 0.0001) in a nanoparticle formulation can lead to a decrease in particle size. HA is a highly hydrophilic polymer that can help stabilize the nanoparticles and prevent them from aggregating or sticking together. HA’s presence can increase the formulation’s osmotic pressure, which can help draw water into the particles and contribute to a smaller particle size distribution. In addition to its stabilizing effects, HA can also influence the properties of the surfactants in the formulation. HA has interacted with nonionic surfactants, TPGS, to promote the formation of smaller and more uniform nanoparticles. This effect may be due to the ability of HA to reduce the interfacial tension between the surfactant and the aqueous phase, which can facilitate the formation of smaller particles.
Finally, in EA type (C) (p < 0.0001), the PS of the formulae containing TPGS was significantly higher compared to Kolliphor RH-40 formulae. The hydrophobicity of TPGS has a longer and more hydrophobic alkyl chain than Kolliphor RH40, which can help to destabilize the lipid bilayer of the nanoparticles and promote the formation of smaller and more uniform particles. Edge activator properties of TPGS have been reported to have edge activator properties in some nanoparticle systems, which means that it can destabilize the edges of the lipid bilayer and promote the formation of smaller particles. This can contribute to a smaller particle size distribution. Higher surfactant concentrations can help stabilize the particles and prevent them from aggregating but may also lead to larger particle sizes. The optimal surfactant concentration will depend on the specific nanoparticle system being formulated.
Impact of formulation parameters on PDI: The uniformity and quality of the produced nanosystem are indicated by the PDI values of the dispersion. A low, nearly zero PDI value denotes homogenous dispersion with a constrained PS range. Conversely, a number close to 1 denotes a substantially polydisperse dispersion.18 The PDI values of all the produced systems ranged from 0.415 ±0.04 to 0.801 ±0.02, as shown in Table 2 of our inquiry; this indicated the polydispersity of some of the created PC.29 These high PDI values could be attributed to the irregular tubulated vesicular morphology of cerosomes; nevertheless, only systems with a narrow range of PS were considered in the optimization process. Each of the parameters that were examined, ceramide amount (mg), HA amount (mg), and EA type (Kolliphor RH40 and TPGS), showed a non-significant effect on the PDI of the systems, with p values of 0.93, 0.19, and 0.41, respectively—factors related to formulation impact ZP. Zeta-potential measurement determines the generated nanodispersion’s overall surface charge, physical stability, and potential interactions with the body. Human skin is negatively charged due to negatively charged protein residues on its surface.15 A positively charged delivery method will actively interact with the skin’s surface to enhance drug retention in the skin’s layers. Higher absolute ZP values also suggest reduced particle-particle interaction, increased surface charge for the produced vesicles, and enhanced physical stability. According to Table 2, all cerosome formulations had positive ZP values between 23.35±0.61 and 29.54±0.34 mV. The positive ZP values seen with the formed vesicles are related to the presence of SA, which caused the positive charge to predominate over the other excipients used.15,19 Statistical analysis of the data revealed that both the kind of edge activator and the amount of ceramide considerably changed ZP values, as illustrated in Figure 2. When ceramide (A) was raised, ZP values were significantly lowered (p <0.0001). Ceramide IIIB possesses an amphiphilic structure that might potentially deposit on the surface of the generated vesicle, shielding the positive charge of the CsA-DTH formulation and reducing ZP.10,15 ZP values were significantly lower (p<0.0001) for CsA-DTH-loaded cerosomes generated using Kolliphore RH40 than those created with TPGS. These lower ZP values could be explained by the fact that Kolliphore RH40 contains 40 PEG units instead of 1000 PEG units in TPGS. It has previously been documented that the presence of hydrophilic PEG molecules in a surfactant can create a steric barrier that screens the charge at the surface of the colloidal particles, increasing Zeta potential (ZP) values.29 PEG chains on the surface of the colloidal particles can create a repulsive force that prevents other particles from approaching too closely and reduces the chance of aggregation—this repulsive force results in a higher ZP value, indicating a more stable colloidal solution.
