Back to Journals » Journal of Multidisciplinary Healthcare » Volume 14
Revisiting Spasticity After Stroke: Clustering Clinical Characteristics for Identifying At-Risk Individuals
Authors Ahmedy F , Mohd Tuah N , Mohamad Hashim N , Sybil Shah S, Ahmedy I, Tan SF
Received 26 May 2021
Accepted for publication 21 July 2021
Published 31 August 2021 Volume 2021:14 Pages 2391—2396
DOI https://doi.org/10.2147/JMDH.S320543
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fatimah Ahmedy,1 Nooralisa Mohd Tuah,2 Natiara Mohamad Hashim,3 Syahiskandar Sybil Shah,4 Ismail Ahmedy,5 Soo Fun Tan2
1Rehabilitation Medicine Unit, Faculty of Medicine & Health Sciences, Universiti Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia; 2Faculty of Computing & Informatics, Universiti Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia; 3Department of Rehabilitation Medicine, Faculty of Medicine, Universiti Teknologi MARA, Sg. Buloh, Selangor, Malaysia; 4Department of Rehabilitation Medicine, Queen Elizabeth Hospital, Kota Kinabalu, Sabah, Malaysia; 5Department of Computer System & Technology, Faculty of Computer Science & Information Technology, University of Malaya, Kuala Lumpur, Malaysia
Correspondence: Fatimah Ahmedy
Rehabilitation Medicine Unit, Faculty of Medicine & Health Sciences, Universiti Malaysia Sabah, Jalan UMS, Kota Kinabalu, 88846, Sabah, Malaysia
Tel +60138805513
Email [email protected]
Purpose: To collectively identify the clinical characteristics determining the risk of developing spasticity after stroke.
Patients and Methods: A cross-sectional study was conducted at a single rehabilitation outpatient clinic from June to December 2019. Inclusion criteria were stroke duration of over four weeks, aged 18 years and above. Exclusion criteria were presence of concurrent conditions other than stroke that could also lead to spasticity. Recruited patients were divided into “Spasticity” and “No spasticity” groups. Univariate analysis was deployed to identify significant predictive spasticity factors between the two groups followed by a two-step clustering approach for determining group of characteristics that collectively contributes to the risk of developing spasticity in the “Spasticity” group.
Results: A total of 216 post-stroke participants were recruited. The duration after stroke (p < 0.001) and the absence of hemisensory loss (p = 0.042) were two significant factors in the “Spasticity” group revealed by the univariate analysis. From a total of 98 participants with spasticity, the largest cluster of individuals (40 patients, 40.8%) was those within less than 20 months after stroke with moderate stroke and absence of hemisensory loss, while the smallest cluster was those within less than 20 months after severe stroke and absence of hemisensory loss (21 patients, 21.4%).
Conclusion: Analyzing collectively the significant factors of developing spasticity may have the potential to be more clinically relevant in a heterogeneous post-stroke population that may assist in the spasticity management and treatment.
Keywords: spasticity, stroke rehabilitation, clinical characteristics, clustering analysis
Introduction
Stroke is one of the leading causes of death globally, with a high rate of complications and physical impairments.1 The condition is associated with an annual 43.7 million lost disability-adjusted-life-years (DALYs) worldwide, signifying the substantial impact and burden of this condition to the affected survivors.2 The most prevalent neurological presentation in stroke is weakness leading to spasticity, affecting 20–30% of stroke survivors.3–5
Spasticity, defined as increased muscle tone due to exaggeration of the stretch reflex, is part of the recovery commonly observed in the affected limbs.6,7 Most strokes typically involve the flexors and extensors of the upper and lower extremity, respectively.6 Spasticity can emerge at any time during the motor recovery but is customarily seen within the first 6 weeks after stroke. However, it has been reported to develop during the chronic stage.8 Being a common complication after stroke, neurological and functional recovery is significant, causing a delay in achieving maximal stroke recovery.
Several works were conducted on determining the factors associated with spasticity in stroke.9–11 Some of the established factors that predict spasticity following stroke include NIHSS score, the severity of weakness, hemianesthesia, and treatment type (surgery or conservative).12 These form the basis for rehabilitation clinicians to predict the outcome, that is to prognosticate the likelihood to acquire spasticity after stroke and anticipate subsequent needs for interventions. On the other hand, many of these established factors were evaluated individually rather than collectively to determine characteristics of stroke survivors that would be at higher risk to develop spasticity complication. Furthermore, these influencing factors are gathered from a heterogeneous condition, but the type of respondents and characteristics of the study are not contextualized.
