Back to Journals » Clinical Ophthalmology » Volume 17
Review of Literature on Intraductal Meibomian Gland Probing with Insights from the Inventor and Developer: Fundamental Concepts and Misconceptions
Received 16 September 2022
Accepted for publication 12 December 2022
Published 8 February 2023 Volume 2023:17 Pages 497—514
DOI https://doi.org/10.2147/OPTH.S390085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Natalia A Warren,1 Steven L Maskin2
1Not A Dry Eye Foundation, Daytona Beach, FL, USA; 2Dry Eye and Cornea Treatment Center, Tampa, FL, USA
Correspondence: Steven L Maskin, Dry Eye and Cornea Treatment Center, 3001 Swann Avenue, Tampa, FL, 33609, USA, Tel +1 8138750000, Email [email protected]
Abstract: Obstructive Meibomian gland dysfunction (MGD) affects millions of patients around the world. Its effective treatment with intraductal meibomian gland probing (MGP), was first reported in 2010. Since then, MGP has provided relief to thousands of patients globally suffering with refractory MGD. The purpose of Meibomian gland probing is restoring the integrity of the gland’s central duct by entering the gland through the natural orifice, releasing fixed obstruction thought to be periductal fibrosis, thereby establishing and/or confirming the patency of the duct, and concurrently equilibrating intraductal pressure as well as promoting gland functionality with meibum production. There may or may not be immediate secretion of meibum upon successful restoration of ductal integrity depending on the gland’s state of function and degree of atrophy. One double-blind placebo-controlled study has been conducted and, with the accumulated evidence of over 12 other peer reviewed articles in the scientific literature, overwhelmingly indicates that MGP is a safe and effective treatment for the MGD patient refractory to prior standard care and as a first-line treatment. This paper describes relevant fundamental concepts, dispels commonly held misconceptions, and provides an objective review of the current understanding and effectiveness of MGP for the treatment of obstructive MGD. Our analysis will better equip clinicians to draw informed conclusions about both subjective and objective findings reported in MGP studies and researchers to design future robust studies that provide meaningful results.
Keywords: meibomian gland dysfunction, MGD, obstructive MGD, MGP clinical trial, MGP studies, dry eye
Introduction
Meibomian gland probing (MGP) was first introduced in 2010 by author SLM.1 This innovative treatment targets firm, focal, fixed, unyielding obstruction thought to be secondary to periductal fibrosis, the apparent root cause of Meibomian gland dysfunction (MGD), and provides evidence of its presence while also confirming or restoring patency of gland ducts.2 MGP has been used to successfully treat patients around the world. Independent studies on the efficacy of MGP have been conducted in the United States,3–5 China,6–8 Turkey,9,10 India,11 Cuba,12 Japan,13 Mexico,14 and Russia.15
One previous literature review summarized study results of a subset of these studies, namely those available in English.16 However, the review had several significant limitations.17
Our paper provides fresh insights from the inventor and developer of MGP into the reviewed MGP research studies and dispels misconceptions that have entered the literature. We seek to establish a foundation for clinicians to draw informed conclusions about both objective and subjective findings reported in those and future MGP studies and to aid researchers studying MGP in designing robust studies that produce meaningful results.
Methods
Ten published peer reviewed studies from the 2021 review16 are included in our review.3–5,7–11,13,14 SLM, the author and inventor of MGP1 did not provide any guidance or input toward these studies. Studies published by SLM1,2,18–21 and included in the 2021 review,16 are not reviewed here to avoid suggestion of bias.