In-Vitro Release Studies
The in-vitro release of free CsA and DTH and CsA-DTH when encapsulated in CsA-DTH cerosomes was measured using a direct dialysis bag method. The cumulative drug release profiles in Figure 3 illustrate the continuous and controlled pattern of CsA-DTH release from cerosomes. Due to the weak contact between the drugs and the surface of the cerosomes, the release of CsA and DTH from the cerosomes occurs faster within the first 10 hours than from free medications.30 After 48 hours, the drug release rate significantly decreases and eventually reaches a constant 93% release without ever reaching a plateau. A zero-order initial burst and a delayed release mediated by one or more mechanisms, such as diffusion, erosion, or a mix of both mechanisms, are typical release profiles of polymeric matrices containing CsA-DTH cerosomes.13,18,31 To determine the most accurate kinetic model for the release of CsA-DTH cerosomes, the most popular kinetic models indicated in Table 3 were run on the release date. The model that modified the cerosomes formulation the best was Korsmeyer-Peppas (r2 = 0.99, Akaike information criterion (AIC) = 59.24).
Selection of the Optimal CsA-DTH-Cerosomes
Design-expert Virtual reality software was used to analyze the data for the dependent factors to determine the proper values for the independent variables. We were able to design the best CsA-DTH loaded Cerosomes system because of the highest EE% and ZP values and the lowest PS and PDI levels. The algorithm determined that A-D12 was the optimal formula since it had the highest desire factor (0.821). Ten milligrams of TPGS and fifteen milligrams of ceramide were used to make the selected formula. It exhibited nanosized vesicles measuring 222.36±0.36 nm, with a reasonable ZP value of 29.36±0.38 mV and a high CsA-DTH EE% of 96.91±0.56% w/w. The agreement between the expected and actual values of the dependent variables for optimal CsA-DTH-loaded cerosomes (A-D12) showed that the statistical approach was adequate for the statistical evaluation and analysis of the various formulation parameters (Table 3).11,19
In-Vitro Evaluation of the Optimized Formula (A-D12)
Fourier Transform Infrared (FT-IR) Spectroscopy
FTIR spectroscopy was used to examine interactions between the medication and the extract. The FTIR study results show how cyclosporine-dithranol cerosomes interact with hyaluronic acid, cyclosporine A, and dithranol, as shown in (Figure 4). There was no indication that Cyclosporine-Dithranol cerosomes and Dithranol had formed a covalent connection. The stretching vibrations of amide I (C = O and C–N) at 1638 cm−1 and amide II (N–H bending with contribution from C–N stretching vibrations) at 1520 cm−1 are two of the most noticeable peaks in the IR spectra. O-H stretching vibration at 3279 cm−1 in water indicates the existence of leftover H2O19. In Cyclosporine A and Dithranol, amide VI exhibits asymmetric stretches such as methylene stretching and polyene C = C and C = O. C-O stretch at 1030−1, C = C stretch at 1520 cm−1, O-H stretch at 3200 cm −1, and Ceramide IIIB Amide I band (C=O stretch) at 1650 cm −1. Ceramide III Amide II band (N-H bend and C-N stretch) at 1550 cm −1. Alkene stretches at 3010 cm−1, Alkane stretches at 2850 cm−1, C-O stretches at 1030 cm−1, and O-H stretches at 3200 cm−1 are the ester C=O stretches.6,19,32
 |
Figure 4 FTIR spectra of pure Cyclosporine, Dithranol, Empty Cerosomes, and CsA-DTH loaded Cerosomes optimized formula separately. |
The Dithranol and Cyclosporine have profiles similar to empty cerosomes and Cyclosporine-Dithranol cerosomes, with the addition of peaks characterized by modest intensity owing to amide I, II, IV, and O–H vibrations in Cyclosporine -A molecule. The combinatory formula (Dithranol and Cyclosporine-Dithranol cerosomes) demonstrates no chemical interactions between Cyclosporine, Dithranol, and the excipients.