The presence of spasticity masks the neurological recovery of the antagonist’s muscle (for example, spasticity in biceps hinders clinically observed recovery of triceps) and deters the ability for stroke individuals to walk and perform activities of daily living (ADLs).13 Hence, it is essential to manage the spasticity for improvement in these functions. The treatments include, but are not limited to, physical stretching, massage therapy, antispasmodic agents (for instance, oral baclofen), phenol neurolysis and intramuscular botulinum toxin injection.14,15 The latter two interventions are considered localized and effective for spasticity after stroke.
In a region where effective treatment of spasticity such as intramuscular botulinum toxin injection is readily available, treating the stroke survivors presented with any of the factors associated with spasticity would be ideal. However, in many rehabilitation facilities where such intervention is scarce, the treating physicians have to prioritize those at risk and benefit from the intervention while reducing disability. Clustering the influencing factors for spasticity to identify those at higher risk to develop this complication would be the next step towards precision medicine in rehabilitation. Based on these factors, the article aimed to analyze individuals at risk of developing spasticity after stroke based on clinical characteristics, including duration after stroke, the severity of stroke, and presence of hemisensory loss.
Patients and Methods
Study Design and Patient Selection
This cross-sectional study was conducted at Rehabilitation Medicine Specialist Clinic at Queen Elizabeth Hospital, Kota Kinabalu, Sabah, from June to December 2019. All stroke patients who attended the clinic within the study period were screened for eligibility, assessed for outcome measures and demographic collection from the data gathered by Tan et al.16 Inclusion criteria were stroke duration of over four weeks and aged 18 years and above. Exclusion criteria were the presence of concurrent conditions that could also lead to spasticity complication such as spinal-related conditions, brain infection and traumatic brain injury.
Outcome Variables
The independent variables are categorized into demography and clinical characteristics. Two demographic variables were age and sex, and variables evaluated as clinical characteristics were duration after stroke, the severity of stroke, type of stroke and presence of hemisensory loss. Duration after stroke was recorded in months, the severity of stroke was categorized as either mild, moderate or severe, and the type of stroke was either ischaemic or haemorrhagic. Sensory assessment was determined based on the ability to perceive pain and fine touch sensation using pin prick and cotton wool testing respectively on both non-affected and affected sides. The presence of hemisensory loss was considered in the absence or reduced pain or fine touch sensation on the affected stroke side compared to the non-affected side.
Spasticity Assessment
Recruited patients were assessed for the presence of spasticity in any of these muscles on the affected limbs; elbow flexors, wrist flexors, knee extensors and ankle plantar flexors using the Modified Ashworth Scale (MAS).17 Patients were categorized as “spasticity not present“ based on spasticity assessment of MAS 0, or “spasticity present“ measured as MAS 1, MAS 1+, MAS 2 or MAS 3.
Study Procedure
Participants’ demography and clinical characteristics data were extracted from clinical notes and transferred onto a separate, private database as secondary data. Among the collected attributes, only data related to the research were selected and included for analysis. Patients were assessed for the presence of hemisensory loss and spasticity using MAS as part of the standard of clinical assessment during the outpatient consultation. Those with no spasticity present in any of the muscles stated earlier were classified as the “No spasticity” group, and those with spasticity present are classified as the “Spasticity” group. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Medical Research & Ethics Committee (MREC) of the National Medical Research Register (protocol code: NMRR-19-1698-48648 [IIR]) of the Ministry of Health Malaysia. Informed consent was obtained from all subjects involved in the study.
Statistical Analysis
Statistical analyses were conducted using SPSS version 22.0. The baseline demography (age and gender) and clinical characteristics (duration after stroke, severity of stroke, type of stroke and presence of hemisensory loss) were analyzed between the “Spasticity” and “No spasticity” groups for determining whether these variables have significant differences between the two groups. For continuous data, Independent T-testing and Mann Whitney U testing were used for normal and skewed data distribution respectively. Chi-square testing was performed for analyzing all categorical data. A p-value of less than 0.05 is considered statistically significant.
