Back to Journals » Drug Design, Development and Therapy » Volume 18
Review of the Protective Mechanism of Curcumin on Cardiovascular Disease
Authors Yang C, Zhu Q, Chen Y, Ji K, Li S, Wu Q, Pan Q, Li J
Received 3 November 2023
Accepted for publication 16 January 2024
Published 30 January 2024 Volume 2024:18 Pages 165—192
DOI https://doi.org/10.2147/DDDT.S445555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Chunkun Yang,1,* Qinwei Zhu,2,* Yanbo Chen,3,* Kui Ji,2 Shuanghong Li,2 Qian Wu,1 Qingquan Pan,2 Jun Li1
1Department of Cardiology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2Department of Emergency, Weifang Hospital of Traditional Chinese Medicine, Weifang, Shandong, People’s Republic of China; 3Department of Arrhythmia, Weifang People’s Hospital, Weifang, Shandong, People’s Republic of China
*These authors contributed equally to this workThese authors contributed equally to this work
Correspondence: Jun Li, Email [email protected]
Abstract: Cardiovascular diseases (CVDs) are the most common cause of death worldwide and has been the focus of research in the medical community. Curcumin is a polyphenolic compound extracted from the root of turmeric. Curcumin has been shown to have a variety of pharmacological properties over the past decades. Curcumin can significantly protect cardiomyocyte injury after ischemia and hypoxia, inhibit myocardial hypertrophy and fibrosis, improve ventricular remodeling, reduce drug-induced myocardial injury, improve diabetic cardiomyopathy(DCM), alleviate vascular endothelial dysfunction, inhibit foam cell formation, and reduce vascular smooth muscle cells(VSMCs) proliferation. Clinical studies have shown that curcumin has a protective effect on blood vessels. Toxicological studies have shown that curcumin is safe. But high doses of curcumin also have some side effects, such as liver damage and defects in embryonic heart development. This article reviews the mechanism of curcumin intervention on CVDs in recent years, in order to provide reference for the development of new drugs in the future.
Keywords: curcumin, intima, endothelial cells, vascular smooth muscle cell, cardiovascular diseases, inflammation, apoptosis
Introduction
CVDs, disorders of the heart and blood vessels, are a major health problem worldwide, causing the largest number of deaths, and its incidence is still rising. It is estimated that nearly 20 million people worldwide died from CVDs in 2016, accounting for one-third of all deaths. It is predicted that by 2030, 40.5% of Americans will have CVDs, which will result in about $818 billion in health care costs and $276 billion in indirect costs.1 However, many people fail to recognize the risk of CVDs. Individuals at risk for CVDs may present high blood pressure, weight issues, and altered glucose or lipid levels. Great progress has been made in the research of the pathogenesis mechanism of CVDs, but the morbidity and mortality of CVDs are still very high. There is an urgent need for drugs that are more effective and have minimal side effects to prevent and treat these diseases.
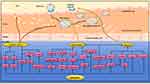
Compared to traditional drugs, natural drugs have many advantages, such as fewer side effects, low long-term toxicity, and variable bioavailability.2 Curcuma originated in India and has a history of 6000 years. About 700 AD, Curcuma was introduced into China and was first recorded in the New Materia Medica. In 1815, curcumin was isolated in an impalured form by Milobedeska and Lampe. It was not until 1910 that the chemical structure of curcumin was determined and could be synthesized chemically. Curcumin is a bioactive component of the curry spice, and its pleiotropic effects in CVDs suggest that it is a promising drug candidate. Specifically, curcumin can significantly alleviate vascular endothelial dysfunction, inhibit foam cell formation, reduce VSMCs proliferation, protect cardiomyocyte injury after ischemia and hypoxia, inhibit myocardial hypertrophy and fibrosis, improve ventricular remodeling, reduce drug-induced myocardial injury, and improve DCM.3,4 The current research on curcumin is scattered. In order to further clarify the mechanism of curcumin in CVDs, this study systematically summarized the research reports on curcumin in recent years (Figure 1).
 |
Figure 1 (A) Curcuma. (B) Curcuma Decoction pieces. (C) New Materia Medica and Picture of Curcuma. (D) Chemical Structure of Curcumin. (E) Blood Vessels and Heart. |
Search Strategy
This review is based on the guidelines and recommendations of the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA). All relevant articles published in PubMed between January 2010 and August 2023 were included in this review. We used the keywords “curcumin”, “atherosclerosis”, “vascular smooth muscle cells”, “vascular endothelium”, “Vascular endothelial cell”, “vascular endothelium”, “vascular endothelium”, “plaque”, “adhesion”, “lipids”, “vasodilation”, “cardiac”, “myocardial ischemia”, “cardiotoxicity”, “myocardial cell” and “cardiomyocyte” to search the database. We did a preliminary screening of the obtained articles by browsing the title and abstract to include eligible articles. We included curcumin pharmacokinetic data, drug safety studies, CVDs-related biological mechanisms, and clinical studies.
Protective Effect of Curcumin on Blood Vessels
Reduce the Oxidative Stress of Vascular Endothelial Cells(VECs)
Oxidation should be mainly stimulated by excessive generation of reactive oxygen species (ROS). It plays an important role in mediating various vascular inflammatory signal pathways.5 Nuclear factor erythroid factor 2 related factor 2 (Nrf2) is a key regulatory factor for redox homeostasis and cellular antioxidant defense. Under homeostatic conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein 1(Keap1). When the oxidative stress system is activated, Nrf2 separates from Keap1 and then translocated to the nucleus to bind to antioxidant response element (ARE) and regulate the expression of antioxidant defense genes such as heme oxygenase-1 (HO-1).6 Zhou et al found that curcumin could activate Nrf2/HO-1 signaling pathway to resist hydrogen peroxide (H2O2)-induced oxidative stress in Ea.hy926 cells.7 H2O2 is a kind of endogenous ROS. In one study, Yang et al found that curcumin (25 μM) alleviated the damage of H2O2 to human umbilical vein endothelial cells (HUVECs) by inhibiting Notch1 signaling pathway.8 In another study, H2O2 decreased cell viability and antioxidant enzyme activity and increased oxidative stress levels in HUVECs. However, curcumin (40 and 50 μM) reversed these changes.9 (Figure 2 and Table 1)
 |
Table 1 Protective Mechanism of Curcumin on Blood Vessels |
Lan et al randomly assigned stroke-prone spontaneously hypertensive rats (SHRs) to receive saline or curcumin(100 mg/kg/day). Finally, it was found that curcumin group may reduce the incidence of stroke by regulating uncoupling protein-2(UCP2)/nitric oxide(NO) signaling pathway to reduce oxidative stress and improve vascular endothelial function.10 In another study, Boonla et al found that curcumin (50 and 100 mg/kg/day) significantly reduced blood pressure and improved endothelial dysfunction and vascular remodeling in 2kidney-1clip(2K-1C) hypertensive rats by reducing matrix metalloproteinases (MMPs) levels and inhibiting oxidative stress.11
Hyperglycemia can induce superoxide anion production.86 The production of superoxide anion in the vasculature is associated with nicotinamide adenine dinucleotide phosphate(NADPH) reduced oxidase, which requires the activation of protein kinase C (PKC).87 Rungseesantivanon et al found that curcumin supplementation (300 mg/kg/day) inhibited PKC activity and reduced superoxide production, thereby improving endothelial function in diabetic mice.12 The cluster of differentiation 40 (CD40)/cluster of differentiation 40 ligand (CD40L) system can upregulate proinflammatory and prothrombotic genes in atherosclerotic vessels, and it also upregulates ROS production in endothelial cells.88,89 Wu et al found that curcumin (10 µM) could inhibit the expression of CD40 in the miR-590-3p dependent pathway, thereby reducing the oxidative stress level and protecting VECs.13 (Figure 2 and Table 1)
Inhibits Inflammation of VECs
Toll-like receptor (TLR)4 is a pattern recognition receptor of the immune system.90 TLR4 stimulation can lead to the activation of nuclear factor kappa-B(NF-κB) transcription factor,91 promote inflammatory response, and lead to atherosclerotic plaque instability.92 Zhang et al found that curcumin (0.1% curcumin added to high-fat diet(HFD)) significantly weakened the development of AS in ApoE−/− mice by inhibiting the expression of TLR4 in atherosclerotic plaque. In addition, curcumin also decreased the expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecule-1(ICAM-1), tumor necrosis factor alpha(TNF-α) and the activity of NF-κB.14 High mobility group box protein 1 (HMGB1) is a potent extracellular proinflammatory mediator.93 In one study, curcumin (5 to 100 μM) down-regulated the expression of TLR2 and TLR4 on the surface of human endothelial cells (HECs), thereby inhibiting lipopolysaccharide(LPS)-mediated HMGB1 release. In addition, curcumin can also down-regulate the cell surface receptor of HMGB1 in HECs.15 In another study, Lv et al found that curcumin (0.5, 1 and 2 μM) could reduce the release of inflammatory factors in HUVECs induced by human cytomegalovirus (HCMV) infection by inhibiting HMGB1/TLR/NF-κB signaling pathway, thereby improving the progression of AS.16 Chen et al found that curcumin (10 μM) promoted cell proliferation and inhibited apoptosis, inflammation and oxidative stress, thereby reducing HUVECs cell damage induced by oxidized low-density lipoprotein (ox-LDL). The mechanism is related to the regulation of miR-599/Myeloid differentiation factor 88(MyD88)/NF-κB signaling pathway by curcumin.17 (Figure 2 and Table 1)
Lipocalin-2 (LCN2) is an inflammatory factor released by activated neutrophils, which is closely related to AS.94 In one study, dietary curcumin(60 and 80 mg/kg/day) improved AS in in ApoE−/− mice by downregulating LCN2 and inhibiting inflammation.18 P-selectin and fractalkine are key molecules in the inflammatory process related to AS. Pirvulescu et al found that curcumin (20 μM) significantly inhibited the expression of p-selectin and fractalkine, and decreased the activation of NADPH oxidase and intracellular ROS levels in HECs exposed to resistin.19 HO-1 is a subtype of heme oxygenase that inhibits AS, inflammation, and oxidative stress.95 Xiao et al found that curcumin (100 μM) induced HO-1 expression by activating p38 mitogen-activated protein kinase(p38 MAPK)/Nrf2 signaling pathway, thereby inhibiting inflammation in human arterial endothelial cells (HAECs).20 In addition, HO-1 can also improve inflammation by inhibiting the expression of endothelial cell adhesion molecules.96 In one study, curcumin (10 and 30 μM) induced HO-1 expression and inhibited ICAM-1 expression in Ea.hy926 cells treated with TNF-α, thereby improving VECs inflammation.21 (Figure 2 and Table 1)
Hyperglycemia can interfere with vascular homeostasis and lead to vascular complications in diabetes. In one study, curcumin (300 mg/kg/day) inhibited NADPH oxidase and reduced ROS and ICAM-1 in a diabetic rat model, thereby improving diabetic vascular inflammation.22 In another study, curcumin (10 μM) attenuated high glucose-induced inflammatory injury in rat thoracic aortic endothelial cells (TAECs) through inhibition of phosphatidyinositol 3-kinase(PI3K)/protein kinase B(Akt)/NF-κB signaling pathway.23 Advanced glycation end products (AGEs) induced by hyperglycemia are the main cause of diabetes-related complications.97 Methylglyoxal (MGO) is a key precursor of AGEs, and the capture of MGO is an effective strategy to alleviate endothelial damage induced by carbonyl stress.98 In one study, curcumin (10 µM) could protect endothelial cells by trapping MGO and inhibiting the formation of AGEs.24 In another study, Hu et al similarly found curcumin (0.25 to 2.5 µM) to be an effective MGO catcher in HUVECs.25 Narra et al evaluated the effects of curcumin and recombinant high-density lipoprotein(HDL) nanoparticles(Cur-rHDLs) on MGO-induced murine cerebrovascular endothelial cells (bEnd.3). They found that Cur-rHDLs(0.2 and 0.4 µM) reduced oxidative stress and endoplasmic reticulum stress(ERS) and played a protective role in the integrity of the endothelial cell barrier.26 AGEs also have an effect on myocardial inflammation. Sowndhar et al found that dietary curcumin could neutralize AGEs-induced adverse reactions and reduce the expression of pro-inflammatory genes.27 Elevated plasma homocysteine (Hcy) can impair VECs and has been an independent risk factor for ischemic CVDs.99 Li et al found that curcumin (17 µM) could protect VECs from Hcy injury by inhibiting the expression of NF-κB and interleukin-8 (IL-8).28 (Figure 2 and Table 1)
Acrolein is a known toxin in tobacco smoke that can cause inflammation in VECs. Lee et al found that curcumin (25 μM) could inhibit acrolein-induced cyclooxygenase (COX)-2 expression and prostaglandin production in HECs by inhibiting PKC/p38 MAPK/cyclic adenosine monophosphate (cAMP)-response element binding protein(CREB) pathway, thereby inhibiting cellular inflammatory response.29 Biodegradable poly-L-lactic acid (PLLA) is often used as major components or coating agents in tissue engineering and scaffolds.100 In one study, PLLA degraded extract significantly inhibited the migration and increased the inflammatory response of HAECs, which was alleviated by curcumin (5 μM) treatment.30 (Figure 2 and Table 1)
Inhibit the Adhesion of Macrophages to VECs
During the progression of AS, monocytes can be trapped by endothelial secretion of adhesion molecules such as ICAM-1, E-selectin, and P-selectin, and then enter the subendothelium.101,102 Li et al found that atorvastatin calcium combined with curcumin effectively reduced E-selectin and ICAM-1, thereby alleviating AS.31 Monocyte chemoattractant protein 1 (MCP-1) also plays an important role in adhesion and migration of monocytes to arterial intima.103 In one study, curcumin (30 μM) decreased MCP-1 expression in rat aortic endothelial cells (RAECs) induced by high glucose by inhibiting the NF-κB pathway.32 In another study, Monfoulet et al exposed HUVECs to curcumin (1 μM) for 3h before TNF-α activation. It was found that curcumin reduced monocyte adhesion and their transendothelial migration.33 ox-LDL can increase the production of endoderm-derived ROS and the expression of adhesion molecules.104,105 In one study, curcumin (67.9 μM) significantly inhibited the expression of ROS, lectin-like ox-LDL receptor-1 (LOX-1) and adhesion molecules in TNF-α-stimulated HUVECs.34 Radiotherapy can increase the expression of adhesion molecules in VECs.106 Soltani et al found that nanoformulation of curcumin could inhibit radiation-induced adhesion molecules (especially ICAM-1) by modulating NF-κB and Nrf2 pathways, thereby inhibiting monocyte adhesion to HUVECs.35 (Figure 2 and Table 1)