Differential Scanning Calorimetry (DSC) Analysis (A-D12)
The thermograms of pure Cyclosporine, pure Dithranol, empty cerosomes, and optimal cerosomes (C-D12) were determined using DSC investigations and shown in Figure 5. The thermogram of cyclosporine reveals an abrupt endothermic accident with a maximum temperature (Tmax) of 65°C associated with a fusion event. Interestingly, the thermogram of CsA-DTH-Cerosomes did not include the accident above. This evidence points to the containment of CsA-DTH as a solid solution or molecular dispersion within the cerosomes.18,32,33 Dithranol melted, resulting in a thermogram peak at 191±3.21 °C. Due to the colligative properties, dithranol’s melting point is lowered in all physical mixtures. All pure medications have significantly lower melting enthalpies of fusion than the physical combination, which suggests the presence of intermolecular hydrogen bonds and the interaction of the treatment with phospholipid. The results demonstrated that the elevated melting point of CsA-DTH-Cerosomes was connected to the functional group augmentation and hydrogen bonding reinforcing influence. Amorphous combination cerosomes may be advantageous since the active substance is more soluble in them.14
 |
Figure 5 Differential scanning calorimetry (DSC) thermograms of pure Cyclosporine, Dithranol, Empty Cerosomes, and CsA-DTH loaded Cerosomes optimized formula separately. |
Morphology and Transmission Electron Microscopy (TEM)
The morphometric characteristics of the improved formulation of CsA-DTH-loaded Cerosomes (optimal formula C-D12) were determined by TEM imaging. Figure 6 presents images. Photos displayed The reason why cerosomes have spherical vesicles next to the tubules may be due to a non-uniform distribution of ceramide in the bilayer, which could result in ceramide-rich domains with flat morphology and ceramide-poor domains with spherical morphology and average particle sizes similar to those reported in morphometry studies.12,15
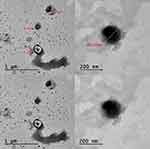 |
Figure 6 Transmission electron microscope (TEM) pictures of an Optimum CsA-DTH -Cerosomes (C-D12). |
Short-Term Stability of Nanoparticles
At the end of the experiment, the stored vesicles did not agglomerate or show any physical changes. Table 4 lists the EE%, PS, PDI, ZP, and Release% data for the optimized formula when it was new and after three months of storage at 4°C and 25°C. We found modest changes (p > 0.05) in EE%, PS, ZP, and Release%. This outcome highlights the excellent stability of the recipe. The strong positive charge of the ideal formula prevents the aggregation/agglomeration of stored Cerosomes. The tiny Particle Size of the ideal formula would also generate a high surface area, allowing the ionizable surface groups on the cerosomes to reveal their charge. The improved formulation was evaluated after 1, 15, 30, and 3 months at 40 and 25 °C.13,15,18
 |
Table 4 Effect of Short-Term Storage on the Physicochemical Properties of the Best Formula CsA-DTH Loaded Cerosomes(C-D12) |
Ex-Vivo Skin Permeation
An ex-vivo permeation study was conducted to compare and evaluate the penetration parameters of CsA-loaded Cerosomes, CsA-solution, DTH-loaded Cerosomes, and DTH solution across the skin. The results are presented in Figures 7a and b, and Table 5. The CsA-DTH loaded Cerosomes formulation demonstrated significant superiority (p<0.05) over the CsA-DTH solution in all evaluated permeation parameters, except for CsA-DTH loaded Cerosomes QR values. CsA-DTH loaded in Cerosomes showed faster skin penetration than CsA-DTH solution, with a J value almost three times higher than that of CsA-DTH solution, which is attributed to the higher lipophilicity of Cerosomes.15,34 The skin’s stratum corneum, comprised of lipids such as ceramides, cholesterol, and free fatty acids, forms a rate-limiting barrier that restricts hydrophilic molecules from entering the skin and affects skin-level medication delivery. The other permeation parameters showed comparable ratios, with CsA-DTH loaded Cerosomes having a higher Kp and Q48h than CsA-DTH solution, indicating better permeation and cumulative drug delivery over 48 hours. Cerosomes promote the action of CsA-DTH on the skin and stratum corneum.28 The study did not show significant changes in CsA-DTH QR between formulations. After applying the Cerosomes formulation, the amount of drug retained in the skin tissue depends on the fraction of CsA-DTH that is not encapsulated and the fraction undergoing first burst ejection from the CsA-DTH weakly attached to the Cerosomes surface.8 By gently releasing CsA-DTH throughout the skin tissue, the Cerosomes formulation can efficiently distribute the drug to the intended location, making it a promising treatment option for skin psoriasis and inflammation caused by the immune system mistakenly attacking healthy skin cells.