Procedure
The MGP procedure19 has been previously described by the author as follows (shown in Figure 1):2
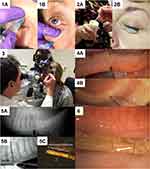 |
Figure 1 (1a and b) Performing supraorbital and infraorbital nerve block using JBP 33 gauge 4 mm long nanoneedles. (2a and b) Jojoba anesthetic ointment consisting of 8% lidocaine and 25% jojoba is taken from a refrigerated jar and applied to the lid margin for 10 minutes. This may be repeated. (3) The probing set-up at the slit lamp with an assistant to steady the patient for good visualization. (4) View through the slit lamp microscope of probing upper (a) and lower (b) lid Meibomian glands. (5) Meibography guided probing using the Mediworks S390L WDR FireFly Digital Slit Lamp from Eyefficient (Aurora, Ohio), demonstrating a 4 mm probe within the central duct (a), a sterile MicroTube Stent within the central duct for retrieval of meibum (b) and the retrieved meibum inside the MicroTube removed from within the gland (c). Reproduced from Maskin SL, Alluri S. Meibography guided intraductal meibomian gland probing using real-time infrared video feed. Br J Ophthalmol. 2020;104(12):1676; with permission from BMJ Publishing Group Ltd.21 (6) An alternative approach to obtaining a virgin sample of meibum by allowing the meibum to travel through the MicroTube Stent for collection and analysis. Arrow shows a drop of meibum at the distal end of the MicroTube Stent.22 Notes: 1a-b, 2a-b, 3, 4a-b: Courtesy of Steven L Maskin MD; 5a-c: Reproduced from Maskin SL, Alluri S Meibography guided intraductal meibomian gland probing using real-time infrared video feed British Journal of Ophthalmology 2020;104:1676–1682 with permission from BMJ; 6: Reproduced from Maskin, SL, Warren NA. Your Dry Eye Mystery Solved: Reversing Meibomian Gland Dysfunction, Restoring Hope. Yale University Press, 2022 with permission from Yale University Press. |
One drop of topical 0.5% tetracaine hydrochloride (Bausch and Lomb, Tampa, FL) is placed in the inferior fornix, followed by placing a bandage contact lens over the cornea. If nerve block is used for anesthetic, supraorbital, supratrochlear and infraorbital nerves are anesthetized using 2% Lidocaine with epinephrine. One mL is injected into each site using JBP 33 gauge nanoneedles of 4mm length (Henry Schein, Inc) after appropriate site preparation with povidone-iodine. Topical anesthetic ointment consisting of 8% lidocaine with 25% jojoba in a petrolatum ointment base (O’Brien Pharmacy, Mission, KS) is also applied to the inferior lid margin. The eye is then closed for 15 minutes. After 15 minutes, one additional drop of topical tetracaine is placed in the eye. The patient is then positioned at the slit lamp and the MG orifices are visualized and examined. To begin the procedure, first the angle of entry is determined with a 1-mm long stainless steel sterile intraductal MG probe (Rhein Medical, a division of Katena Products, Denville, NJ). The probe is inserted into each orifice, perpendicular to the lid margin using a dart-throwing motion. As the probe passes through the orifice lumen and into the distal duct, it typically encounters resistance. The resistance is characterized as firm, fixed, focal and unyielding, which requires additional probing force to relieve, analogous to relief of canalicular fibrosis with a punctal dilator. Relieving the obstruction creates a tactile sensation of pressure release and an audible firm pop (FP) and/or firm gritty (FG) (multiple pops) sounds which can be heard by both the patient and physician. When the tight band of contracting periductal fibrosis releases and resistance gives way, the probe can advance freely further into and then out of the duct when it is retracted.
The audible FP and gritty sounds and sensations are thought to be indicative of fibrosis’ severity, extent, and position along the lumen.2 For example, a single audible FP is typically accompanied by a single focus of pressure release, whereas an audible FG is typically accompanied by multiple foci of pressure release. In addition, variation in the intensity level of tactile intraductal resistance at times can be heard across the room, suggesting advanced fibrosis. Both the FP and FG sounds occur as sudden bursts, rather than as prolonged sounds, suggesting release of narrow bands of fibrosis. In fact, the multiple individual FP sounds that make up a FG, can occur immediately after each other in rapid succession or after a short delay as the probe advances through span of non-strictured duct lumen.
Less commonly, a mild back pressure or “soft” resistance, which is not fixed, not firm, and does not easily yield, may be noted. This mild pressure allows the probe to advance into the lumen without significant additional mechanical pressure and does not generate an audible sound. It does, on the other hand, “drag” on the “to and fro” movement of the wire probe. Soft resistance is not focal in contrast to the firm resistance which characteristically is focal or multifocal. Infrequently, there is a lack of resistance designated as no resistance where the probe enters the orifice and duct without any resistance or drag.
Imaging guided probing with use of infrared video meibography can dynamically visualize real time probing and tube devices within the central duct.21
Results from Literature
Summary of Previous Independent Study Results
Table 1 summarizes the results of independent peer-reviewed studies3-5,7–11,13,14 included in the 2021 review.16
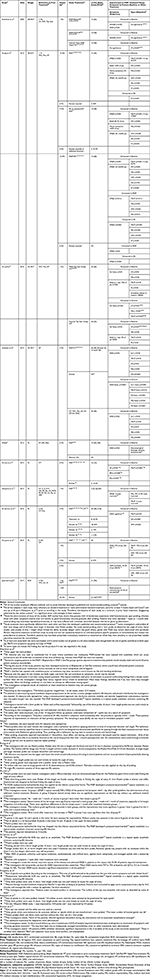 |
Table 1 Summary of Independent MGP Studies and Their Result |
The table includes details about the study designs and describes deviations from established MGP protocol.19 The table also lists p-values and improvements in subjective symptoms and objective signs in the MGP treatment group compared to control, baseline or other treatment. (SLM’s published studies are not included in the table to avoid any suggestion of bias.)