Regulate Lipid Metabolism and Inhibit Lipid Plaque Formation
Lipid metabolism disorders are closely related to AS.107 In one study, curcumin (500 and 1000 mg/kg/day) effectively reduced fatty streak formation and inhibited IL-6 expression in the descending aorta of LDLR−/−mice fed a HFD.108 In another study, curcumin reduced serum levels of total cholesterol (TC), total triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), ox-LDL cholesterol, circulating inflammatory cytokines and soluble adhesion molecules in New Zealand white rabbits.109 Lv et al found that curcumin (15 and 25 mg/kg/day) decreased serum LDL-C, TC, and TG levels, significantly reduced aortic atheromatous plaque formation, and reduced lipid deposition in the liver in ApoE−/− mice.16 Zingg et al found that curcumin (500, 1000 and 1500 mg/kg/day for 4 months) reduced plasma and tissue lipid levels in LDLR−/− mice fed HFD. Further studies have found that curcumin can induce the increase of cAMP level in liver, which may be one of its mechanisms of lowering blood lipids and anti-AS.36 Apolipoprotein B (Apo B) is a major component of LDL in mammals, and it comes in two subtypes: apoB-100 and apoB-48. Lipoprotein particles containing apoB-48 were cleared from plasma more quickly than those containing apoB-100 (10 mins vs 3 days).110 Tian et al found that curcumin (15 μM) increased the level of apoB-48 and decreased the level of ApoB-100 by increasing the expression of APOBEC-1 in rat hepatocytes.37 The spleen also plays an important role in lipid metabolism.111 Splenectomy can lead to hyperlipidemia with macrophage dysfunction.112 Altinel et al found that curcumin (20 mg/kg/day for 28 days) partially improved lipid metabolism disorders by regulating NF-κB, superoxide dismutase (SOD) and glutathione(GSH) peroxidase (GSH-Px) in splenectomy rats.38 (Figure 3 and Table 1)
Monocytes/macrophages migrate to the inner membrane and subsequently clear cholesterol and secrete proinflammatory cytokines.113,114 Excessive LDL uptake or impaired cholesterol efflux in macrophages accelerates foam cell formation. Scavenger receptor class A(SR-A) and cluster of differentiation 36(CD36) are responsible for modifying LDL internalization.115,116 In contrast, the class B scavenging receptor type I (SR-BI) and the ATP-binding cassette transporters A1(ABCA1) and G1(ABCG1) are responsible for intracellular cholesterol efflux to HDL or apolipoprotein AI (apo AI).117,118 In one study, curcumin (40 μM) treatment significantly improved ox-LDL-induced intracellular cholesterol accumulation in macrophages by decreasing protein expression of SR-A and increasing protein expression of ABCA1.39 In other studies, researchers made similar findings. Tan et al found that curcumin (40 μM) could promote cholesterol efflux in human myeloid leukemia mononuclear cells (THP-1) macrophages by increasing ABCA1 expression via the miR-125a-5p/Sirtuin 6(Sirt 6) signaling axis.40 Min et al found that curcumin (10 μM) could inhibit the expression of CD36 through p38 MAPK signaling pathway.41 Liver X receptors (LXRs) are ligand-dependent transcription factors that can be activated by cholesterol.119 In one study, curcumin (10, 20 and 40 μM) enhanced cholesterol efflux from foam cells by inhibiting the c-Jun N-terminal kinase(JNK) pathway and activating the LXR/ABCA1/SR-BI pathway.42 Lin et al also found that curcumin (10 μM) promoted cholesterol efflux in THP-1 macrophage-derived foam cells by upregulating ABCA1 expression through activation of AMP-activated protein kinase(AMPK)/Silent information regulator 1(Sirt 1)/LXR signaling.43 Not all cell experiments have reached the same conclusion. Chen et al found that curcumin (6.25 and 12.5 μM) enhanced ox-LDL-induced cholesterol esterification and foam cell formation by upregulating CD36 and ABCA1 expression in M1 macrophages.120 In another study, curcumin (5 μM) increased lipid levels in THP-1 monocytes and RAW264.7 macrophages, associated with up-regulation of CD36/fatty acid translocase(FAT) and fatty acid binding protein 4(FABP4). However, in vivo experiments, curcumin (500, 1000, and 1500 mg/kg/day) reduced lipid levels in peritoneal macrophages of LDL-receptor knockout mice after 4 months of HFD.121 (Figure 3 and Table 1)
Curcumin also inhibits macrophage release of inflammatory cytokines. In one study, curcumin (10, 20 and 40 μM) inhibited ox-LDL-induced MCP-1 production by inhibiting JNK and NF-κB pathways.44 In another study, curcumin (6.25 and 12.5 μM) significantly reduced ox-LDL-induced production of inflammatory cytokines, such as IL-1β, IL-6 and TNF-α.45 Hypoxia inducible factor-1α (HIF-1α) is also closely related to inflammation. Curcumin (20 and 40 µM) can inhibit HIF-1α-induced macrophage inflammation and apoptosis by inhibiting extracellular regulated protein kinase(ERK) signaling pathway.46 According to the activation pattern and function, macrophages can be differentiated into two subtypes: classically activated M1 macrophages and alternatively activated M2 macrophages. M1 macrophages are characterized by proinflammation and tissue damage, while M2 macrophages are involved in anti-inflammatory and tissue remodeling.122 In one study, curcumin (6.25, 12.5, and 25 μM) polarized macrophages to the M2 phenotype by activating peroxisome proliferator-activated receptor γ (PPAR γ).47 In another study, curcumin (7.5 to 30 μM) promoted the transformation of M1 macrophages into M2 phenotype by inhibiting the TLR4/MAPK/NF-κB pathway.48 Cadmium is a non-essential element in the human body. It does not degrade easily, so it is easy to accumulate in crops. Some surveys have shown an increased incidence of AS in areas exposed to cadmium.123 Zhang et al found that cadmium exposure led to changes in microbial community composition, resulting in increased trimethylamine-N-oxide (TMAO) synthesis, lipid metabolism imbalance and M1-type macrophage polarization in ApoE−/− mice, thus promoting the progression of AS. Intragastric administration of curcumin (100 mg/kg/day) reverses cadmium-induced AS progression by restoring intestinal flora.49 (Figure 3 and Table 1)
MMP-9 and extracellular matrix metalloproteinase inducer (EMMPRIN) in macrophages are closely related to atherosclerotic plaque rupture.124 In one study, curcumin (25 μM) inhibited EMMPRIN and MMP-9 expression in ox-LDL-stimulated macrophages by down-regulating NF-κB and p38 MAPK signaling pathways.50 In another study, curcumin (6.25, 25 and 50 µM) inhibited the expression of EMMPRIN, MMP-9, and MMP-13 in THP-1 cells stimulated by phorpol 12 myristic acid 13 acetate (PMA) through PKC and AMPK pathways.51 Thrombospondins(THBS) plays a role in stabilizing atherosclerotic plaques.125 Curcumin (5, 15 and 25 µM) could significantly inhibit the reduction of THBS-4 expression in macrophages induced by ox-LDL.52 All these three studies indicated that curcumin could stabilize atherosclerotic plaque. (Figure 3 and Table 1)
Promote Angiogenesis
Diabetes mellitus (DM)-induced hyperglycemia can cause nonhealing ulcers and impaired wound healing. Endothelial progenitor cells (EPCs) play an important role in wound healing. In one study, curcumin (10 μM) reversed DM-induced EPCs dysfunction (EPCD) by increasing the expression of manganese superoxide dismutase (MnSOD).53 In another study, curcumin promoted angiogenesis, migration, and proliferation of EPCs and reversed the number of aging EPCs by a mechanism related to up-regulation of vascular endothelial growth factor(VEGF)-A and angiotensin I(Ang I;). In vivo studies, curcumin can significantly improve blood reperfusion in ischemic hind limbs of type 1 diabetic mice by increasing capillary density.54 Chen et al found that curcumin (10 μM) may enhance the migration and healing of HUVECs by enhancing autophagy. In vivo, curcumin (25 and 75 mg/kg/day) can promote re-endothelization and intimal hyperplasia after carotid artery balloon injury in rats by inducing autophagy, inhibiting oxidative stress levels and apoptosis.55 miR-93 has been reported to induce angiogenesis in peripheral arterial disease (PAD) mice.126 Zhang et al found that curcumin (1000 mg/kg/day) can promote angiogenesis by upregulating miR-93 and exert beneficial effects on non-diabetic PAD.56 It is worth mentioning that the effect of curcumin on angiogenesis may be dose-dependent. In one study, curcumin (50 μM) may inhibit the proliferation and migration rate of HUVECs by regulating focal adhesion kinase(FAK)/p-38 MAPK signaling pathway.127
Reduce Drug and Environmental Damage to VECs
Cyclosporine A(CsA) is a commonly used immunosuppressant in clinical practice, but it also has some side effects, such as causing hypertension.128 Sagiroglu et al found that curcumin (200 mg/kg/day) could alleviate CsA-induced endothelial dysfunction in rats by anti-oxidative stress.57 Methotrexate, the most commonly used antirheumatic drug, also has the side effect of damaging the endothelium.129 Sankrityayan et al found that curcumin (200 and 400 mg/kg/day) could eliminate the vascular side effects of methotrexate by inhibiting oxidative stress and the reduction of physiological NO levels.58
Rapamycin eluting scaffolds can inhibit the proliferation and migration of VSMCs and reduce vascular restenosis. But rapamycin can inhibit the proliferation and migration of endothelial cells and induce apoptosis of EPCs, thereby destroying re-endothelialization.130 Guo et al found that curcumin (30 μM) antagonizes the harmful effects of rapamycin on RAECs by upregulating endothelial nitric oxide synthase(eNOS).59 PLLA is a candidate material for biodegradable vascular scaffolds.131 In one study, there was a significant increase in inflammatory cytokines around PLLA stents after they were implanted into porcine coronary arteries. In vitro studies, curcumin (20 μM) can reduce foam cell inflammation caused by PLLA degradation through PPAR γ signaling pathway.60 Epidemiological studies have shown that air pollution is associated with the development of vascular diseases. Shi et al found that curcumin attenuates particulate matter 2.5(PM 2.5) damage to human microvascular endothelial cells (HMECs) by reducing the levels of ROS, ox-LDL, ICAM-1 and VCAM-1.61
Other Protective Effects of Curcumin on VECs
ERS is involved in endothelial cell injury. LOX-1 is a major receptor for ox-LDL in endothelial cells.132 Excessive binding of ox-LDL to LOX-1 can lead to endothelial dysfunction.133 Luo et al found that curcumin (2.5 μM) may block the upregulation of LOX-1 in HUVECs by inhibiting ERS, thereby reducing subcutaneous lipid deposition.62 In another study, curcumin (2.5 to 20 μM) inhibited ERS and subsequently inhibited the JNK/insulin receptor substrate 1(IRS-1) pathway, thereby improving insulin resistance and protecting endothelial function in diabetic patients.134 (Figure 3 and Table 1)
Basal level autophagy maintains cellular homeostasis by clearing and recycling damaged cytoplasmic contents.135 Han et al found that curcumin (5 µM) could inhibit PI3K/Akt/mammalian target of rapamycin(mTOR) signaling pathway and increase free beclin1(BECN1) in the cytoplasm, thereby promoting autophagy and protecting H2O2-intervened HUVECs.63 Interestingly, Guo et al had similar findings in another study. They found that curcumin (5 and 20 µM) could inhibit apoptosis and induce autophagy through the Akt/mTOR pathway, thereby protecting Ea.hy926 cells from oxidative stress-induced damage.64 Curcumin can also induce autophagy through other pathways. Zhao et al found that curcumin (5 μM) ameliorated ox-LDL-induced endogenous toxicity and attenuated endothelial dysfunction by regulating AMPK/mTOR/p70S6K autophagy signaling pathway.65 Previous studies have shown that ox-LDL can cause abnormal crosstalk between autophagy and inflammation in macrophages. Li et al found that curcumin(20 μM) regulates ox-LDL-induced macrophage autophagy and inflammation via the transcription factor EB(TFEB)/P300/bromodomain-containing protein 4(BRD4) pathway.66 (Figure 3 and Table 1)
NOD-, LRR- and pyrin domain-containing protein 3(NLRP3) inflammasome is an apoptosis-associated protein.136 In one study, curcumin (6.25, 12.5 and 25 μM) reduced NLRP3 inflammasome production in PMA-stimulated macrophages by inhibiting TLR4/MyD 88/NF-κB signaling.67 Pyroptosis is a kind of programmed cell death, which is closely related to inflammatory response.137 Yuan et al found that curcumin(25 µM) inhibited H2O2-induced pyroptosis of HUVECs by inhibiting NLRP3 activation.68
Cellular senescence is a state of cell growth arrest, which is irreversible.138 Studies have shown that Sirt1 is involved in the process of age-related vascular diseases, such as AS.139 Sun et al found that curcumin (25 μM) could attenuate oxidative stress-induced premature senescence in HUVECs by activating Sirt1.69
The endothelial-to-mesenchymal transition (EndMT) is one of the mechanisms of myofibroblast generation in fibrous tissues or organs.140 Transforming growth factor-β1 (TGF-β1) can induce EndMT.141 Chen et al found that curcumin (5 and 10 μM) could inhibit TGF-β1-induced EndMT by regulating nuclear factor erythroid-2-related factor 2(Nrf-2)/dimethylarginine dimethylaminohydrolase(DDAH)/asymmetric dimethylarginine(ADMA)/NO pathway, thereby attenuating endothelial cell fibrosis.70
Transient receptor potential vanilloid 4 (TRPV4), as a member of Ca2+ -permeable ion channels, which plays an important role in vasodilatation.142,143 Shao et al found that curcumin (1 to 300 μM) could stimulate Ca2+ entry into endothelial cells and improve vasodilation function by activating TRPV4 channels.71
Protection of VSMCs by Curcumin
Migration of VSMCs from media to intima is a key pathogenic event in the formation and development of AS and vascular restenosis.144 In one study, curcumin (20 μM) inhibited angiotensin II(Ang II)-induced migration of VSMCs in SHRs, possibly by inhibiting NF-κB/NLRP3 signaling.72 Mintz et al found that curcumin (10 μM) could inhibit the proliferation and migration of VSMCs after arterial balloon injury in rats by regulating miR-22/specific protein 1(SP1) pathway.73 Chemerin is a novel adipokine that is positively correlated with the severity of AS.145 He et al found that curcumin (60 mg/kg/day) alleviated the proliferation and migration of VSMCs during AS by inhibiting chemerin/chemokine-like receptor 1 (CMKLR1)/LCN2 pathway.74 Insulin-like growth factor 1 (IGF-1) is a mitogen associated with the proliferation, hypertrophy and migration of VSMCs.146 Youreva et al found that curcumin (5, 25 and 50 μM) attenuates IGF-1-induced VSMCs proliferation and migration by inhibiting the protein kinase B (PKB)/glycogen synthase kinase-3β(GSK-3β)/early growth response protein 1(Egr-1) pathway.75 MMP-2, a member of the Zinc-dependent protease family, plays a key role in promoting the proliferation and migration of VSMCs and weakening the stability of atherosclerotic plaques. Zhong et al found that curcumin (20 and 40 μM) inhibited the migration of VSMCs by inhibiting TNF-α-induced MMP-2 expression and activity through the NF-κB pathway.76 In another study, Zhong et al also found that curcumin (20 and 40 μM) inhibited LPS-induced MMP-2 activity through rat sarcoma(Ras)/mitogen-activated protein kinase kinase 1/2(MEK1/2) pathway.77 (Figure 4 and Table 1)