 |
Table 5 Ex-Vivo Pharmacokinetic Parameters of the CsA-Loaded Cerosomes, DTH Loaded Cerosomes, and Ex-Vivo Skin Penetration Profile Compared to Their Solution Estimated by Linear Regression |
 |
Figure 7 Ex-vivo Skin permeation profile (a) for Cycosporine and (b) for Dithranol, from CsA-DTH loaded Cerosomes compared to CsA-DTH solution. |
In silico Study
The immunosuppressive agent cyclosporine and the hydroxy anthracene derivative dithranol were tested for their binding and inhibitory activity against the most common targets for improving skin healing and inhibiting the pro-inflammatory mediator well related to psoriasis. Three targets were chosen: the pro-inflammatory mediators TNF-α and IL-1β with the anti-itching protein TRPV3.5–7
Binding energy scores in kcal/mol. Cyclosporine and dithranol versus the co-crystallized ligand against TNF-α, IL-1β, and TRPV3 proteins are summarized in Table 6.
 |
Table 6 The Docking Energy Binding Scores in Kcal/Mol for Cyclosporine and Dithranol |
Both cyclosporine and dithranol showed good binding forces, especially with GLU121 through H-bonds. In addition to supporting interactions, cyclosporine has many functional groups and so showed a higher binding score (−11.9 kcal/mol.), even more than the co-crystallized ligand (−9.7)8 (Figure 8a). Similarly, cyclosporine and dithranol showed the key binding force against ASN1089 and other hydrogen and hydrophobic interactions. Cyclosporine also showed a higher binding score against IL-1β (−9.1 kcal/mol.) than the co-crystallized ligand (Figure 8b). Finally, the binding of both compounds was tested against TRPV3; both can bind to GLY600 (hydrogen bond) and LEU657 (hydrophobic interaction).10 Cyclosporine also showed a higher binding energy score than dithranol (−7.4 kcal/mol. to −4.5 kcal/mol., respectively) (Figure 8c).
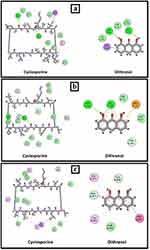 |
Figure 8 Binding interactions of cyclosporine and dithranol in (a)TNF-α binding site,(b) IL-1β, and (c) TRPV3 binding site. |
Cell Culture Assays
Cell Toxicity
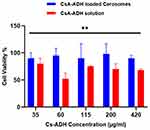
The safety of cerosomes loaded with CsA-DTH was tested using HSE-2 cells. Figure 9 displays the findings. No cytotoxic effects were seen when CsA-DTH-loaded cerosomes were incubated for 24 hours at doses up to 450 µg/mL. Over 90% of the cells were alive. Consequently, it can be said that, at all tested concentrations, the CsA-DTH solution exhibited no harmful effects. One class of lipid molecule essential to preserving the skin’s barrier function is ceramide IIIB. They play a crucial role in the stratum corneum, the skin’s outermost layer, which guards against environmental stressors, including pollution, UV rays, infections, and excessive water loss from the skin. Ceramides function by creating a shield that keeps the skin hydrated and stops dangerous chemicals from penetrating the skin.15,19 Because the formulation contains three times the quantity of Ceramide II found in healthy Skin, it increased rapidly in response to a stimulus like acute inflammation or oxidative stress after delivery of CsA-DTH loaded cerosomes. Cerosomes loaded with CsA-DTH may produce a long-lasting protein concentration. In cases where the skin’s CsA-DTH solution composition is impaired, this raises its bioavailability. It’s possible that the TPGS surfactant’s presence somewhat reduced cell viability. In contrast, surfactants are often used to prevent protein aggregation during manufacturing, transportation, and storage. TPGS (d-alpha-tocopheryl polyethylene glycol 1000 succinate) is widely employed as a surfactant in biological products and is generally recognized as safe (GRAS) due to its well-known breakdown. It is widely utilized as a food additive and an element in pharmaceutical and cosmetic items. Extensive studies have been conducted on the safety and toxicity of TPGS. Considering that the formulation’s ingredients are often considered safe, this finding confirms the biocompatibility of CsA-DTH-loaded Cerosomes when in touch with skin tissue.18,32,35
Cerosomes’ Anti-Inflammatory Efficacy on HSE-2 Cells