The clustering method was adopted for analyzing and predicting specific characteristics that may be at higher risk to develop spasticity after stroke. Two-step clustering approach was used for this purpose for 2 reasons: this form of clustering methods has the aptitude to analyze large data, while permitting analyses of both scale and ordinal data in the same model with automatic selection of number of clusters due to the expected heterogeneous type of the extracted data. Thus, by applying this method, it will provide a model that can help to predict the presence of spasticity on other prospective patients.
Results
Characteristics
A total of 216 stroke patients were recruited and consented for the study. Based on the spasticity assessment, 118 patients were categorized into the “No spasticity” group and the remaining 98 patients into the “Spasticity” group (Figure 1). Patients baseline characteristics are summarized in Table 1. Duration after stroke was further re-grouped into four categories; 1–20 months, 21–40 months, 41–60 months, 61 months and more. Comparisons between the “Spasticity“ group and “No spasticity” group revealed significant differences in duration after stroke and the presence of hemisensory loss (p < 0.05). Univariate analysis of characteristics is summarized in Table 2.
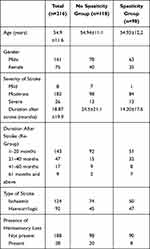 |
Table 1 Demography and Clinical Characteristics of All Stroke Subjects |
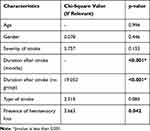 |
Table 2 Univariate Analysis of Characteristics |
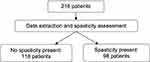 |
Figure 1 Recruited stroke patients and grouping for the study. |
Two-Step Clustering Analysis
The three main characteristics included as attributed for the two-step clustering analysis were duration after stroke (re-group), hemisensory loss, and severity of stroke. The former two were included based on the significant differences found in our data analysis. However, the severity of stroke was added in the analyses based on the previous works.15 It would be imperative to include the latter characteristic to explore its relevance in our data cohort.
By selecting only patients with spasticity in the two-step clustering analysis (n = 98), there were a total of 3 clusters with good cluster quality (Average Silhouette = 0.7) and a ratio of sizes of 1.90. The hierarchy based on the predictor of importance for these three variables are as follows: duration after stroke (1.00), the severity of stroke (0.79) and absence of hemisensory loss (0.47). Table 3 summarizes the characteristics in respective clusters. The largest cluster of individuals who developed spasticity after stroke were those within less than 20 months after stroke having moderate stroke severity and the absence of hemisensory loss. On the other hand, those who were less than 20 months after stroke with severe stroke and absence of hemisensory loss made up of the smallest cluster in spasticity group.
 |
Table 3 Clustering Analysis of Characteristics |
Discussion
In our center, only two out of the five studied clinical characteristics were found to be significantly different: duration after stroke (both in “months” and “re-group” categories) and presence of hemisensory loss. The severity of stroke was not significant in our study although this characteristic has been evaluated and proven to be an important predictor.18 Based on the clustering analysis, stroke survivors within 1–20 months duration having moderate stroke severity in the absence of hemisensory loss made up the largest cluster among those who developed post-stroke spasticity.
The NIHSS score was used for direct analysis in determining its significance as a predictor for spasticity after stroke.18 A higher NIHSS score increased the risk for developing spasticity. In our analysis, we did not analyze NIHSS directly. Instead, we categorized patients into three different categories; mild, moderate or severe. Nevertheless, the severity of stroke is a significant predictor based on the two-step clustering analysis.
From the data gathered among patients attending our clinic, the absence of hemisensory loss was an essential characteristic between the two groups. The number of patients with hemisensory loss were much higher in the “No spasticity” group compared to those in “Spasticity” group. This finding is contrary to hemasthesia, or hemisensory loss, being a predictor for spasticity development in stroke.9–11,18 Our own interpretation has to be cautious in view that only 28 patients (<13%) of the studied population have hemisensory loss even though this impairment is common in stroke.