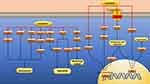 |
Figure 4 Pharmacological effects of curcumin on vascular smooth muscle cells. Curcumin can inhibit the proliferation, migration and inflammation of vascular smooth muscle cells. |
VSMCs also release inflammatory cytokines that accelerate AS.147 In one study, curcumin (5, 10 and 20 μM) inhibited endothelin-induced TNF-α, IL-6, and NO production and cell proliferation in VSMCs by increasing PPAR γ activity and inhibiting NADPH oxidase-mediated intracellular ROS production.78 In another study, curcumin (5 μM) inhibited C-reactive protein (CRP) production in aldosterone-induced VSMCs by interfering with ROS/ERK1/2 signaling.79 LPS and ox-LDL also stimulated VSMCs to overexpress inflammatory factors, including TNF-α and MCP-1. In one study, curcumin (5, 10 and 30 μM) inhibited overexpression of inflammatory mediators in LPS-induced VSMCs by a mechanism related to inhibition of the TLR4/MAPK/NF-κB pathway.80 In another study, curcumin (10 and 30 μM) inhibited ox-LDL-induced MCP-1 expression by inhibiting p38 MAPK and NF-κB pathways in VSMCs.81 In addition, Ruan et al found that curcumin(135.7 μM) can reduce LPS-induced inflammatory damage by reducing apoptosis, increasing VSMCs activity and inhibiting the release of inflammatory cytokines, the mechanism of which is related to the inhibition of NF-κB and JNK signaling pathways.82 (Figure 4 and Table 1)
The activation of renin aniotension aldosterone system(RAAS) is associated with the pathogenesis of hypertension.148 Yao et al found that curcumin (1 μM) down-regulated the expression of angiotensin type 1 receptor(AT1R) in VSMCs by inhibiting SP1/AT1R pathway, and then inhibited the occurrence of Ang II–induced hypertension.83 Endothelin-1(ET-1) is a potent vasoconstrictor peptide that plays a key role in inducing growth and hypertrophy of VSMCs. Kapakos et al found that curcumin (25 μM) could counteract the biological response of ET-1 and play an important role in vascular protection by inhibiting the c-Raf/ERK1/2/Egr-1 and IGF-1 receptor(IGF-1R)/PKB pathways.84 There are two phenotypes of VSMCs, the contractile type and the synthetic type. The switch of VSMCs from a contractile to a synthetic phenotype plays a deleterious role in the development of AS and intimal hyperplasia. Sun et al found that Nicotinic-curcumin(1 μM) and curcumin(10 μM) could inhibit Ang II–induced VSMCs switching from a contractile to a synthetic phenotype by regulating the phosphatase and tensin homolog(PTEN)/Akt pathway.85 (Figure 4 and Table 1)
Clinical Trials
Flow-mediated dilation (FMD) can reflect the biological activity of endothelial NO and is a good indicator of vascular endothelial function. Choi et al administered curcumin (150 mg) (n=6) or placebo (n=8) to 14 healthy, sedentary young men before performing eccentric elbow flexor exercises on the nondominant arm. The study found that acute curcumin supplementation can reduce the decrease of FMD of brachial artery.149 In another randomized controlled trial, curcumin (0.2 g/day for 8 weeks) increased FMD and thus improved endothelial function in healthy young subjects.150 A meta-analysis by Hallajzadeh et al also showed that curcumin (0.045 to 2 g/day) improved FMD.151 Santos-Parker et al randomly assigned 39 healthy men and postmenopausal women to receive curcumin (N=20) or placebo (N=19) for 12 weeks. Studies have found that curcumin (2 g/day longvida®) can increase vascular NO bioavailability, reduce oxidative stress, and improve arterial endothelial function.152
Protect Cardiomyocytes
Alleviate Ischemia and Reperfusion Injury
Myocardial ischemia and reperfusion(I/R) leads to increased oxidative stress, which aggravates cardiomyocyte injury. Sirt1, a histone deacetylase, whose activation reduces oxidative stress.153 Yang et al found that curcumin (0.25, 0.5 and 1 μM) could reduce I/R-induced mitochondrial oxidative damage by activating Sirt1 signaling pathway.154 Janus kinase(JAK)/signal transducer and activator of transcription(STAT) signaling pathway plays an important role in the myocardial response to various cardiac injuries.155 Duan et al found that curcumin (1 μM) could alleviate I/R injury in isolated rat hearts by activating JAK2/STAT3 signaling pathway.156 Liu et al obtained the same conclusion in vivo. They found that curcumin (10, 20 and 30 mg/kg/day) alleviated myocardial I/R injury in rats by activating JAK2/STAT3 signaling pathway.157 TLRs are key signaling receptors mediating innate immune recognition.158 Kim et al found that curcumin (300 mg/kg/day) could reduce myocardial I/R injury in Sprague Dawley(SD) rats by selectively inhibiting TLR2.159 Fiorillo et al found that curcumin (10 μM) could protect cardiomyocytes from I/R injury, and the mechanism may be related to antioxidative stress, inhibition of nuclear translocation of NF-κB and JNK phosphorylation.160 Zhu et al found that curcumin (10 µM) attenuates hypoxia/reoxygenation(H/R)-induced injury in H9C2 cells by down-regulating Notch pathway.161 m6A modification, the most common type of RNA methylation, is involved in myocardial I/R by influencing RNA stability and translation to regulate gene expression. Cui et al found that curcumin (10 µM) attenuated myocardial I/R damage in H9c2 cardiomyocytes by regulating total RNA m6A levels.162 (Figure 5 and Table 2)
 |
Table 2 Protective Mechanism of Curcumin on Cardiomyocytes |
Reduce Cardiomyocyte Apoptosis
Excessive catecholamines can cause some damage to myocardium. Izem-Meziane et al found that curcumin (60 mg/kg/day) reduced isoproterenol (ISO) -induced cardiomyocyte apoptosis in Wistar rats by preventing mitochondrial damage and mitochondrial permeability transition pore(mPTP) opening.163 In another study, Boarescu et al found that curcumin (200 mg/kg/day) attenuates ISO-induced cardiomyocyte apoptosis in rats.200 Heat shock proteins (Hsps) are a type of stress proteins that help maintain protein stability and mediate proper folding, segmentation, and degradation of unfolded proteins when cells are subjected to harmful stimuli. Tanwar et al found that curcumin pretreatment (100 and 200 mg/kg/day) increased the level of Hsp27 after ISO induced myocardial ischemic injury in rats, thereby protecting cardiomyocytes.164 SP1 is a widely expressed transcription factor that is closely related to cell differentiation, growth and apoptosis.201 Geng et al found that curcumin(50 mg/kg/day) can protect hypoxia-induced cardiomyocyte apoptosis by up-regulating miR-7a/b and down-regulating SP1 expression.165 Coronary microembolism (CME) is closely related to cardiomyocyte apoptosis. In a study, curcumin (150 mg/kg/day) alleviated myocardial inflammation and inhibited myocardial apoptosis in rats after CME by inhibiting TLR4/MyD88/NF-κB signaling pathway.166 Chronic intermittent hypoxia can lead to increased infarct size in patients with myocardial infarction, and HIF-1α seems to be a key player in this vicious cycle. Moulin et al found that chronic curcumin (100 mg/kg/day) treatment could inhibit HIF-1α activation induced by intermittent hypoxia, thereby reducing infarct size in mice.167 (Figure 5 and Table 2)
Inhibit Cardiomyocyte Hypertrophy
Left ventricular hypertrophy is a physiologically adaptive response to increased hemodynamic pressure load that initially improves systolic function but ultimately leads to heart failure.202 The histone acetyltransferase (HAT) activity of p300 is closely related to cardiac hypertrophy and heart failure. GATA binding protein 4(GATA4) is a hypertrophic response transcription factor that can be activated by p300-HAT.203 Sunagawa et al found that curcumin (20 μM) could alleviate cardiomyocyte hypertrophy by inhibiting p300-HAT activity.168 In another study, Sunagawa et al also found that curcumin (50 mg/kg/day) reduced cardiac hypertrophy induced by hypertension in Dahl salt-sensitive(DS) rats by reducing p300-HAT activity and GATA4 acetylation levels.169 Two other studies reached similar conclusions. Chowdhury et al found that curcumin (0.1 mg/kg, intraperitoneal injection) attenuates LPS-induced cardiac hypertrophy in mice by inhibiting p300-HAT activity.170 Ahuja et al found that curcumin (8 μM) inhibited cardiomyocyte hypertrophy induced by norepinephrine by inhibiting the nuclear localization and DNA-binding activity of GATA-4.171 (Figure 5 and Table 2)
Enhanced expression of Na+/Ca2+ exchanger(NCX) in cardiomyocytes can improve systolic function in heart failure rats by increasing cellular Ca2+ load.204 Bai et al found that curcumin (50 mg/kg/day) inhibited myocardial hypertrophy in male Wistar rats treated with transverse aortic constriction(TAC) by upregulating NCX expression.172 Myocardial hypertrophy is common in uremic patients. Ghosh et al found that curcumin (150 mg/kg/day) attenuated myocardial hypertrophy and remodeling in nephrectomy rats by inhibiting GSK-3β/catenin, calcineurin/nuclear factor of activated T cells(NFAT) and ERK1/2 pathways.173 Cardiomyocyte hypertrophy is also common in patients with diabetes.205 Sudirman found that curcumin (150 mg/kg/day) significantly improved left ventricular cell hypertrophy in mice with type 1 diabetes.206 (Figure 5 and Table 2)
Inhibit Myocardial Fibrosis
Myocardial fibrosis is characterized by increased extracellular matrix (ECM) in the myocardial interstitium.207 Cardiac fibroblasts (CFs) are the main effector cells of myocardial fibrosis.208 TGF-β1 is a potent pro-fibrotic cytokine that can induce CFs to transform into myofibroblasts.209 Fang et al found that curcumin (20 μM) inhibited TGF-β1-induced CFs proliferation and collagen deposition by inhibiting mothers against decapentaplegic homolog(Smad)2/3, p38 MAPK and ERK signaling pathways.174 In another study, Liu et al found that curcumin (5, 10 and 20 μM) significantly inhibited the expression of α-smooth muscle actin (α-SMA) and collagen in TGF-β1-induced CFs by inhibiting Smad-2 and p38 MAPK signaling pathways.175 Zhao et al. Found that curcumin (20 μM) reduced the expression of pro-fibrotic protein in CFs co-cultured with LPS-stimulated macrophages. The mechanism is related to the down-regulation of proinflammatory cytokines in macrophages by curcumin.210 (Figure 5 and Table 2)
In the setting of hypertension, CFs can up-regulate cardiac ECM deposition, resulting in ventricular remodeling. Meng et al found that curcumin (100 mg/kg/day) could reduce the heart weight/body weight ratio, Ang II concentration and cardiac fibrosis of SHRs. In vitro, curcumin (5, 10 and 20 μM) inhibited the expression of Ang II–induced connective tissue growth factor (CTGF), collagen III and fibronectin (FN) by increasing the expression and activity of PPAR γ, thus exerting an antifibrotic effect.176 In another study, curcumin (10 and 20 μM) reduced Ang II–induced fibroblast proliferation by decreasing TGF-β1 and MMPs/tissue inhibitor of matrix metalloproteinases(TIMPs).177 There are two types of Ang II receptors. Activation of AT1Rs can induce inflammation, vasoconstriction, interstitial collagen deposition, and tissue fibrosis, while activation of angiotensin type 2 receptor(AT2R) may protect the heart by counteracting the harmful effects of AT1R stimulation.211 Pang et al found that curcumin (150 mg/kg/day) could reduce the expression of AT1R and enhance the expression of AT2R in rats, thus exerting the effect of myocardial fibrosis.178 (Figure 5 and Table 2)
Myocardial fibrosis is the initial change in DCM.212 Guo et al found that curcumin (300 mg/kg/day) significantly inhibited the deposition of type I and III collagen in the heart tissue of diabetic rats, decreased the production of TGF-β1 and the phosphorylation of Smad2/3, and increased the expression of Smad7. In vitro studies, curcumin (25 μM) inhibited collagen synthesis induced by high glucose in fibroblasts by inhibiting TGF-β1/Smad and AMPK/p38 MAPK signaling pathways.179 In the early stage of myocardial infarction(MI), MMPs activated by local oxidative stress degrade ECM, and subsequently CFs proliferate and deposit collagen to form repairable fibrosis and noncontractile scars.213 Although fibrosis is necessary for normal healing, excessive fibrosis can gradually impair ventricular function.214 Wang et al found that curcumin (150 mg/kg/day) could reduce ECM degradation and inhibit collagen synthesis by inhibiting TGF-β1/Smad signaling pathway, thereby alleviating poor cardiac repair after myocardial I/R.180 In another study, Xiao et al found that curcumin (100 mg/kg/day) could inhibit CFs fibrosis in MI rats by activating Sirt1.181 (Figure 5 and Table 2)
Inhibition of Ventricular Remodeling
MMPs are proteolytic enzymes involved in ECM degradation after MI, causing ventricular remodeling.215 Boarescu et al found that curcumin pretreatment (100,150 and 200 mg/kg/day) could reduce the levels of MMP-2 and MMP-9 in ISO-induced MI rats, thereby improving ventricular remodeling.182 In another study, Kohli et al found that curcumin (8 μM) inhibited norepinephrine induced MMP-9 expression and activity in H9C2 cardiomyocytes, which may help prevent ECM remodeling.183 (Figure 5 and Table 2)
Pulmonary arterial hypertension(PAH) is pathologically characterized by progressive obstruction of the pulmonary artery, resulting in hypertrophy of the right ventricle(RV). Rice et al found that curcumin nanoparticles (50 mg/kg/day) inhibited the development of RV remodeling in PAH rats by reducing TNF-α levels and oxidative stress.184 Hypertension can also lead to ventricular remodeling. In one study, Hernandez et al found that curcumin (120 mg/kg/day) restored systolic blood pressure, attenuated septal thickening, reduced end-systolic left ventricular size, and restored ejection fraction in nephrectomy rats.216 Dickkopf related protein 3 (DKK-3) plays a crucial role in cell growth and proliferation.217 In animal models, DKK-3 depletion can aggravate ventricular remodeling induced by pressure overload.218 In one study, Cao et al found that curcumin (100 mg/kg/day) may inhibit ventricular remodeling in rabbits with chronic heart failure by inhibiting p38 and JNK pathways and increasing DKK-3 expression.185 (Figure 5 and Table 2)
Treatment of Autoimmune Myocardial Injury and Diabetic Cardiomyopathy