Cytokine levels of IL-6, IL-1β, and TNF-α were assessed to assess Cerosomes’ anti-inflammatory ability against Imiquimod-induced inflammation in HSE-2 cells. The results are shown in Figures 10a–c). The introduction of CsA-DTH-loaded cerosomes considerably reduced the production of cytokines to a level equivalent to CsA solution (P <0.05), demonstrating that CsA-DTH-loaded cerosomes had a considerable anti-inflammatory impact. The positive control revealed a large cytokine release. Prolonged inflammatory skin disorders are distinguished by the overproduction of inflammatory markers, specifically cytokines like TNF-α, IL-1β, and IL-6. It is well established that lowering IL-6 levels in the skin can trigger neutrophil, basophil, and T cell migration, which can worsen symptoms.15,36 TNF-α and IL-1β release is reported to be significantly higher in individuals with various dermopathies, and these molecules are thought to be indicators of the general inflammatory status of the skin surface. Through its interaction with inflammatory cell surface receptors, such as C-D-12, which inhibits IL-1β and TNF-α-induced transcription of many inflammatory mediator genes, CsA-DTH has been demonstrated to influence the expression of various cytokines. The administration of both drugs may have a synergistic anti-inflammatory impact since CsA-DTH can also control the oxidative bursts of neutrophils and macrophages that initiate the inflammatory response. Often used to treat psoriasis, cyclosporine, and dithranol have been demonstrated to modify levels of proinflammatory cytokines, such as IL-6, IL-1β, and TNF-α, which contributes to their anti-inflammatory and immunosuppressive effects in psoriasis patients.18,37 While dithranol inhibits skin cell growth and the skin’s immunological response, cyclosporine decreases T-cell activation and cytokine generation. It has been demonstrated that both medications reduce the expression of IL-6, IL-1β, and TNF-α in skin lesions.
In-Vivo Study
Ear Thickness
In numerous investigations, the thickness of the ear has been employed as a pharmacological indicator to assess the degree of psoriasis and skin inflammation caused by IMQ. As illustrated in Figure 11, every group in this study with 5% IMQ applied to their right ear pinna had thicker ears than the control group. Figure 12 illustrates how the substantial erythema and scaling that followed this increase in ear thickness developed throughout the experiment. The groups that underwent the greatest changes in ear thickness, redness, and scaling were those who received only 5% IMQ (group B) and those treated with 5% IMQ with CsA-DTH (group C). On the other hand, group E, which received CsA-DTH loaded cerosome treatment, had the least amount of change in ear thickness, with an average of 9 x10−2mm. This change was notably less than the 18x10−2mm change in group D, which was treated with the commercial BetnovateRV ointment and received 5% IMQ.1,33 According to these findings, cerosomes loaded with CsA-DTH have a more potent anti-psoriatic action than commercial products. Nevertheless, a one-way ANOVA statistical test and a Tukey post hoc test (p<0.05) revealed that groups D and E differed significantly from the sick groups.
 |
Figure 11 There was a change in ear thickness in all groups on day 7. For unpaired t-test #Means p < 0.0317 and ###Means p < 0.0001 relative to control group. For ANOVA ****Means p-value < 0.0001. |
 |
Figure 12 Changes in the right ear pinna receiving IMQ on days (A) 2, (B) 4, and (C) 7. |
PASI Score
For many years, the PASI scoring system has been used to measure the severity of psoriasis and track its recovery following treatment. To evaluate the course of the disease, this approach evaluates three essential characteristics of psoriatic lesions: erythema (redness), infiltration (thickness), and desquamation (scaling), as shown in Figures 13 and 14 that the various groups experienced differing rates of illness development based on these three factors. With the most severe thickness, redness, and scaling, groups B and C, which received 5% IMQ cream alone and 5% IMQ cream followed by CsA-DTH treatment, respectively, showed the worst prognosis. However, groups D and E, which received the commercial lotion and the tested Cerosomes formulation, had a better prognosis, as evidenced by their lower cumulative PASI scores.8,38 According to a Kruskal–Wallis statistical test, there was a significant difference (p<0.05) between the two and the ill groups.