Significant spasticity can emerge at any time and commonly develops between 1–6 weeks after stroke. However, some develop in the chronic stage (i.e., 6 months after stroke).19 Our resultant predicted clusters were in line with the previous research by Li & Francisco, whereby these individuals developed spasticity complication between 1–20 months after stroke.20 Similarly, this finding also added to the arguments of detecting spasticity early within 12 months post-stroke.21
Therefore, our predicted determinant has shown that the significant relationship of few clinical characteristics that led to spasticity include the duration of a stroke, presence of hemisensory loss, and stroke severity. These factors, although they have been studied before, to our knowledge, they were not explicitly linked together in clustering analysis to produce a predictive model for post-stroke spasticity. Here, we attempted to bridge this gap in our study. Thus, the findings from this study might provide further emphasis on prioritizing patients at-risk who presented with these characteristics for early spasticity intervention using effective management in addition to existing evidence.8–11,19,21 This measure would likely enhances the services in regions or areas where such effective treatments are scarce, whereby patient selection is crucial.
In the healthcare field, the use of clustering analysis allows the segregation of certain patients’ clinical characteristics in predicting a complication.22 Our predictive analysis was deployed based on the historical data to conceptualize our prediction for future events. At the current application, the data we modeled showed that spasticity is predicted to be present if the duration of stroke is between 1 and 20 months with moderate stroke severity in the absence of hemisensory loss. This model will need further validation in prospective study design.
This study has few limitations. Firstly, it was conducted at a specialized outpatient clinic, and the majority of stroke patients who attended this setting were mainly moderate and severe stroke. Those with mild stroke severity do receive a consultation at our setting but mostly were followed up until 1-year post-stroke due to their excellent neurological and functional recovery without significant spasticity complication. Secondly, previous studies have evaluated many predictors, including but not exhaustive to pre-morbid diseases, lesion location, type of operative treatment, and early functional assessments, including Motricity Index and Modified Barthel Index (MBI). The latter measures were more rehabilitation-orientated and highly relevant for this speciality. However, such data were not readily available for all studied populations in our analyses; for example, functional measures such as MBI were documented in separate inpatient clinical records. The Motricity Index is not commonly used as a standard assessment measure in our setting.
Next, the duration after stroke was further grouped into four categories with even duration interval for each. This division was applied based on the understanding that spasticity is less likely to occur very early in stroke and spasticity-related functional impairments are commonly seen in chronic stage. With diverse patients attending the studied site, it would be useful for the treating rehabilitation physician to review the pattern in chronicity of years as described, as ways to foresee the chronic cases attending the clinic setting and prioritize the limited available effective medication for treating spasticity. Assessment of hemisensory loss may also present some limitation on generalizing the data findings. Here, we used either this clinical feature is present, which meant had hemisensory loss at the time of study, or there was no hemisensory loss detected. However, our study did not discriminate whether presence of hemisensory loss was a completely loss or reduced in sensation, and whether pain or fine touch or both sensation were affected. Finally, the extracted data were largely dependent on physicians’ clinical records and accuracy that assessed the patients. There was no inter-rater reliability testing conducted for this study between physicians on spasticity assessment using the MAS. However, all three rehabilitation physicians involved in patients’ assessment received their speciality training from a single medical training center.
Conclusion
Spasticity requires effective treatments for promoting neurological and functional recovery after stroke. Identifying individuals at-risk allow better patient selection to prioritize treatment allocations when such therapies are scarce. Segregating potential clinical characteristics as predictors through clustering analyses is a reliable solution for identifying these individuals.
Acknowledgments
The authors thank the participants of this research and rehabilitation medicine team at the Outpatient Rehabilitation Clinic of Queen Elizabeth Hospital, Kota Kinabalu. Authors also thank the Director General of Ministry of Health Malaysia for permission to publish the results. The research received funding from the Ministry of Higher Education (Dana Penyelidikan, Kementerian Pengajian Tinggi/RACER/1/2019/SKK02/UMS//1). The funders had no role in the study design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Feigin VL, Abajobir AA, Abate KH, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017;16(11):877–879. doi:10.1016/S1474-4422(17)30299-5
2. Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6):S85–90. doi:10.1016/j.wneu.2011.07.023
3. Bani-Ahmed A. The evidence for prolonged muscle stretching in ankle joint management in upper motor neuron lesions: considerations for rehabilitation–a systematic review. Top Stroke Rehabil. 2019;26(2):153–161. doi:10.1080/10749357.2018.1550958
4. Sommerfeld DK, Gripenstedt U, Welmer AK. Spasticity after stroke: an overview of prevalence, test instruments, and treatments. Am J Phys Med Rehabil. 2012;91(9):814–820. doi:10.1097/PHM.0b013e31825f13a3
5. Bhimani RH, Peden-McAlpine C, Gaugler J, Anderson LC. Spasticity over time during acute rehabilitation: a study of patient and clinician scores. Appl Nurs Res. 2016;30:16–23. doi:10.1016/j.apnr.2015.08.007
6. Trompetto C, Marinelli L, Mori L, et al. Pathophysiology of spasticity: implications for neurorehabilitation. BioMed Res Int. 2014;2014:1–8. doi:10.1155/2014/354906
7. Li S, Francisco GE, Rymer WZ. A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil Neural Repair. 2021;15459683211011214.