Autoimmune myocarditis(AM) is a potentially fatal disease that usually develops prior to acute and chronic heart failure.219 Mito et al found that curcumin (50 mg/kg/day) significantly inhibited the expressions of inducible nitric oxide synthase (iNOS), NADPH oxidase and ERS signaling proteins in rats with experimental AM(EAM), thereby improving cardiac function.186 In another study, Mito et al also found that curcumin (50 mg/kg/day) could reduce heart weight, inflammatory area, and myocardial protein levels of NF-κB, IL-1β, TNF-α and GATA-4 in experimental AM rats.220 M2 macrophages have the function of secreting anti-inflammatory cytokines. Gao et al found that curcumin (50 mg/kg/day) could induce M2 polarization of macrophages, thereby attenuating EAM.187
DCM can increase the risk of heart failure in diabetic patients, mainly caused by cardiomyocyte apoptosis.221,222 Ren et al found that curcumin (100 mg/kg/day) could reduce cardiomyocyte apoptosis in diabetic rats by regulating Sirt1/forkhead box protein O1(FOXO1) and PI3K/Akt pathways.188 Yu et al found that curcumin (100 and 200 mg/kg/day) attenuates myocardial fibrosis, oxidative stress, inflammation and apoptosis in rats with DCM, possibly by regulating Akt/GSK-3β signaling pathway.189 Abdelsamia et al found that metformin (200 mg/kg/day) combined with curcumin (100 mg/kg/day) could inhibit myocardial fibrosis in diabetic rats by activating Nrf2/HO-1 pathway and inhibiting JAK2/STAT3 pathway.190 Aziz et al found that curcumin (20 mg/kg/day) alleviated cardiomyocyte hypertrophy by inhibiting P300 upregulation, thereby improving left ventricular function in diabetic rats.191 PPAR γ plays an important role in regulating lipid and glucose metabolism.223 Calmodulin-dependent protein kinase II (CaMKII) is a multifunctional serine/threonine kinase that is closely related to the pathogenesis of DCM. Gbr et al found that curcumin (100 mg/kg/day) could reduce oxidative stress, inflammation and fibrosis in the heart of diabetic rats by activating PPAR γ and inhibiting CaMKII/NF-κB/TGF-β1 pathway.192 HMGB1 is a highly conserved nuclear protein associated with inflammatory diseases. Yan et al found that curcumin (50 and 100 mg/kg/day) alleviated inflammatory damage in DCM by inhibiting HMGB1/NLRP3/NF-κB pathway.193 Beclin 1 is an important autophagy protein.224 Yao et al found that curcumin (200 mg/kg/day) disrupted the interaction between apoptotic proteins and Beclin1 by activating AMPK and JNK1, thereby promoting autophagy and reducing cardiomyocyte apoptosis. In addition, curcumin can improve DCM by regulating cardiomyocyte autophagy through AMPK/mTOR pathway in diabetic mice.194
Treatment of Drug-Induced Myocardial Injury
Clinically, some drugs can induce cardiotoxicity.225 Doxorubicin (DOX), a cancer treatment drug, under the action of NADPH oxidase, can be converted into semi-quinone to produce active hydroxyl radicals, causing damage to cells.226 Soliman et al found that curcumin (200 mg/kg/day) could alleviate doxorubicin induced cardiotoxicity in rats by regulating ras related C3 botulinum toxin substrate 1(Rac 1)/tumor necrosis factor-like weak inducer of apoptosis(TWEAK)/fibroblast growth factor-inducible protein 14(Fn14)/NF-κB intricate network.195 In another study, Swamy et al found that curcumin (200 mg/kg/day) could increase the decreased levels of GSH, SOD and catalase (CAT), and thus significantly protect rat myocardes from the toxic effects of DOX.227 14-3-3γ, a member of the 14-3-3 protein family, can interact with Bad to block mPTP opening and reduce drug-induced cardiomyocyte apoptosis. He et al found that curcumin (50 mg/kg/day) protected myocardium from DOX-induced injury by upregulating 14-3-3γ expression.196
Cisplatin is one of the commonly used chemotherapy drugs in the treatment of cancer.228 In one study, Bahadhir et al found that cisplatin (a single dose injected twice, once a week, 5 mg/kg/week) could significantly increase the levels of malondialdehyde(MDA), TNF-α, IL-1β and IL-6 and significantly decrease the activities of CAT and SOD in rat myocardium. Pretreatment with curcumin (200 mg/kg) for 30 min before cisplatin injection could significantly improve the above changes.229 In another study, Khadrawy et al found that cisplatin significantly reduced GSH levels and Na+, K+-ATPase activity in rat hearts, while treatment with curcumin (50 mg/kg/day) nanoparticles ameliorated these changes.230 Cyclophosphamide (CP) has a strong immunosuppressive effect on autoimmune diseases, but it also has a wide range of side effects, including cardiotoxicity.231 Avci et al found that CP(30 mg/kg/day) significantly increased the MDA content and DNA fragmentation level, decreased the SOD level, and impaired the histopathological status of heart tissue in Wistar rats. However, curcumin (100 mg/kg/day) significantly improved the above changes.232 Irinotecan(CPT-11) and bevacizumab(BEV) are also important anticancer drugs with some cardiotoxicity. In one study, Ciftci et al found that curcumin (100 mg/kg/day) reversed CPT-11-induced oxidative stress and myocardial tissue damage.233 Sabet et al demonstrated that curcumin (10, 50 and 100 μM) prevented BEV-induced mitochondrial damage and elevated oxidative stress in cardiomyocytes.234 Abemacilib (ABE) is a cyclin-dependent kinase inhibitor commonly used to treat cancer. Huyut et al found that curcumin (30 mg/kg/day) combined with ABE inhibited the elevation of creatine kinase-MB(CK-MB) and cardiac troponin I(cTnI) in rats, and also inhibited cardiac fibrosis.235
Other Protective Effects of Curcumin on Myocardium
Aging is a multi-factor physiological process. One of the common features of cellular aging is the accumulation of damaged proteins within the cell. Autophagy can digest its own low-efficiency components through a lysosomal mediated catabolic process, and studies have shown that autophagy can delay aging. Another important mechanism regulating the cellular aging process is protein acetylation. Sirt 1, also known as NAD-dependent deacetylase, mediates life extension in organisms. Aziz et al found that autophagy, Sirt 1 levels were significantly reduced in the hearts of older rats compared to younger rats, and curcumin(50 mg/kg/day) ameliorated these undesirable changes.236 In another study, Yang et al found that curcumin (1,5 and 10 μM) increased the expression of Sirt 1 and phosphorylated AMPK in a dose-dependent manner, and decreased phosphorylated mTOR, thereby promoting autophagy to achieve anti-cardiac aging effects.197
With age, the formation of blood vessels in the heart is gradually impaired, and endothelial dysfunction increases significantly. Studies have shown that VEGF can induce angiogenesis. Some angiointegrins such as thrombospondin (TSP) inhibit angiogenesis by inhibiting VEGF receptor(VEGFR) activity or increasing its degradation.237 Ghorbanzadeh et al found that curcumin (50 mg/kg/day) promoted the formation of cardiac blood vessels in rats, and its mechanism was related to the regulation of TSP 1/VEGF-A signaling pathway.198 PM2.5 activates ERS, which can increase the risk of heart problems. El Tabaa et al found that nano-emulsion curcumin(NEC) inhibits ERS by activating sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a(SERCA2a), thereby improving PM2.5-associated cardiac inflammation and cardiomyocyte apoptosis.199
Pharmacokinetics and Toxicology of Curcumin
The solubility and stability of curcumin are related to the type and pH of the solvent. Curcumin is very insoluble in water, but soluble in organic solvents. Under acidic to neutral conditions, curcumin is relatively stable, while under alkaline conditions, curcumin is very unstable and easy to be decomposed. Studies have shown that curcumin is poorly absorbed in the gastrointestinal tract after oral administration, and only a small amount enters the peripheral blood circulation through the portal vein. Wahktrom et al administered 1000 mg/kg of curcumin orally to SD rats and then tested for curcumin in urine and feces, resulting in about 75% curcumin detected in feces and only trace curcumin detected in urine.238 Ravindranath et al administered 400, 80 and 10 mg of radio-labeled curcumin orally to Wistar rats, respectively. When curcumin is administered at a dose of 10 or 80 mg, more than 90% of the drug is excreted from the stool and only 3% is detected in the blood. When curcumin was administered at 400mg, 60% of the drug was excreted in the stool and only 13% was detected in the blood239. In one clinical trial, 19 subjects took curcumin orally (4g/day for 30 days). The results showed that trace curcumin could be detected in the serum of only 2 subjects, and the average blood concentration was only (3.8±1.3) ng.mL−1 240, which was related to the hepatoenteric first pass effect after curcumin was taken orally. Compared with oral administration, the plasma curcumin concentration was significantly increased by injection. Pan et al injected mice with curcumin (100 mg/kg) peritoneally, and found that high levels of curcumin (6.1 μM) appeared in plasma 15 minutes later. In addition, curcumin concentrations in intestine, spleen, liver and kidney were 480.59, 70.74, 73.02 and 20.39 μM, respectively, 1 hour after administration.241 With the development and application of nanotechnology, some preparations such as liposomes, microemulsion, hydrophilic prodrugs, solid nanoparticles, polymer micelles and nanogels can improve the water solubility and bioavailability of curcumin.
The metabolic pathways of curcumin compounds in vivo include Phase I reduction metabolism, Phase II binding metabolism, autooxidation and intracellular catalytic oxidation metabolism.242 The main products of curcumin I phase reduction metabolism are tetrahydrocurcumin, hexahydrocurcumin and a small amount of ferulic acid, which is a step by step hydrogenation process. In phase I reductive metabolism, ethanol dehydrogenase in the liver and small intestine cytoplasm is involved in the reaction.243 Because the phase I metabolites have the structure of phenol hydroxyl group and alcohol hydroxyl group, the combination reaction of gluconaldehyde and sulfation occurs in phase II metabolism. Most of the final metabolites of curcumin exist in the form of glucosylation, and the sulfation products are relatively few. Glucosylation of curcumin occurs in intestinal and hepatic microparticles, while sulfation occurs in intestinal and hepatic solutes.244
Long-term studies have shown that curcumin is safe and protective when used in the diet.In one study, high doses of curcumin (8 g/day) do not cause side effects. In another study, curcumin was administered at doses up to 12 g/day for three months with no apparent toxicity.245 However, some studies have found that high concentrations of curcumin have some side effects. Tanwar et al found that curcumin had antioxidant effects at lower concentrations (100 or 200 mg/kg/day), but curcumin had side effects of promoting lipid peroxidation at higher concentrations (400 mg/kg/day) in a rat model of myocardial necrosis induced by ISO.246 In another study, Sun et al found that curcumin (75 mg/kg/day) can induce histone hypoacetylation and inhibit the expression of transcription factors such as GATA4 early in heart development, ultimately leading to defects in heart development, such as thinning of the ventricular wall and septum. In general, curcumin may be detrimental to embryonic heart development during early pregnancy.247 Clinically, the common side effects of curcumin are flatulence,248 changes in liver enzymes and blood biochemical parameters.249 The metabolism and lipid-lowering effects of curcumin are mainly carried out in the liver, which may be the main reason for the increase of liver enzymes.243
Discussion
The therapeutic effect and mechanism of curcumin have become the focus of pharmacokinetic research. Curcumin has a good therapeutic effect, especially in the cardiovascular field. It can significantly protect cardiomyocyte injury after ischemia and hypoxia, inhibit myocardial hypertrophy and fibrosis, improve ventricular remodeling, reduce drug-induced myocardial injury, improve DCM, alleviate vascular endothelial dysfunction, inhibit foam cell formation, and reduce VSMCs proliferation. The targets and signaling pathways of CVDs are complex. The pharmacology and mechanism of action of curcumin have not been studied very deeply. It’s worth noting that not all studies come to the same conclusion. Min et al found that curcumin (10 μM) can reduce the accumulation of cholesterol in macrophages by inhibiting the expression of CD36 on the surface of macrophages.41 However, Chen et al found that curcumin (6.25 and 12.5 μM) enhances ox-LDL-induced cholesterol esterification and foam cell formation by upregulating CD36 expression in M1 macrophages.120 The two studies reached inconsistent conclusions, which may be related to the dose of curcumin.
Previous reviews have summarized the protective effects of curcumin on CVDs. In 2020, Li et al4 reviewed the preclinical studies of curcumin in CVDs such as cardiac hypertrophy, heart failure, abdominal aortic aneurysm, stroke, drug-induced cardiotoxicity, and diabetic cardiovascular complications, and reviewed the potential molecular targets of curcumin. Pourbagher-Shahri et al250 also reviewed the effects of curcumin on the cardiovascular system in 2021. They classified curcumin according to the possible cellular targets of action, which is different from the classification of curcumin in our review. Neither of the above two teams had a complete summary of the cardiovascular protective pathway of curcumin, and the dosage of curcumin was not indicated in the article. Our review classifies the effects of curcumin on different cells in the cardiovascular system, and we provide a complete review of the signaling pathways. It is well known that different doses of the drug are not equally effective, so curcumin dosages are also noted in our review.
The protective mechanism of curcumin against CVDs is complex and networked. In the past, researchers have not studied the pharmacological mechanism of curcumin deeply. The signaling pathways mentioned in this review may only be part of the downstream signaling pathways following curcumin action on specific molecular targets. Therefore, we propose to investigate specific molecular targets of curcumin using a systems biology approach. For example, Ma et al251 used a photosensitive probe to label metformin and eventually identified PEN2 as a direct target of metformin. Finally, the effect of curcumin in CVDs is mostly studied in animal models and cell models, and there are few clinical trial data. Therefore, we suggest that large-scale clinical trials should be conducted to evaluate the efficacy of curcumin in protecting against CVDs.
In conclusion, curcumin has a good protective effect on CVDs. It is important to note that curcumin is a complementary or alternative medicine, not a replacement for the main treatment, and needs to be used under the guidance of a doctor. To be sure, curcumin is an important drug that is worth exploring.