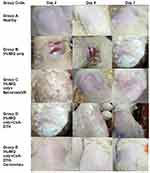 |
Figure 13 Progression of psoriasis in all five groups on days 2.4 and 7. |
 |
Figure 14 Graphical representation of the progression of erythema, thickness, scaling, and PASI score in all groups over the seven days of the experimental model.For ANOVA ****Mean p < 0.0001. |
Histopathological Study
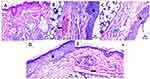
Microscopic examinations of the dorsal back skin samples were performed to evaluate the impact of various treatments on psoriasis. These are the outcomes: As shown in Figure 15A, Group A, the healthy control displayed normal skin layer morphology, including an intact structure of epidermis and dermis, well-organized collagen fibers, and no abnormal inflammatory cell infiltrates, with intact subcutaneous tissue. All tissue sections in Group B, which received only 5% IMQ cream treatment, showed a considerable increase in thickness of the epidermis (arrowhead), the presence of dermal infiltration by mononuclear inflammatory cells (blue arrow), and severe congestion of dermal blood vessels (black arrow). However, there were more instances of isolated subepidermal aggregation of inflammatory cells than more widespread reports of inflammatory cell infiltration throughout the sample, as shown in Figure 15B.25,28
The 5% IMQ and CsA-DTH- solution treated Group C displayed a significant increase in thickness of the epidermis (arrowhead) and infiltration of the dermis by mononuclear inflammatory cells (blue arrow), as well as focal encapsulated subcutaneous aggregates of inflammatory cells as shown in Figure 15C. The 5% IMQ and commercial ointment Betnovate treated Group D increase the thickness of the epidermis (arrowhead) and infiltration of the dermis by a low number of mononuclear inflammatory cells (blue arrow), as shown in Figure 15D. The 5% IMQ and CsA-DTH-cerosomes treated Group E, normal epidermis, presence of mild congestion of dermal blood vessels (black arrow) with infiltration of dermis by a low number of mononuclear inflammatory cells (blue arrow) as shown in Figure 15E. depicts the minor sporadic focal subcutaneous inflammatory cell aggregation and the mildly congested BVs that were seen. Interestingly, there was a substantial difference between the commercial Betnovate product and the dispersion of CsA-DTH cerosomes, indicating the tested formula had a stronger antiproliferative effect.8,34,39
Conclusions and Prospects
Our study aimed to optimize the therapeutic efficacy of cyclosporine (CsA) and dithranol (DTH), first-line treatments for psoriasis, despite their low systemic bioavailability. We introduced ceramide/phospholipid composite (CCPCs) cerosomes, a novel delivery system loaded with CsA and DTH for topical application, to enhance systemic bioavailability and improve psoriasis treatment outcomes.
The optimized CCPCs cerosomes efficiently dissolved CsA and DTH, enhancing skin permeability and exerting antiproliferative effects. With a particle size of 222.36±0.36 and a PDI of 0.415±0.04, the cerosomes demonstrated robust potential for topical skin penetration, supported by a zeta potential of 29.36±0.38 indicating electrostatic stability of CsA-DTH-loaded cerosomes. The combination of CsA and DTH in cerosomes significantly improved skin penetration, as evidenced by ex-vivo, in-silico, in-vitro, and in-vivo permeation experiments.
Topical delivery of CsA and DTH through cerosomes markedly enhanced efficacy in an IMQ-induced psoriatic-like mouse model, reducing inflammation, down-regulating proinflammatory cytokines, minimizing skin thickness changes, and exhibiting protective histopathological effects. Blank cerosomes displayed mild anti-inflammatory action, suggesting potential adjuvant use in psoriasis treatment.
Our findings establish CsA/DTH cerosomes as a reliable and efficient method for psoriasis treatment. Future research should explore the system’s potential at lower CsA doses or altered dosing frequencies, further advancing the understanding and applicability of this novel topical drug delivery method for psoriasis management.
Institutional Review Board Statement
The Faculty of Pharmacy, Cairo University, Cairo, Egypt’s animal care and use committee accepted the animal study protocol (no. PI 3219, 2023).
Data Sharing Statement
Data is contained within the article.
Disclosure
The authors declare no conflict of interest.
References
1. Campanati A, Marani A, Martina E, et al. Psoriasis as an immune-mediated and inflammatory systemic disease: from pathophysiology to novel therapeutic approaches. Biomedicines. 2021;9(11):1511. doi:10.3390/biomedicines9111511
2. Khan R, Mirza MA, Aqil M, et al. In vitro and in vivo investigation of a dual-targeted nanoemulsion gel for the amelioration of psoriasis. Gels. 2023;9(2):112. doi:10.3390/gels9020112
3. Yiu ZZN, Becher G, Kirby B, et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA dermatol. 2022;158(10):1131–1141. doi:10.1001/jamadermatol.2022.2909
4. Georgaki M, Piperi E, Theofilou VI, et al. A randomized clinical trial of topical dexamethasone vs. cyclosporine treatment for oral lichen planus. Med Oral Patol Oral Cir Bucal. 2022;27:e113–e124. doi:10.4317/medoral.25040
5. Benezeder T, Painsi C, Patra V, et al. Dithranol targets keratinocytes in their crosstalk with neutrophils and inhibits the IL-36 inflammatory loop in psoriasis. Elife. 2020;9:1–31. doi:10.7554/eLife.56991
6. Sathe P, Saka R, Kommineni N, Raza K, Khan W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev Ind Pharm. 2019;45(5):826–838. doi:10.1080/03639045.2019.1576722