8. Li S. Spasticity, motor recovery, and neural plasticity after stroke. Front Neurol. 2017;8:120. doi:10.3389/fneur.2017.00120
9. Wissel J, Verrier M, Simpson DM, et al. Post-stroke spasticity: predictors of early development and considerations for therapeutic intervention. PM R. 2015;7(1):60–67. doi:10.1016/j.pmrj.2014.08.946
10. Opheim A, Danielsson A, Murphy MA, Persson HC, Sunnerhagen KS. Early prediction of long-term upper limb spasticity after stroke: part of the SALGOT study. Neurology. 2015;85(10):873–880. doi:10.1212/WNL.0000000000001908
11. Sunnerhagen KS. Predictors of spasticity after stroke. Curr Phys Med Rehabil Rep. 2016;4(3):182–185. doi:10.1007/s40141-016-0128-3
12. Glaess-Leistner S, Ri SJ, Audebert HJ, Wissel J. Early clinical predictors of post-stroke spasticity. Top Stroke Rehabil. 2020;9:1. doi:10.1080/10749357.2020.1843845
13. Ward AB. A literature review of the pathophysiology and onset of post-stroke spasticity. Eur J Neurol. 2012;19(1):21–27. doi:10.1111/j.1468-1331.2011.03448.x
14. Patel AT, Wein T, Bahroo LB, Wilczynski O, Rios CD, Murie-Fernández M. Perspective of an international online patient and caregiver community on the burden of spasticity and impact of botulinum neurotoxin therapy: survey study. J Med Internet Res Public Health Surveill. 2020;6(4):e17928. doi:10.2196/17928
15. Cabanas-Valdés R, Calvo-Sanz J, Serra-Llobet P, Alcoba-Kait J, González-Rueda V, Rodríguez-Rubio PR. The effectiveness of massage therapy for improving sequelae in post-stroke survivors. A systematic review and meta-analysis. Int J Env Res Pub Health. 2021;18(9):4424. doi:10.3390/ijerph18094424
16. Tan SF, Ahmedy F, The SY. Designing and developing a predictive rehabilitation management system for patient registry in northern Borneo. Int J Adv Trends Comp Sci Eng. 2019;6(6):3588–3595. doi:10.30534/ijatcse/2019/142862019
17. Gregson JM, Leathley M, Moore AP, Sharma AK, Smith TL, Watkins CL. Reliability of the tone assessment scale and the modified ashworth scale as clinical tools for assessing poststroke spasticity. Am J Phys Med Rehabil. 1999;80(9):1013–1016. doi:10.1016/S0003-9993(99)90053-9
18. Seok Ryu J, Woo Lee J, Il Lee S, Ho Chun M. Factors predictive of spasticity and their effects on motor recovery and functional outcomes in stroke patients. Top Stroke Rehabil. 2010;17(5):380–388. doi:10.1310/tsr1705-380
19. Triccas LT, Kennedy N, Smith T, Pomeroy V. Predictors of upper limb spasticity after stroke? A systematic review and meta-analysis. Physiotherapy. 2019;105(2):163–173. doi:10.1016/j.physio.2019.01.004
20. Li S, Francisco GE. New insights into the pathophysiology of post-stroke spasticity. Front Hum Neurosci. 2015;9:192. doi:10.3389/fnhum.2015.00192
21. Sunnerhagen KS, Opheim A, Murphy MA. Onset, time course and prediction of spasticity after stroke or traumatic brain injury. Ann Phys Rehabil Med. 2019;62(6):431–434. doi:10.1016/j.rehab.2018.04.004
22. Malik MM, Abdallah S, Ala’raj M. Data mining and predictive analytics applications for the delivery of healthcare services: a systematic literature review. Ann Oper Res. 2018;270(1):287–312. doi:10.1007/s10479-016-2393-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