Abbreviations
CVDs, Cardiovascular diseases; DCM, Diabetic cardiomyopathy; VSMCs, Vascular smooth muscle cells; VECs, vascular endothelial cells; ROS, Reactive oxygen species; Nrf2, Nuclear factor erythroid factor 2 related factor 2; Keap1, Kelch-like ECH-associated protein 1; ARE, antioxidant response element; HO-1, Heme oxygenase-1; H2O2, Hydrogen peroxide; HUVECs, Human umbilical vein endothelial cells; AS, atherosclerosis; SHRs, Spontaneously hypertensive rats; UCP2, uncoupling protein-2; NO, nitric oxide; 2K-1C, 2kidney-1clip; MMPs, matrix metalloproteinases; NADPH, nicotinamide adenine dinucleotide phosphate; PKC, protein kinase C; CD40, cluster of differentiation 40; CD40L, cluster of differentiation 40 ligand; TLR, Toll-like receptor; NF-κB, Nuclear factor kappa-B; HFD, high-fat diet; VCAM-1, Vascular cell adhesion molecule-1; ICAM-1, Intercellular cell adhesion molecule-1; TNF-α, Tumor necrosis factor α; HMGB1, High mobility group box protein 1; HECs, human endothelial cells; LPS, Lipopolysaccharide; HCMV, human cytomegalovirus; ox-LDL, moxidized low-density lipoprotein; MyD88, Myeloid differentiation factor 88; LCN2, Lipocalin-2; p38 MAPK, p38 mitogen-activated protein kinase; HAECs, Human arterial endothelial cells; TAECs, Thoracic aortic endothelial cells; PI3K, phosphatidyinositol 3-kinase; Akt, protein kinase B; AGEs, Advanced glycation end products; MGO, Methylglyoxal; HDL, high-density lipoprotein; ERS, endoplasmic reticulum stress; IL-8, interleukin-8; COX-2, Cyclooxygenase; CREB, cyclic adenosine monophosphate(cAMP)-response element binding protein; PLLA, poly-L-lactic acid; MCP-1, Monocyte chemoattractant protein 1; RAECs, Rat aortic endothelial cells; LOX-1, lectin-like ox-LDL receptor-1; TC, total cholesterol; TG, Total triglycerides; LDL-C, low-density lipoprotein cholesterol; Apo B, Apolipoprotein B; GSH, glutathione; GSH-Px, glutathione peroxidase; SR-A, Scavenger receptor class A; CD36, differentiation 36; SR-BI, Class B scavenging receptor type I; ABCA1, ATP-binding cassette transporters A1; ABCG1, ATP-binding cassette transporters G1; Sirt 6, Silent information regulator 6; LXRs, Liver X receptors; JNK, c-Jun N-terminal kinase; AMPK, AMP-activated protein kinase; FAT, fatty acid translocase; FABP4, Fatty acid binding protein 4; HIF-1α, Hypoxia inducible factor-1α; ERK, extracellular regulated protein kinase; PPAR γ, Proliferator-activated receptor γ; TMAO, trimethylamine-N-oxide; EMMPRIN, Extracellular matrix metalloproteinase inducer; PMA, Phorpol 12 myristic acid 13 acetate; THBS, Thrombospondins; DM, Diabetes mellitus; EPCs, Endothelial progenitor cells; EPCD, Endothelial progenitor cell dysfunction; MnSOD, Manganese superoxide dismutase; VEGF, Vascular endothelial cell growth factor; Ang-1, Angiotensin 1; PAD, Peripheral arterial disease; FAK, focal adhesion kinase; CsA, Cyclosporine A; eNOS, endothelial nitric oxide synthase; HMEC, Human microvascular endothelial cells; IRS-1, insulin receptor substrate 1; mTOR, mammalian target of rapamycin; BECN1, beclin1; TFEB, transcription factor EB; BRD4, bromodomain-containing protein 4; NLRP3, NOD- LRR- and pyrin domain-containing protein 3; EndMT, Endothelial-to-mesenchymal transition; TGF-β1, Transforming growth factor-β1; Nrf-2, nuclear factor erythroid-2-related factor 2; DDAH, dimethylarginine dimethylaminohydrolase; ADMA, asymmetric dimethylarginine; TRPV4, Transient receptor potential vanilloid 4; Ang II, Angiotensin II; SP1, specific protein 1; CMKLR1, chemokine-like receptor 1; IGF-1, Insulin-like growth factor 1; PKB, protein kinase B; GSK-3β, glycogen synthase kinase-3β; Egr-1, early growth response protein 1; Ras, rat sarcoma; MEK1/2, mitogen-activated protein kinase kinase 1/2; CRP, C-reactive protein; RAAS, renin aniotension aldosterone system; AT1R, angiotensin type 1 receptor; ET-1, Endothelin-1; IGF-1R, IGF-1 receptor; PTEN, phosphatase and tensin homolog; FMD, Flow-mediated dilation; I/R, Ischemia and reperfusion; JAK, Janus kinase; STAT, Signal transducer and activator of transcription; SD, Sprague Dawley; H/R, Hypoxia/reoxygenation; ISO, Isoproterenol; mPTP, mitochondrial permeability transition pore; Hsps, Heat shock proteins; CME, Coronary microembolism; HAT, Histone acetyltransferase; GATA4, GATA binding protein 4; DS, Dahl salt-sensitive; NCX, Na+/Ca2+ exchanger; TAC, Transverse aortic constriction; NFAT, nuclear factor of activated T cells; ECM, Extracellular matrix; CFs, Cardiac fibroblasts; Smad, mothers against decapentaplegic homolog; α-SMA, α-smooth muscle actin; CTGF, connective tissue growth factor; FN, fibronectin; TIMPs, tissue inhibitor of matrix metalloproteinases; AT2R, type 2 receptor; MI, myocardial infarction; PAH, Pulmonary arterial hypertension; RV, Right ventricular; DKK-3, Dickkopf related protein 3; AM, Autoimmune myocarditis; iNOS, inducible nitric oxide synthase; FOXO1, forkhead box protein O1; EAM, Experimental AM; CaMKII, Calmodulin-dependent protein kinase II; DOX, Doxorubicin; Rac 1, ras related C3 botulinum toxin substrate 1; TWEAK, tumor necrosis factor-like weak inducer of apoptosis; Fn14, fibroblast growth factor-inducible protein 14; CAT, Catalase; MDA, Malondialdehyde; CP, Cyclophosphamide; CPT-11, Irinotecan; BEV, Bevacizumab; ABE, Abemacilib; CK-MB, creatine kinase-MB; cTnI, cardiac troponin I; TSP, thrombospondin; VEGFR, VEGF receptor; NEC, nano-emulsion curcumin; SERCA2a, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a.
Credit Authorship Contribution Statement
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study was funded by grants from General Program of National Natural Science Foundation of China (Grant no. 81973836), Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (Grant no. CI2021A00902).
Disclosure
The authors declare that they have no competing interests.
References
1. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi:10.1161/CIR.0b013e31820a55f5
2. Kim BM. The role of saikosaponins in therapeutic strategies for age-related diseases. Oxid Med Cell Longev. 2018;2018:8275256. doi:10.1155/2018/8275256
3. Campbell MS, Fleenor BS. The emerging role of curcumin for improving vascular dysfunction: a review. Crit Rev Food Sci Nutr. 2018;58(16):2790–2799. doi:10.1080/10408398.2017.1341865
4. Li H, Sureda A, Devkota HP, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2020;38:107343. doi:10.1016/j.biotechadv.2019.01.010
5. Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3(2):254–267. doi:10.1111/j.1538-7836.2004.01085.x
6. Nie P, Meng F, Zhang J, et al. Astragaloside IV exerts a myocardial protective effect against cardiac hypertrophy in rats, partially via activating the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. 2019;2019:4625912. doi:10.1155/2019/4625912
7. Zhou X, Afzal S, Zheng YF, et al. Synergistic protective effect of curcumin and resveratrol against oxidative stress in endothelial EA.hy926 cells. Evid Based Complement Alternat Med. 2021;2021:2661025. doi:10.1155/2021/2661025
8. Yang Y, Duan W, Liang Z, et al. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cell Signal. 2013;25(3):615–629. doi:10.1016/j.cellsig.2012.11.025
9. Ouyang J, Li R, Shi H, et al. Curcumin protects human umbilical vein endothelial cells against H2 O2 -induced cell injury. Pain Res Manag. 2019;2019:3173149. doi:10.1155/2019/3173149
10. Lan C, Chen X, Zhang Y, et al. Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC Cardiovasc Disord. 2018;18(1):43. doi:10.1186/s12872-018-0768-6
11. Boonla O, Kukongviriyapan U, Pakdeechote P, et al. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53. doi:10.1016/j.niox.2014.09.001
12. Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, et al. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med. 2010;10:57. doi:10.1186/1472-6882-10-57
13. Wu T, Xiang Y, Lv Y, Li D, Yu L, Guo R. miR-590-3p mediates the protective effect of curcumin on injured endothelial cells induced by angiotensin II. Am J Transl Res. 2017;9(2):289–300.
14. Zhang S, Zou J, Li P, et al. Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression. J Agric Food Chem. 2018;66(2):449–456. doi:10.1021/acs.jafc.7b04260
15. Kim DC, Lee W, Bae JS. Vascular anti-inflammatory effects of curcumin on HMGB1-mediated responses in vitro. Inflamm Res. 2011;60(12):1161–1168. doi:10.1007/s00011-011-0381-y
16. Lv YL, Jia Y, Wan Z, et al. Curcumin inhibits the formation of atherosclerosis in ApoE-/- mice by suppressing cytomegalovirus activity in endothelial cells. Life Sci. 2020;257:117658. doi:10.1016/j.lfs.2020.117658
17. Chen D, Zhu C, Ye S, et al. Curcumin ameliorates oxidized low-density lipoprotein (ox-LDL)-caused damage in human umbilical vein endothelial cells (HUVECs) through the miR-599/MYD88/NF-κB axis. Toxicol In Vitro. 2022;85:105481. doi:10.1016/j.tiv.2022.105481
18. Wan Q, Liu ZY, Yang YP, et al. Effect of curcumin on inhibiting atherogenesis by down-regulating lipocalin-2 expression in apolipoprotein E knockout mice. Biomed Mater Eng. 2016;27(6):577–587. doi:10.3233/BME-161610
19. Pirvulescu MM, Gan AM, Stan D, et al. Curcumin and a Morus alba extract reduce pro-inflammatory effects of resistin in human endothelial cells. Phytother Res. 2011;25(12):1737–1742. doi:10.1002/ptr.3463
20. Xiao Y, Xia J, Wu S, et al. Curcumin inhibits acute vascular inflammation through the activation of heme oxygenase-1. Oxid Med Cell Longev. 2018;2018:3295807. doi:10.1155/2018/3295807
21. Olszanecki R, Gebska A, Korbut R. The role of haem oxygenase-1 in the decrease of endothelial intercellular adhesion molecule-1 expression by curcumin. Basic Clin Pharmacol Toxicol. 2007;101(6):411–415. doi:10.1111/j.1742-7843.2007.00151.x
22. Wongeakin N, Bhattarakosol P, Patumraj S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: txnip, ICAM-1, and NOX2 expressions. Biomed Res Int. 2014;2014:161346. doi:10.1155/2014/161346
23. Zhang Z, Li K. Curcumin attenuates high glucose-induced inflammatory injury through the reactive oxygen species-phosphoinositide 3-kinase/protein kinase B-nuclear factor-κB signaling pathway in rat thoracic aorta endothelial cells. J Diabetes Investig. 2018;9(4):731–740. doi:10.1111/jdi.12767
24. Sun YP, Gu JF, Tan XB, et al. Curcumin inhibits advanced glycation end product-induced oxidative stress and inflammatory responses in endothelial cell damage via trapping methylglyoxal. Mol Med Rep. 2016;13(2):1475–1486. doi:10.3892/mmr.2015.4725
25. Hu TY, Liu CL, Chyau CC, et al. Trapping of methylglyoxal by curcumin in cell-free systems and in human umbilical vein endothelial cells. J Agric Food Chem. 2012;60(33):8190–8196. doi:10.1021/jf302188a
26. Narra SS, Rosanaly S, Rondeau P, et al. ApoA-I nanoparticles as curcumin carriers for cerebral endothelial cells: improved cytoprotective effects against methylglyoxal. Pharmaceuticals (Basel). 2022;15(3):347. doi:10.3390/ph15030347
27. Sowndhar Rajan B, Krishnan K, Vellaichamy E. Diet-derived advanced glycation end products (dAGEs) induce proinflammatory cytokine expression in cardiac and renal tissues of experimental mice: protective effect of curcumin. Cardiovasc Toxicol. 2022;22(1):35–51. doi:10.1007/s12012-021-09697-4
28. Li J, Luo M, Xie N, Wang J, Chen L. Curcumin protects endothelial cells against homocysteine induced injury through inhibiting inflammation. Am J Transl Res. 2016;8(11):4598–4604.
29. Lee SE, Park HR, Jeon S, Han D, Park YS. Curcumin attenuates acrolein-induced COX-2 expression and prostaglandin production in human umbilical vein endothelial cells. J Lipid Atheroscler. 2020;9(1):184–194. doi:10.12997/jla.2020.9.1.184
30. Chen D, Weng L, Chen C, et al. Inflammation and dysfunction in human aortic endothelial cells associated with poly-l-lactic acid degradation in vitro are alleviated by curcumin. J Biomed Mater Res A. 2019;107(12):2756–2763. doi:10.1002/jbm.a.36778
31. Li X, Xiao H, Lin C, et al. Synergistic effects of liposomes encapsulating atorvastatin calcium and curcumin and targeting dysfunctional endothelial cells in reducing atherosclerosis. Int J Nanomed. 2019;14:649–665. doi:10.2147/IJN.S189819
32. Panicker SR, Kartha CC. Curcumin attenuates glucose-induced monocyte chemoattractant protein-1 synthesis in aortic endothelial cells by modulating the nuclear factor-kappaB pathway. Pharmacology. 2010;85(1):18–26. doi:10.1159/000262325
33. Monfoulet LE, Mercier S, Bayle D, et al. Curcumin modulates endothelial permeability and monocyte transendothelial migration by affecting endothelial cell dynamics. Free Radic Biol Med. 2017;112:109–120. doi:10.1016/j.freeradbiomed.2017.07.019
34. Lee HS, Lee MJ, Kim H, et al. Curcumin inhibits TNFalpha-induced lectin-like oxidised LDL receptor-1 (LOX-1) expression and suppresses the inflammatory response in human umbilical vein endothelial cells (HUVECs) by an antioxidant mechanism. J Enzyme Inhib Med Chem. 2010;25(5):720–729. doi:10.3109/14756360903555274
35. Soltani B, Bodaghabadi N, Mahpour G, et al. Nanoformulation of curcumin protects HUVEC endothelial cells against ionizing radiation and suppresses their adhesion to monocytes: potential in prevention of radiation-induced atherosclerosis. Biotechnol Lett. 2016;38(12):2081–2088. doi:10.1007/s10529-016-2189-x
36. Zingg JM, Hasan ST, Nakagawa K, et al. Modulation of cAMP levels by high-fat diet and curcumin and regulatory effects on CD36/FAT scavenger receptor/fatty acids transporter gene expression. Biofactors. 2017;43(1):42–53. doi:10.1002/biof.1307
37. Tian N, Li X, Luo Y, et al. Curcumin regulates the metabolism of low density lipoproteins by improving the C-to-U RNA editing efficiency of apolipoprotein B in primary rat hepatocytes. Mol Med Rep. 2014;9(1):132–136. doi:10.3892/mmr.2013.1754
38. Altinel Y, Kose E, Karacaglar A, et al. Systemic Amelioration via Curcumin in Rats following Splenectomy: lipid Profile, Endothelial and Oxidative Damage. J Invest Surg. 2021;34(6):627–636. doi:10.1080/08941939.2020.1834651
39. Zhao JF, Ching LC, Huang YC, et al. Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol Nutr Food Res. 2012;56(5):691–701. doi:10.1002/mnfr.201100735
40. Tan C, Zhou L, Wen W, et al. Curcumin promotes cholesterol efflux by regulating ABCA1 expression through miR-125a-5p/SIRT6 axis in THP-1 macrophage to prevent atherosclerosis. J Toxicol Sci. 2021;46(5):209–222. doi:10.2131/jts.46.209
41. Min KJ, Um HJ, Cho KH, et al. Curcumin inhibits ox-LDL-induced CD36 expression and foam cell formation through the inhibition of p38 MAPK phosphorylation. Food Chem Toxicol. 2013;58:77–85. doi:10.1016/j.fct.2013.04.008
42. Liu T, Li C, Sun H, et al. Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm Res. 2014;63(10):841–850. doi:10.1007/s00011-014-0758-9
43. Lin XL, Liu MH, Hu HJ, et al. Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRα signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol. 2015;34(9):561–572. doi:10.1089/dna.2015.2866
44. Liu T, Li C, Sun H, et al. Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm Res. 2014;63(10):841–850.
45. Chen FY, Zhou J, Guo N, et al. Curcumin retunes cholesterol transport homeostasis and inflammation response in M1 macrophage to prevent atherosclerosis. Biochem Biophys Res Commun. 2015;467(4):872–878.