7. Poloxamer: handbook of Pharmaceutical Excipients, Sixth Edition. Handbook Pharm Excipients;2009.110–113.
8. Yang X, Tang Y, Wang M, et al. Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy. Int J Pharm. 2021;605:120826. doi:10.1016/j.ijpharm.2021.120826
9. Effect of sphingosine and phytosphingosine ceramide ratio on lipid arrangement and barrier function in skin lipid models – PubMed; 2024. Available from: https://pubmed.ncbi.nlm.nih.gov/37301511/.
10. Yilmaz E, Borchert HH. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity, and erythema--an in vivo study. Int J Pharm. 2006;307(2):232–238. doi:10.1016/j.ijpharm.2005.10.002
11. Fouad SA, Teaima MH, Gebril MI, et al. Formulation of novel niosomal repaglinide chewable tablets using co-processed excipients: in vitro characterization, optimization and enhanced hypoglycemic activity in rats. Drug Deliv. 2023;30(1):doi:10.1080/10717544.2023.2181747
12. Nadaban A, Gooris GS, Beddoes CM, Dalgliesh RM, Bouwstra JA. Phytosphingosine ceramide mainly localizes in the central layer of the unique lamellar phase of skin lipid model systems. J Lipid Res. 2022;63(9):100258. doi:10.1016/j.jlr.2022.100258
13. Abdelmonem R, Elhabal SF, Abdelmalak NS, El-Nabarawi MA, Teaima MH. Formulation and characterization of acetazolamide/carvedilol niosomal gel for glaucoma treatment: in vitro, and in vivo study. Pharmaceutics. 2021;13(2):1–20. doi:10.3390/pharmaceutics13020221
14. Bhardwaj P, Tripathi P, Pandey S, Gupta R, Ramchandra Patil P. Cyclosporine and pentoxifylline laden tailored niosomes for the effective management of psoriasis: in-vitro optimization, ex-vivo and animal study. Int J Pharm. 2022;626:122143. doi:10.1016/j.ijpharm.2022.122143
15. Fathy Elhabal S, El-Nabarawi MA, Abdelaal N, et al. Development of canagliflozin nanocrystals sublingual tablets in the presence of sodium caprate permeability enhancer: formulation optimization, characterization, in-vitro, in silico, and in-vivo study. Drug Deliv. 2023;30(1):2241665. doi:10.1080/10717544.2023.2241665
16. Drira C, Ben Ayed W, Soussi MA, Razgallah Khrouf M, Fradi I. Validation of routine analytical method for injectable cyclosporine preparation control using HPLC-FIA assay. Ann Pharm Fr. 2021;79(3):266–274. doi:10.1016/j.pharma.2020.10.006
17. Ormerod A, Winfield A, Priprem A, et al. The development and clinical efficacy of a dithranol-impregnated hydrogel in treating psoriasis. J DermatolTreat. 1992;2(4):133–136. doi:10.3109/09546639209092739
18. Elhabal SF. Development of thermosensitive hydrogel of amphotericin-b and lactoferrin combination-loaded PLGA-PEG-PEI nanoparticles for potential eradication of ocular fungal infections: in-vitro, ex-vivo and in-vivo studies. Int J Pharm X. 2023;5:1.
19. Elhabal SF, Elwy HM, Hassanin S, et al. Biosynthesis and characterization of gold and copper nanoparticles from salvadora persica fruit extracts and their biological properties. Int J Nanomed. 2022;17:6095–6112. doi:10.2147/IJN.S385543
20. Gajera BY, Shah DA, Dave RH. Development of an amorphous nanosuspension by sonoprecipitation-formulation and process optimization using design of experiment methodology. Int J Pharm. 2019;559:348–359. doi:10.1016/j.ijpharm.2019.01.054
21. Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J Chem Inf Model. 2021;61:3891–3898. doi:10.1021/acs.jcim.1c00203
22. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:NA–NA. doi:10.1002/jcc.21334
23. Keller A, Chavez JD, Tang X, Bruce JE. Leveraging the entirety of the protein data bank to enable improved structure prediction based on cross-link data. J Proteome Res. 2021;20:1087–1095. doi:10.1021/acs.jproteome.0c00495
24. Hamza AA, Khasawneh MA, Elwy HM, et al. Salvadora persica attenuates DMBA-induced mammary cancer through the downregulation oxidative stress, estrogen receptor expression and proliferation and augmenting apoptosis. Biomed Pharmacother. 2022;147:112666. doi:10.1016/j.biopha.2022.112666
25. Strati F, Mukhina T, Neubert RHH, et al. Cerosomes as skin repairing agent: mode of action studies with a model stratum corneum layer at liquid/air and liquid/solid interfaces. BBA Adv. 2021;2:doi:10.1016/j.bbadva.2021.100039
26. Tagami T, Ernsting MJ, Li SD. Optimization of a novel and improved thermosensitive liposome formulated with DPPC and a Brij surfactant using a robust in vitro system. J Control Release. 2011;154(3):290–297. doi:10.1016/j.jconrel.2011.05.020
27. Anzengruber M, Nepustil LM, Kurtaj F, et al. A versatile brij-linker for one-step preparation of targeted nanoparticles. Pharmaceutics. 2023;15(5):1403. doi:10.3390/pharmaceutics15051403
28. Yokose U, Ishikawa J, Morokuma Y, et al. The stratum corneum’s ceramide [NP]/[NS] ratio is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020;20(1):doi:10.1186/s12895-020-00102-1
29. Auría-Soro C, et al. Interactions of nanoparticles and biosystems: microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomater. 2019;9:1.
30. Carvajal-Vidal P, Fábrega MJ, Espina M, Calpena AC, García ML. Development of halobetasol-loaded nanostructured lipid carrier for dermal administration: optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy. Nanomedicine. 2019;20. doi:10.1016/j.nano.2019.102026
31. Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye - part I - barriers and determining factors in ocular delivery. Eur J Pharm Biopharm. 2017;110:70–75. doi:10.1016/j.ejpb.2016.10.009
32. Mohammed MHH, Hamed ANE, Elhabal SF, et al. Chemical composition and anti-proliferative activities of Hyophorbe lagenicaulis aerial parts and their biogenic nanoparticles supported by network pharmacology study. South Afr J Bot. 2023;156:398–410. doi:10.1016/j.sajb.2023.03.018
33. Fathalla D, Youssef EMK, Soliman GM. Liposomal and ethosomal gels for the topical delivery of anthralin: preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics. 2020;12(5):446. doi:10.3390/pharmaceutics12050446
34. Trombino S, Servidio C, Laganà AS, et al. Viscosified solid lipidic nanoparticles based on naringenin and linolenic acid to release cyclosporine a on the skin. Molecules. 2020;25(15):3535. doi:10.3390/molecules25153535
35. Gu Y, Xu C, Wang Y, et al. Multifunctional nanocomposites based on liposomes and layered double hydroxides conjugated with glycylsarcosine for efficient topical drug delivery to the posterior segment of the eye. Mol Pharm. 2019;16(7):2845–2857. doi:10.1021/acs.molpharmaceut.8b01136
36. Gao J, Chen F, Fang H, et al. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol Res. 2020;53(1):doi:10.1186/s40659-020-00316-0
37. Mizutani H, Ohmoto Y, Mizutani T, Murata M, Shimizu M. Role of increased production of monocytes TNF-alpha, IL-1beta and IL-6 in psoriasis: relation to focal infection, disease activity and responses to treatments. J Dermatol Sci. 1997;14(2):145–153. doi:10.1016/s0923-1811(96)00562-2
38. Nădăban A, Rousel J, El Yachioui D, et al. Effect of sphingosine and phytosphingosine ceramide ratio on lipid arrangement and barrier function in skin lipid models. J Lipid Res. 2023;64(8):100400. doi:10.1016/j.jlr.2023.100400
39. Herculano RD, Dos Santos TO, de Barros NR, et al. Aloe vera-loaded natural rubber latex dressing as a potential complementary treatment for psoriasis. Int J Biol Macromol. 2023;242:124779. doi:10.1016/j.ijbiomac.2023.124779
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.