46. Ouyang S, Yao YH, Zhang ZM, et al. Curcumin inhibits hypoxia inducible factor-1α-induced inflammation and apoptosis in macrophages through an ERK dependent pathway. Eur Rev Med Pharmacol Sci. 2019;23(4):1816–1825. doi:10.26355/eurrev_201902_17145
47. Chen F, Guo N, Cao G, Zhou J, Yuan Z. Molecular analysis of curcumin-induced polarization of murine RAW264.7 macrophages. J Cardiovasc Pharmacol. 2014;63(6):544–552. doi:10.1097/FJC.0000000000000079
48. Zhou Y, Zhang T, Wang X, et al. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways. Cell Physiol Biochem. 2015;36(2):631–641. doi:10.1159/000430126
49. Zhang J, Ou C, Chen M. Curcumin attenuates cadmium-induced atherosclerosis by regulating trimethylamine-N-oxide synthesis and macrophage polarization through remodeling the gut microbiota. Ecotoxicol Environ Saf. 2022;244:114057. doi:10.1016/j.ecoenv.2022.114057
50. Cao J, Ye B, Lin L, et al. Curcumin Alleviates ox-LDL Induced MMP-9 and EMMPRIN Expression through the Inhibition of NF-κB and MAPK Pathways in Macrophages. Front Pharmacol. 2017;8:62. doi:10.3389/fphar.2017.00062
51. Cao J, Han Z, Tian L, et al. Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J Transl Med. 2014;12:266. doi:10.1186/s12967-014-0266-2
52. Zhou ZY, Chen YQ, Wang FY, et al. Effect of curcumin on down-expression of thrombospondin-4 induced by oxidized low-density lipoprotein in mouse macrophages. Biomed Mater Eng. 2014;24(1):181–189. doi:10.3233/BME-130798
53. Kadam S, Kanitkar M, Dixit K, et al. Curcumin reverses diabetes-induced endothelial progenitor cell dysfunction by enhancing MnSOD expression and activity in vitro and in vivo. J Tissue Eng Regen Med. 2018;12(7):1594–1607. doi:10.1002/term.2684
54. You J, Sun J, Ma T, et al. Curcumin induces therapeutic angiogenesis in a diabetic mouse hindlimb ischemia model via modulating the function of endothelial progenitor cells. Stem Cell Res Ther. 2017;8(1):182. doi:10.1186/s13287-017-0636-9
55. Chen D, Tao X, Wang Y, et al. Curcumin accelerates reendothelialization and ameliorates intimal hyperplasia in balloon-injured rat carotid artery via the upregulation of endothelial cell autophagy. Int J Mol Med. 2015;36(6):1563–1571. doi:10.3892/ijmm.2015.2365
56. Zhang J, Wang Q, Rao G, et al. Curcumin improves perfusion recovery in experimental peripheral arterial disease by upregulating microRNA-93 expression. Exp Ther Med. 2019;17(1):798–802. doi:10.3892/etm.2018.7000
57. Sagiroglu T, Kanter M, Yagci MA, et al. Protective effect of curcumin on cyclosporin A-induced endothelial dysfunction, antioxidant capacity, and oxidative damage. Toxicol Ind Health. 2014;30(4):316–327. doi:10.1177/0748233712456065
58. Sankrityayan H, Majumdar AS. Curcumin and folic acid abrogated methotrexate induced vascular endothelial dysfunction. Can J Physiol Pharmacol. 2016;94(1):89–96. doi:10.1139/cjpp-2015-0156
59. Guo N, Chen F, Zhou J, et al. Curcumin Attenuates Rapamycin-induced Cell Injury of Vascular Endothelial Cells. J Cardiovasc Pharmacol. 2015;66(4):338–346. doi:10.1097/FJC.0000000000000285
60. Chen D, Xi Y, Zhang S, et al. Curcumin attenuates inflammation of Macrophage-derived foam cells treated with Poly-L-lactic acid degradation via PPARγ signaling pathway. J Mater Sci Mater Med. 2022;33(4):33. doi:10.1007/s10856-022-06654-7
61. Shi J, Deng H, Zhang M. Curcumin pretreatment protects against PM2.5-induced oxidized low-density lipoprotein-mediated oxidative stress and inflammation in human microvascular endothelial cells. Mol Med Rep. 2017;16(3):2588–2594. doi:10.3892/mmr.2017.6935
62. Luo R, Zhao L, Li S, et al. Curcumin alleviates palmitic acid-induced LOX-1 upregulation by suppressing endoplasmic reticulum stress in HUVECs. Biomed Res Int. 2021;2021:9983725. doi:10.1155/2021/9983725
63. Han J, Pan XY, Xu Y, et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8(5):812–825. doi:10.4161/auto.19471
64. Guo S, Long M, Li X, et al. Curcumin activates autophagy and attenuates oxidative damage in EA.hy926 cells via the Akt/mTOR pathway. Mol Med Rep. 2016;13(3):2187–2193. doi:10.3892/mmr.2016.4796
65. Zhao L, Luo R, Yu H, et al. Curcumin protects human umbilical vein endothelial cells against high oxidized low density lipoprotein-induced lipotoxicity and modulates autophagy. Iran J Basic Med Sci. 2021;24(12):1734–1742. doi:10.22038/IJBMS.2021.59969.13297
66. Li X, Zhu R, Jiang H, et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis. Acta Pharm Sin B. 2022;12(5):2280–2299. doi:10.1016/j.apsb.2021.12.014
67. Kong F, Ye B, Cao J, et al. Curcumin Represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R Signaling in PMA-induced macrophages. Front Pharmacol. 2016;7:369. doi:10.3389/fphar.2016.00369
68. Yuan Y, Zhang C, He Y, et al. Curcumin improves the function of umbilical vein endothelial cells by inhibiting H2O2-induced pyroptosis. Mol Med Rep. 2022;25(6):214. doi:10.3892/mmr.2022.12730
69. Sun Y, Hu X, Hu G, et al. curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of SIRT1 in human umbilical vein endothelial cells. Biol Pharm Bull. 2015;38(8):1134–1141. doi:10.1248/bpb.b15-00012
70. Chen X, Chen X, Shi X, et al. Curcumin attenuates endothelial cell fibrosis through inhibiting endothelial-interstitial transformation. Clin Exp Pharmacol Physiol. 2020;47(7):1182–1192. doi:10.1111/1440-1681.13271
71. Shao J, Han J, Zhu Y, et al. Curcumin induces endothelium-dependent relaxation by activating endothelial TRPV4 channels. J Cardiovasc Transl Res. 2019;12(6):600–607. doi:10.1007/s12265-019-09928-8
72. Han Y, Sun HJ, Tong Y, et al. Curcumin attenuates migration of vascular smooth muscle cells via inhibiting NFκB-mediated NLRP3 expression in spontaneously hypertensive rats. J Nutr Biochem. 2019;72:108212. doi:10.1016/j.jnutbio.2019.07.003
73. Zhang M, Li Y, Xie H, et al. Curcumin inhibits proliferation, migration and neointimal formation of vascular smooth muscle via activating miR-22. Pharm Biol. 2020;58(1):610–619. doi:10.1080/13880209.2020.1781904
74. He Y, Wang R, Zhang P, et al. Curcumin inhibits the proliferation and migration of vascular smooth muscle cells by targeting the chemerin / CMKLR1 / LCN2 axis. Aging (Albany NY). 2021;13(10):13859–13875. doi:10.18632/aging.202980
75. Youreva V, Kapakos G, Srivastava AK. Insulin-like growth-factor-1-induced PKB signaling and Egr-1 expression is inhibited by curcumin in A-10 vascular smooth muscle cells. Can J Physiol Pharmacol. 2013;91(3):241–247. doi:10.1139/cjpp-2012-0267
76. Aoyagi M, Yamamoto M, Azuma H, et al. Immunolocalization of matrix metalloproteinases in rabbit carotid arteries after balloon denudation. Histochem Cell Biol. 1998;109(2):97–102. doi:10.1007/s004180050206
77. Zhong Y, Feng J, Li J, Fan Z. Curcumin prevents lipopolysaccharide-induced matrix metalloproteinase-2 activity via the Ras/MEK1/2 signaling pathway in rat vascular smooth muscle cells. Mol Med Rep. 2017;16(4):4315–4319. doi:10.3892/mmr.2017.7037
78. Han Y, Sun HJ, Tong Y, et al. Curcumin attenuates migration of vascular smooth muscle cells via inhibiting NFκB-mediated NLRP3 expression in spontaneously hypertensive rats. J Nutr Biochem. 2019;72:108212.
79. Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res. 2013;56(1):131–142. doi:10.1007/s12026-013-8384-0
80. Meng Z, Yan C, Deng Q, et al. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol Sin. 2013;34(7):901–911. doi:10.1038/aps.2013.24
81. Zhong Y, Liu T, Guo Z. Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK and NF-κB pathways in rat vascular smooth muscle cells. Inflamm Res. 2012;61(1):61–67. doi:10.1007/s00011-011-0389-3
82. Ruan H, Huang Q, Wan B, et al. Curcumin alleviates lipopolysaccharides-induced inflammation and apoptosis in vascular smooth muscle cells via inhibition of the NF-κB and JNK signaling pathways. Inflammopharmacology. 2022;30(2):517–525. doi:10.1007/s10787-021-00912-w
83. Yao Y, Wang W, Li M, et al. Curcumin exerts its anti-hypertensive effect by down-regulating the AT1 receptor in vascular smooth muscle cells. Sci Rep. 2016;6:25579. doi:10.1038/srep25579
84. Kapakos G, Youreva V, Srivastava AK. Attenuation of endothelin-1-induced PKB and ERK1/2 signaling, as well as Egr-1 expression, by curcumin in A-10 vascular smooth muscle cells. Can J Physiol Pharmacol. 2012;90(9):1277–1285. doi:10.1139/y2012-059
85. Sun SY, Cao YM, Huo YJ, et al. Nicotinate-curcumin inhibits AngII-induced vascular smooth muscle cell phenotype switching by upregulating Daxx expression. Cell Adh Migr. 2021;15(1):116–125. doi:10.1080/19336918.2021.1909899
86. Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NADPH oxidase and endothelial nitric oxide synthase. Circulation. 2002;105(14):1656–1662. doi:10.1161/01.CIR.0000012748.58444.08
87. Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88(2):E14–22. doi:10.1161/01.RES.88.2.e14
88. Antoniades C, Bakogiannis C, Tousoulis D, et al. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009;54(8):669–677. doi:10.1016/j.jacc.2009.03.076
89. Bhogal RH, Weston CJ, Curbishley SM, et al. Activation of CD40 with platelet derived CD154 promotes reactive oxygen species dependent death of human hepatocytes during hypoxia and reoxygenation. PLoS One. 2012;7(1):e30867. doi:10.1371/journal.pone.0030867
90. Molteni M, Gemma S, Rossetti C. The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediators Inflamm. 2016;2016:6978936. doi:10.1155/2016/6978936
91. den Dekker WK, Cheng C, Pasterkamp G, et al. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2010;209(2):314–320. doi:10.1016/j.atherosclerosis.2009.09.075
92. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi:10.1038/nri1391
93. Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–51. doi:10.1126/science.285.5425.248
94. Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–21. doi:10.1038/nature03104
95. Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi:10.1038/nm0302-240
96. Soares MP, Seldon MP, Gregoire IP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172(6):3553–3563. doi:10.4049/jimmunol.172.6.3553
97. Brownlee M, Cerami A, Vlassara H, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318(20):1315–1321. doi:10.1056/NEJM198805193182007
98. van Eupen MG, Schram MT, Colhoun HM, et al. The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia. 2013;56(8):1845–1855. doi:10.1007/s00125-013-2919-8
99. Selhub J, D’Angelo A. Relationship between homocysteine and thrombotic disease. Am J Med Sci. 1998;316(2):129–141. doi:10.1097/00000441-199808000-00008
100. Mallick SP, Singh BN, Rastogi A, Srivastava P. Design and evaluation of chitosan/poly(l-lactide)/pectin based composite scaffolds for cartilage tissue regeneration. Int J Biol Macromol. 2018;112:909–920. doi:10.1016/j.ijbiomac.2018.02.049
101. Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord. 2003;27:S35–40. doi:10.1038/sj.ijo.0802498
102. Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160(6):2145–2155. doi:10.1016/S0002-9440(10)61163-7
103. Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(11):1997–2008. doi:10.1161/01.ATV.0000142812.03840.6f
104. Xu X, Gao X, Potter BJ, et al. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27(4):871–877. doi:10.1161/01.ATV.0000259358.31234.37
105. Stewart BW, Nagarajan S. Recombinant CD36 inhibits ox-LDL-induced ICAM-1-dependent monocyte adhesion. Mol Immunol. 2006;43(3):255–267. doi:10.1016/j.molimm.2005.02.007
106. Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174(6):865–869. doi:10.1667/RR1862.1
107. Poznyak A, Grechko AV, Poggio P, et al. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835. doi:10.3390/ijms21051835
108. Hasan ST, Zingg JM, Kwan P, Noble T, Smith D, Meydani M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis. 2014;232(1):40–51. doi:10.1016/j.atherosclerosis.2013.10.016
109. Um MY, Hwang KH, Choi WH, et al. Curcumin attenuates adhesion molecules and matrix metalloproteinase expression in hypercholesterolemic rabbits. Nutr Res. 2014;34(10):886–893. doi:10.1016/j.nutres.2014.09.001
110. Innerarity TL, Mahley RW. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978;17(8):1440–1447. doi:10.1021/bi00601a013
111. Petroianu A, Veloso DF, Costa GR, Alberti LR. Effects of splenic surgeries on lipidogram of rats. Rev Assoc Med Bras. 2006;52(1):56–59. doi:10.1590/S0104-42302006000100024
112. Gonçalves TB, Yamaki VN, Feijó DH, et al. Effects of splenic allograft in lipid profile of non-splenectomized rats: the immune and metabolic role of the ”double spleen”. Rev Col Bras Cir. 2014;41(2):122–127. English, Portuguese. doi:10.1590/S0100-69912014000200009
113. Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–41. doi:10.1038/35025203
114. Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79(3):360–376. doi:10.1093/cvr/cvn120
115. Collot-Teixeira S, Martin J, McDermott-Roe C, et al. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75(3):468–477. doi:10.1016/j.cardiores.2007.03.010
116. Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277(51):49982–49988. doi:10.1074/jbc.M209649200
117. Cheng LC, Su KH, Kou YR, et al. α-Lipoic acid ameliorates foam cell formation via liver X receptor α-dependent upregulation of ATP-binding cassette transporters A1 and G1. Free Radic Biol Med. 2011;50(1):47–54. doi:10.1016/j.freeradbiomed.2010.10.706
118. Ji A, Meyer JM, Cai L, et al. Scavenger receptor SR-BI in macrophage lipid metabolism. Atherosclerosis. 2011;217(1):106–112. doi:10.1016/j.atherosclerosis.2011.03.017
119. Bonamassa B, Moschetta A. Atherosclerosis: lessons from LXR and the intestine. Trends Endocrinol Metab. 2013;24(3):120–128. doi:10.1016/j.tem.2012.10.004
120. Chen FY, Zhou J, Guo N, et al. Curcumin retunes cholesterol transport homeostasis and inflammation response in M1 macrophage to prevent atherosclerosis. Biochem Biophys Res Commun. 2015;467(4):872–878. doi:10.1016/j.bbrc.2015.10.051
121. Zingg JM, Hasan ST, Nakagawa K, et al. Modulation of cAMP levels by high-fat diet and curcumin and regulatory effects on CD36/FAT scavenger receptor/fatty acids transporter gene expression. Biofactors. 2017;43(1):42–53.
122. Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22(8):1376–1385. doi:10.1021/tx900086v
123. Fagerberg B, Barregard L, Sallsten G, et al. Cadmium exposure and atherosclerotic carotid plaques--results from the Malmö diet and Cancer study. Environ Res. 2015;136:67–74. doi:10.1016/j.envres.2014.11.004
124. Fuster V, Kovacic JC. Acute coronary syndromes: pathology, diagnosis, genetics, prevention, and treatment. Circ Res. 2014;114(12):1847–1851. doi:10.1161/CIRCRESAHA.114.302806
125. Forslöw A, Liu Z, Sundqvist KG. Receptor communication within the lymphocyte plasma membrane: a role for the thrombospondin family of matricellular proteins. Cell Mol Life Sci. 2007;64(1):66–76. doi:10.1007/s00018-006-6255-8
126. Elavarasu S, Suthanthiran T, Thangavelu A, et al. Evaluation of superoxide dismutase levels in local drug delivery system containing 0.2% curcumin strip as an adjunct to scaling and root planing in chronic periodontitis: a clinical and biochemical study. J Pharm Bioallied Sci. 2016;8(Suppl 1):48. doi:10.4103/0975-7406.191967
127. Hosseini A, Rasmi Y, Rahbarghazi R, et al. Curcumin modulates the angiogenic potential of human endothelial cells via FAK/P-38 MAPK signaling pathway. Gene. 2019;688:7–12. doi:10.1016/j.gene.2018.11.062
128. Textor SC, Taler SJ, Canzanello VJ, Schwartz L, Augustine JE. Posttransplantation hypertension related to calcineurin inhibitors. Liver Transpl. 2000;6(5):521–530. doi:10.1053/jlts.2000.9737
129. Soultati A, Mountzios G, Avgerinou C, et al. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38(5):473–483. doi:10.1016/j.ctrv.2011.09.002
130. Liu HT, Li F, Wang WY, et al. Rapamycin inhibits re-endothelialization after percutaneous coronary intervention by impeding the proliferation and migration of endothelial cells and inducing apoptosis of endothelial progenitor cells. Tex Heart Inst J. 2010;37(2):194–201.
131. Verdoia M, Kedhi E, Suryapranata H, et al. Poly (l -lactic acid) bioresorbable scaffolds versus metallic drug-eluting stents for the treatment of coronary artery disease: a meta-analysis of 11 randomized trials. Catheter Cardiovasc Interv. 2020;96(4):813–824. doi:10.1002/ccd.28594
132. Kataoka H, Kume N, Miyamoto S, et al. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99(24):3110–3117. doi:10.1161/01.CIR.99.24.3110
133. Xu HS, Duan J, Dai S, et al. Phytoestrogen alpha-zearalanol antagonizes oxidized LDL-induced inhibition of nitric oxide production and stimulation of endothelin-1 release in human umbilical vein endothelial cells. Endocrine. 2004;25(3):235–245. doi:10.1385/ENDO:25:3:235
134. Ye M, Qiu H, Cao Y, et al. Curcumin improves palmitate-induced insulin resistance in human umbilical vein endothelial cells by maintaining proteostasis in endoplasmic reticulum. Front Pharmacol. 2017;8:148. doi:10.3389/fphar.2017.00148
135. Sreejayan Rao MN, Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49(1):105–107. doi:10.1111/j.2042-7158.1997.tb06761.x
136. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi:10.1038/nri3452
137. Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi:10.1038/ni.1960
138. Campisi J, d’Adda Di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi:10.1038/nrm2233
139. Ota H, Akishita M, Eto M, et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43(5):571–579. doi:10.1016/j.yjmcc.2007.08.008
140. Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi:10.1038/nm1613
141. Xiang Y, Zhang Y, Tang Y, Li Q. MALAT1 modulates TGF-β1-induced endothelial-to-mesenchymal transition through downregulation of miR-145. Cell Physiol Biochem. 2017;42(1):357–372. doi:10.1159/000477479
142. Peixoto-Neves D, Wang Q, Leal-Cardoso JH, et al. Eugenol dilates mesenteric arteries and reduces systemic BP by activating endothelial cell TRPV 4 channels. Br J Pharmacol. 2015;172(14):3484–3494. doi:10.1111/bph.13156
143. Bubolz AH, Mendoza SA, Zheng X, et al. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca 2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol. 2012;302(3):H634–H642. doi:10.1152/ajpheart.00717.2011
144. Willis AI, Pierre-Paul D, Sumpio BE, et al. Vascular smooth muscle cell migration: current research and clinical implications. Vasc Endovascular Surg. 2004;38(1):11–23. doi:10.1177/153857440403800102
145. Zylla S, Dörr M, Völzke H, et al. Association of circulating chemerin with subclinical parameters of atherosclerosis: results of a population-based study. Arterioscler Thromb Vasc Biol. 2018;38(7):1656–1664. doi:10.1161/ATVBAHA.118.311219
146. Meng D, Shi X, Jiang BH, et al. Insulin-like growth factor-I (IGF-I) induces epidermal growth factor receptor transactivation and cell proliferation through reactive oxygen species. Free Radic Biol Med. 2007;42(11):1651–1660. doi:10.1016/j.freeradbiomed.2007.01.037
147. Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 2015;214(1):33–50. doi:10.1111/apha.12466
148. Moon JY. Recent update of renin-angiotensin-aldosterone system in the pathogenesis of hypertension. Electrolyte Blood Press. 2013;11(2):41–45. doi:10.5049/EBP.2013.11.2.41
149. Choi Y, Tanabe Y, Akazawa N, et al. Curcumin supplementation attenuates the decrease in endothelial function following eccentric exercise. J Exerc Nutrition Biochem. 2019;23(2):7–12. doi:10.20463/jenb.2019.0010
150. Oliver JM, Stoner L, Rowlands DS, et al. Novel form of curcumin improves endothelial function in young, healthy individuals: a double-blind placebo controlled study. J Nutr Metab. 2016;2016:1089653. doi:10.1155/2016/1089653
151. Hallajzadeh J, Milajerdi A, Kolahdooz F, et al. The effects of curcumin supplementation on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2019;33(11):2989–2995. doi:10.1002/ptr.6477
152. Santos-Parker JR, Strahler TR, Bassett CJ, et al. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017;9(1):187–208. doi:10.18632/aging.101149
153. Xu Y, Nie L, Yin YG, et al. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259(3):395–401. doi:10.1016/j.taap.2011.09.028
154. Yang Y, Duan W, Lin Y, et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013;65:667–679. doi:10.1016/j.freeradbiomed.2013.07.007
155. Barry SP, Townsend PA, Latchman DS, et al. Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med. 2007;13(2):82–89. doi:10.1016/j.molmed.2006.12.002
156. Duan W, Yang Y, Yan J, et al. The effects of curcumin post-treatment against myocardial ischemia and reperfusion by activation of the JAK2/STAT3 signaling pathway. Basic Res Cardiol. 2012;107(3):263. doi:10.1007/s00395-012-0263-7
157. Liu H, Wang C, Qiao Z, Xu Y. Protective effect of curcumin against myocardium injury in ischemia reperfusion rats. Pharm Biol. 2017;55(1):1144–1148. doi:10.1080/13880209.2016.1214741
158. van den Berg TK, Yoder JA, Litman GW. On the origins of adaptive immunity: innate immune receptors join the tale. Trends Immunol. 2004;25(1):11–16. doi:10.1016/j.it.2003.11.006
159. Kim YS, Kwon JS, Cho YK, et al. Curcumin reduces the cardiac ischemia-reperfusion injury: involvement of the toll-like receptor 2 in cardiomyocytes. J Nutr Biochem. 2012;23(11):1514–1523. doi:10.1016/j.jnutbio.2011.10.004
160. Fiorillo C, Becatti M, Pensalfini A, et al. Curcumin protects cardiac cells against ischemia-reperfusion injury: effects on oxidative stress, NF-kappaB, and JNK pathways. Free Radic Biol Med. 2008;45(6):839–846. doi:10.1016/j.freeradbiomed.2008.06.013
161. Zhu P, Yang M, He H, et al. Curcumin attenuates hypoxia/reoxygenation-induced cardiomyocyte injury by downregulating Notch signaling. Mol Med Rep. 2019;20(2):1541–1550. doi:10.3892/mmr.2019.10371
162. Cui JK, Wang X, Fan M, et al. Curcuminoids attenuate myocardial ischemia-reperfusion injury by regulating total RNA M6a levels: in vitro study. Comb Chem High Throughput Screen. 2023;26(10):1848–1855. doi:10.2174/1386207325666220929141003
163. Izem-Meziane M, Djerdjouri B, Rimbaud S, et al. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening: protective effect of curcumin. Am J Physiol Heart Circ Physiol. 2012;302(3):H665–H674. doi:10.1152/ajpheart.00467.2011
164. Tanwar V, Sachdeva J, Golechha M, et al. Curcumin protects rat myocardium against isoproterenol-induced ischemic injury: attenuation of ventricular dysfunction through increased expression of Hsp27 along with strengthening antioxidant defense system. J Cardiovasc Pharmacol. 2010;55(4):377–384. doi:10.1097/FJC.0b013e3181d3da01
165. Geng HH, Li R, Su YM, et al. Curcumin protects cardiac myocyte against hypoxia-induced apoptosis through upregulating miR-7a/b expression. Biomed Pharmacother. 2016;81:258–264. doi:10.1016/j.biopha.2016.04.020
166. Liu Y, Liu Y, Huang X, et al. Protective effects and mechanism of curcumin on myocardial injury induced by coronary microembolization. J Cell Biochem. 2019;120(4):5695–5703. doi:10.1002/jcb.27854
167. Moulin S, Arnaud C, Bouyon S, et al. Curcumin prevents chronic intermittent hypoxia-induced myocardial injury. Ther Adv Chronic Dis. 2020;11:2040622320922104. doi:10.1177/2040622320922104
168. Sunagawa Y, Funamoto M, Sono S, et al. Curcumin and its demethoxy derivatives possess p300 HAT inhibitory activity and suppress hypertrophic responses in cardiomyocytes. J Pharmacol Sci. 2018;136(4):212–217. doi:10.1016/j.jphs.2017.12.013
169. Sunagawa Y, Funamoto M, Shimizu K, et al. Curcumin, an inhibitor of p300-HAT activity, suppresses the development of hypertension-induced left ventricular hypertrophy with preserved ejection fraction in dahl rats. Nutrients. 2021;13(8):2608. doi:10.3390/nu13082608
170. Chowdhury R, Nimmanapalli R, Graham T, et al. Curcumin attenuation of lipopolysaccharide induced cardiac hypertrophy in rodents. ISRN Inflamm. 2013;2013:539305. doi:10.1155/2013/539305
171. Ahuja S, Kohli S, Krishnan S, et al. Curcumin: a potential therapeutic polyphenol, prevents noradrenaline-induced hypertrophy in rat cardiac myocytes. J Pharm Pharmacol. 2011;63(12):1604–1612. doi:10.1111/j.2042-7158.2011.01363.x
172. Bai XJ, Hao JT, Wang J, et al. Curcumin inhibits cardiac hypertrophy and improves cardiovascular function via enhanced Na+/Ca2+ exchanger expression after transverse abdominal aortic constriction in rats. Pharmacol Rep. 2018;70(1):60–68. doi:10.1016/j.pharep.2017.07.014
173. Ghosh SS, Salloum FN, Abbate A, et al. Curcumin prevents cardiac remodeling secondary to chronic renal failure through deactivation of hypertrophic signaling in rats. Am J Physiol Heart Circ Physiol. 2010;299(4):H975–H984. doi:10.1152/ajpheart.00154.2010
174. Fang G, Chen S, Huang Q, et al. Curcumin suppresses cardiac fibroblasts activities by regulating the proliferation and cell cycle via the inhibition of the p38 MAPK/ERK signaling pathway. Mol Med Rep. 2018;18(2):1433–1438. doi:10.3892/mmr.2018.9120
175. Liu H, Liu A, Shi C, et al. Curcumin suppresses transforming growth factor-β1-induced cardiac fibroblast differentiation via inhibition of Smad-2 and p38 MAPK signaling pathways. Exp Ther Med. 2016;11(3):998–1004. doi:10.3892/etm.2016.2969
176. Meng Z, Yu XH, Chen J, et al. Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-γ activation. Acta Pharmacol Sin. 2014;35(10):1247–1256. doi:10.1038/aps.2014.63
177. Ma J, Ma SY, Ding CH. Curcumin reduces cardiac fibrosis by inhibiting myofibroblast differentiation and decreasing transforming growth factor β1 and matrix metalloproteinase 9 / tissue inhibitor of metalloproteinase 1. Chin J Integr Med. 2017;23(5):362–369. doi:10.1007/s11655-015-2159-5
178. Pang XF, Zhang LH, Bai F, et al. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des Devel Ther. 2015;9:6043–6054. doi:10.2147/DDDT.S95333
179. Guo S, Meng XW, Yang XS, et al. Curcumin administration suppresses collagen synthesis in the hearts of rats with experimental diabetes. Acta Pharmacol Sin. 2018;39(2):195–204. doi:10.1038/aps.2017.92
180. Wang NP, Wang ZF, Tootle S, et al. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br J Pharmacol. 2012;167(7):1550–1562. doi:10.1111/j.1476-5381.2012.02109.x
181. Xiao J, Sheng X, Zhang X, et al. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des Devel Ther. 2016;10:1267–1277. doi:10.2147/DDDT.S104925
182. Boarescu PM, Chirilă I, Bulboacă AE, et al. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid Med Cell Longev. 2019;2019:7847142. doi:10.1155/2019/7847142
183. Kohli S, Chhabra A, Jaiswal A, et al. Curcumin suppresses gelatinase B mediated norepinephrine induced stress in H9c2 cardiomyocytes. PLoS One. 2013;8(10):e76519. doi:10.1371/journal.pone.0076519
184. Rice KM, Manne ND, Kolli MB, et al. Curcumin nanoparticles attenuate cardiac remodeling due to pulmonary arterial hypertension. Artif Cells Nanomed Biotechnol. 2016;44(8):1909–1916. doi:10.3109/21691401.2015.1111235
185. Cao Q, Zhang J, Gao L, et al. Dickkopf-3 upregulation mediates the cardioprotective effects of curcumin on chronic heart failure. Mol Med Rep. 2018;17(5):7249–7257. doi:10.3892/mmr.2018.8783
186. Mito S, Thandavarayan RA, Ma M, et al. Inhibition of cardiac oxidative and ERS-mediated apoptosis by curcumin treatment contributes to protection against acute myocarditis. Free Radic Res. 2011;45(10):1223–1231. doi:10.3109/10715762.2011.607252
187. Gao S, Zhou J, Liu N, et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015;85:131–139. doi:10.1016/j.yjmcc.2015.04.025
188. Ren BC, Zhang YF, Liu SS, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med. 2020;24(21):12355–12367. doi:10.1111/jcmm.15725
189. Yu W, Wu J, Cai F, et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One. 2012;7(12):e52013. doi:10.1371/journal.pone.0052013
190. Abdelsamia EM, Khaleel SA, Balah A, et al. Curcumin augments the cardioprotective effect of metformin in an experimental model of type I diabetes mellitus; Impact of Nrf2/HO-1 and JAK/STAT pathways. Biomed Pharmacother. 2019;109:2136–2144. doi:10.1016/j.biopha.2018.11.064
191. Aziz MT, El Ibrashy IN, Mikhailidis DP, et al. Signaling mechanisms of a water soluble curcumin derivative in experimental type 1 diabetes with cardiomyopathy. Diabetol Metab Syndr. 2013;5(1):13. doi:10.1186/1758-5996-5-13
192. Gbr AA, Abdel Baky NA, Mohamed EA, et al. Cardioprotective effect of pioglitazone and curcumin against diabetic cardiomyopathy in type 1 diabetes mellitus: impact on CaMKII/NF-κB/TGF-β1 and PPAR-γ signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(2):349–360. doi:10.1007/s00210-020-01979-y
193. Yan X, Xu P, Zhou L, et al. Blockade of high mobility group box 1 involved in the protective of curcumin on myocardial injury in diabetes in vivo and in vitro. IUBMB Life. 2020;72(5):931–941. doi:10.1002/iub.2226
194. Yao Q, Ke ZQ, Guo S, et al. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J Mol Cell Cardiol. 2018;124:26–34. doi:10.1016/j.yjmcc.2018.10.004
195. Soliman NA, Abo El Gheit RE, Abdel Ghafar MT, et al. Unraveling the biomechanistic role of Rac1/TWEAK/Fn14/NF-κB intricate network in experimentally doxorubicin-induced cardiotoxicity in rats: the role of curcumin. J Biochem Mol Toxicol. 2021;35(8):e22829. doi:10.1002/jbt.22829
196. He H, Luo Y, Qiao Y, et al. Curcumin attenuates doxorubicin-induced cardiotoxicity via suppressing oxidative stress and preventing mitochondrial dysfunction mediated by 14-3-3γ. Food Funct. 2018;9(8):4404–4418. doi:10.1039/C8FO00466H
197. Yang L, Shi J, Wang X, et al. Curcumin alleviates D-galactose-induced cardiomyocyte senescence by promoting autophagy via the SIRT1/AMPK/mTOR pathway. Evid Based Complement Alternat Med. 2022;2022:2990843. doi:10.1155/2022/2990843
198. Ghorbanzadeh V, Pourheydar B, Dariushnejad H, et al. Curcumin improves angiogenesis in the heart of aged rats: involvement of TSP1/NF-κB/VEGF-A signaling. Microvasc Res. 2022;139:104258. doi:10.1016/j.mvr.2021.104258
199. El Tabaa MM, Habib EI, Zahran A, et al. SERCA2a directs the cardioprotective role of nano-emulsion curcumin against PM2.5-induced cardiac injury in rats by prohibiting PERK-eIF2α pathway. Life Sci. 2022;311(Pt B):121160. doi:10.1016/j.lfs.2022.121160
200. Boarescu PM, Boarescu I, Bocșan IC, et al. Curcumin nanoparticles protect against isoproterenol induced myocardial infarction by alleviating myocardial tissue oxidative stress, electrocardiogram, and biological changes. Molecules. 2019;24(15):2802. doi:10.3390/molecules24152802
201. Tan NY, Khachigian LM. Sp1 phosphorylation and its regulation of gene transcription. Mol Cell Biol. 2009;29(10):2483–2488. doi:10.1128/MCB.01828-08
202. Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109(13):1580–1589. doi:10.1161/01.CIR.0000120390.68287.BB
203. Morin S, Paradis P, Aries A, et al. Serum response factor-GATA ternary complex required for nuclear signaling by a G-protein-coupled receptor. Mol Cell Biol. 2001;21(4):1036–1044. doi:10.1128/MCB.21.4.1036-1044.2001
204. Sipido KR, Volders PG, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res. 2002;53(4):782–805. doi:10.1016/S0008-6363(01)00470-9
205. Wang J, Song Y, Wang Q, et al. Causes and characteristics of diabetic cardiomyopathy. Rev Diabet Stud. 2006;3(3):108–117. doi:10.1900/RDS.2006.3.108
206. Sudirman S, Lai CS, Yan YL, et al. Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model. Sci Rep. 2019;9(1):15233. doi:10.1038/s41598-019-51821-6
207. Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–574. doi:10.1007/s00018-013-1349-6
208. Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225(3):631–637. doi:10.1002/jcp.22322
209. Ivey MJ, Tallquist MD. Defining the Cardiac Fibroblast. Circ J. 2016;80(11):2269–2276. doi:10.1253/circj.CJ-16-1003
210. Zhao J, Chen Y, Chen Q, et al. Curcumin ameliorates cardiac fibrosis by regulating macrophage-fibroblast crosstalk via IL18-P-SMAD2/3 signaling pathway inhibition. Front Pharmacol. 2022;12:784041. doi:10.3389/fphar.2021.784041
211. Crowley MJ, Powers BJ, Myers ER, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treatment of ischemic heart disease: future research needs prioritization. Am Heart J. 2012;163(5):777–782.e8. doi:10.1016/j.ahj.2012.02.016
212. Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118(6):1021–1040. doi:10.1161/CIRCRESAHA.115.306565
213. Spinale FG, Gunasinghe H, Sprunger PD, et al. Extracellular degradative pathways in myocardial remodeling and progression to heart failure. J Card Fail. 2002;8(6 Suppl):S332–8. doi:10.1054/jcaf.2002.129259
214. Schelbert EB, Piehler KM, Zareba KM, et al. Myocardial Fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc. 2015;4(12):e002613. doi:10.1161/JAHA.115.002613
215. Reinhardt D, Sigusch HH, Hensse J, et al. Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart. 2002;88(5):525–530. doi:10.1136/heart.88.5.525
216. Hernández-Reséndiz S, Correa F, García-Niño WR, et al. Cardioprotection by curcumin post-treatment in rats with established chronic kidney disease. Cardiovasc Drugs Ther. 2015;29(2):111–120. doi:10.1007/s10557-015-6581-x
217. Ochiai K, Watanabe M, Ueki H, et al. Tumor suppressor REIC/Dkk-3 interacts with the dynein light chain, Tctex-1. Biochem Biophys Res Commun. 2011;412(2):391–395. doi:10.1016/j.bbrc.2011.07.109
218. Zhang Y, Liu Y, Zhu XH, et al. Dickkopf-3 attenuates pressure overload-induced cardiac remodelling. Cardiovasc Res. 2014;102(1):35–45. doi:10.1093/cvr/cvu004
219. Nimata M, Okabe TA, Hattori M, et al. MCI-186 (edaravone), a novel free radical scavenger, protects against acute autoimmune myocarditis in rats. Am J Physiol Heart Circ Physiol. 2005;289(6):H2514–H2518. doi:10.1152/ajpheart.00661.2005
220. Mito S, Watanabe K, Harima M, et al. Curcumin ameliorates cardiac inflammation in rats with autoimmune myocarditis. Biol Pharm Bull. 2011;34(7):974–979. doi:10.1248/bpb.34.974
221. Lee C, Joseph L, Colosimo A, et al. Mortality in diabetes compared with previous cardiovascular disease: a gender-specific meta-analysis. Diabetes Metab. 2012;38(5):420–427. doi:10.1016/j.diabet.2012.04.002
222. Kuethe F, Sigusch HH, Bornstein SR, et al. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy? Horm Metab Res. 2007;39(9):672–676. doi:10.1055/s-2007-985823
223. Liu HJ, Liao HH, Yang Z, et al. Peroxisome proliferator-activated receptor- γ is critical to cardiac fibrosis. PPAR Res. 2016;2016:2198645. doi:10.1155/2016/2198645
224. Zhu H, He L. Beclin 1 biology and its role in heart disease. Curr Cardiol Rev. 2015;11(3):229–237. doi:10.2174/1573403X10666141106104606
225. Deavall DG, Martin EA, Horner JM, et al. Drug-induced oxidative stress and toxicity. J Toxicol. 2012;2012:645460. doi:10.1155/2012/645460
226. Damiani RM, Moura DJ, Viau CM, et al. Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 2016;90(9):2063–2076. doi:10.1007/s00204-016-1759-y
227. Swamy AV, Gulliaya S, Thippeswamy A, et al. Cardioprotective effect of curcumin against doxorubicin-induced myocardial toxicity in albino rats. Indian J Pharmacol. 2012;44(1):73–77. doi:10.4103/0253-7613.91871
228. van Laar M, Feltbower RG, Gale CP, et al. Cardiovascular sequelae in long-term survivors of young peoples’ cancer: a linked cohort study. Br J Cancer. 2014;110(5):1338–1341. doi:10.1038/bjc.2014.37
229. Bahadır A, Ceyhan A, Öz Gergin Ö, et al. Protective effects of curcumin and beta-carotene on cisplatin-induced cardiotoxicity: an experimental rat model. Anatol J Cardiol. 2018;19(3):213–221. doi:10.14744/AnatolJCardiol.2018.53059
230. Khadrawy YA, Hosny EN, El-Gizawy MM, et al. The effect of curcumin nanoparticles on cisplatin-induced cardiotoxicity in male wistar albino rats. Cardiovasc Toxicol. 2021;21(6):433–443. doi:10.1007/s12012-021-09636-3
231. Bjelogrlic SK, Radic J, Jovic V, et al. Activity of d,l-alpha-tocopherol (vitamin E) against cardiotoxicity induced by doxorubicin and doxorubicin with cyclophosphamide in mice. Basic Clin Pharmacol Toxicol. 2005;97(5):311–319. doi:10.1111/j.1742-7843.2005.pto_166.x
232. Avci H, Epikmen ET, Ipek E, et al. Protective effects of silymarin and curcumin on cyclophosphamide-induced cardiotoxicity. Exp Toxicol Pathol. 2017;69(5):317–327. doi:10.1016/j.etp.2017.02.002
233. Ciftci O, Turkmen NB, Taslıdere A. Curcumin protects heart tissue against irinotecan-induced damage in terms of cytokine level alterations, oxidative stress, and histological damage in rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(8):783–791. doi:10.1007/s00210-018-1495-3
234. Sabet NS, Atashbar S, Khanlou EM, et al. Curcumin attenuates bevacizumab-induced toxicity via suppressing oxidative stress and preventing mitochondrial dysfunction in heart mitochondria. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(8):1447–1457. doi:10.1007/s00210-020-01853-x
235. Huyut Z, Uçar B, Yıldızhan K, et al. The protective effect of curcumin on cardiac markers and fibrosis in abemaciclib-induced cardiac damage in rats. J Biochem Mol Toxicol. 2023;37(1):e23226. doi:10.1002/jbt.23226
236. Aziz SG, Pourheydar B, Chodari L, et al. Effect of exercise and curcumin on cardiomyocyte molecular mediators associated with oxidative stress and autophagy in aged male rats. Microvasc Res. 2022;143:104380. doi:10.1016/j.mvr.2022.104380
237. Bazzazi H, Isenberg JS, Popel AS. Inhibition of VEGFR2 activation and its downstream signaling to ERK1/2 and calcium by thrombospondin-1 (TSP1): in. Silico Investigation Front Physiol. 2017;8:48.
238. Wahlström B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol. 1978;43(2):86–92. doi:10.1111/j.1600-0773.1978.tb02240.x
239. Ravindranath V, Chandrasekhara N. Metabolism of curcumin--studies with [3H]curcumin. Toxicology. 1981;22(4):337–344. doi:10.1016/0300-483X(81)90027-5
240. Carroll RE, Benya RV, Turgeon DK, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila). 2011;4(3):354–364. doi:10.1158/1940-6207.CAPR-10-0098
241. Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–494.
242. Liu J, Huang YH, Wang BH, et al. Research progress on metabolic pathways and metabolites of curcumin compounds in vivo. Mod Med Clin. 2015;12:1553–1557.
243. Hoehle SI, Pfeiffer E, Sólyom AM, et al. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem. 2006;54(3):756–764. doi:10.1021/jf058146a
244. Ireson C, Orr S, Jones DJ, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–1064.
245. Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480.
246. Tanwar V, Sachdeva J, Kishore K, et al. Dose-dependent actions of curcumin in experimentally induced myocardial necrosis: a biochemical, histopathological, and electron microscopic evidence. Cell Biochem Funct. 2010;28(1):74–82. doi:10.1002/cbf.1623
247. Sun H, Zhu J, Lu T, et al. Curcumin-mediated cardiac defects in mouse is associated with a reduced histone H3 acetylation and reduced expression of cardiac transcription factors. Cardiovasc Toxicol. 2014;14(2):162–169. doi:10.1007/s12012-013-9240-0
248. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4(12):1502–1506. doi:10.1016/j.cgh.2006.08.008
249. Na LX, Li Y, Pan HZ, et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res. 2013;57(9):1569–1577.
250. Pourbagher-Shahri AM, Farkhondeh T, Ashrafizadeh M, et al. Curcumin and cardiovascular diseases: focus on cellular targets and cascades. Biomed Pharmacother. 2021;136:111214.
251. Ma T, Tian X, Zhang B, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603(7899):159–165. doi:10.1038/s41586-022-04431-8
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.