Note that all patients were refractory to prior standard pre-study treatment and treatment protocols varied widely. Nevertheless, all studies reported that either subjective symptoms, objective signs, or both showed improvement in MGP-treated patients. Furthermore, no adverse events were reported by MGP-treated patients (Table 1).
Effect on Subjective and Objective Measures
As summarized in Table 1, investigators3–5,7–11,13,14 reported improvement on these subjective measures in MGP-treated patients: Symptom Assessment in Dry Eye questionnaire (SANDE),3 Ocular Surface Disease Index (OSDI),3,5,9–11 Standard Patient Evaluation of Eye Dryness Questionnaire (SPEED),7 various symptoms,4,7,8 Dry Eye-Related Quality-of-Life Score (DEQS),13 visual acuity,14 comfort when blinking,14 and photophobia.14
Also as summarized in Table 1, investigators5,7–11,13,14 reported improvement on these objective measures in MGP-treated patients: lid tenderness,7 tear break-up time (TBUT),7–11,13,14 lid margin abnormalities,8 meibum grade,7,8 corneal fluorescein stain (CFS),7 Schirmer 1 test,9 fluorescein stain (FS),8 ocular surface Oxford score,9 meibum quality,9 meibum expressibility,9 gland blockage,5 keratitis,5 thickened lids,5 meibum lipid level,13 meibum viscosity,13 lipid interferometry,13 conjunctival hyperemia,10 lid margin vascularization,7,10 orifice abnormality,7 and lid margin congestion.11
Benefits of MGP Reported by Investigators
Studies showed that MGP consistently improved symptoms and signs in the overwhelming majority of patients, even with deviations in protocols (as noted in Table 1). Patients refractory to prior standard care showed the most improvement only after receiving MGP. Researchers arrived at the following conclusions:
Wladis:5
- “Intraductal meibomian gland probing is a safe, effective technique to address the ocular surface disease, tearing, and discomfort associated with ocular rosacea, and this intervention results in a dramatic improvement in these symptoms”.
Syed and Sutula:4
- “Immediately after the procedure, 91.4% of cases experienced symptomatic improvement, and no complications were noted”.
- “Dynamic intraductal meibomian probing is an effective and safe treatment for obstructive meibomian gland dysfunction that is resistant to traditional therapies”.
Incekalan et al:9
- “This study showed that intraductal meibomian gland probing seems to provide rapid symptom relief and clinical improvement for patients with obstructive-MGD (O-MGD)”.
- “Hereby, probing may become part of primary treatment as well as being an option in the patients resistant to conventional therapy in the future”.
Ma and Lu:8
- “Intraductal meibomian gland probing demonstrated significant efficacy in symptom relief and tear film stabilization. Probing helped release accumulated meibum and could help increase the accessibility of disease meibomian glands to topical corticosteroids”.
- “In group I [MGP + fluorometholone], 76% (19/25) of patients had symptom relief 1 day after probing before the administration of any additional medical treatment. However, in group II [fluorometholone], there was no significant improvement on the first day without any additional medical treatment”.
Nirupama et al:11
- “This interventional pilot study has shown that intraductal probing into the meibomian gland duct, with Maskins Meibomian gland probe is safe and reliable. After probing the orifice of the gland becomes patent and the sequestered meibum will release through the orifice. There were no adverse effects on follow-up over 6 months”.
- “Meibomian gland probing seems is a safe and effective method in patients with obstructive meibomian gland dysfunction. Following probing there is a significant improvement in signs and symptoms of the patients”.
Fermon et al:14
- “…it can be concluded that MGP is a promising procedure for blepharitis cases exhibiting resistance to conservative treatment. In the experience of the authors, it was a safe procedure which produced significant improvements in 100% of patients who exhibited improvements after 6 months, with only 2 requiring retreatment”.
Kheirkhah et al:3
- “Compared to baseline, the MG probing/Blephamide group showed significant improvements in both OSDI and SANDE scores and the MG probing/GenTeal group demonstrated a significant improvement only in SANDE score”.
Sik Sarman et al:10
- Conclusions (from Abstract): “A procedure using modified Maskin probes was effective and reliable in the short term for patients with meibomian gland dysfunction”.
Nakayama et al:13
- Conclusions (from Abstract): “Intraductal meibomian gland probing seems to improve meibomian gland lipid levels, and it may be a good treatment option for cases of o-MGD that are resistant to conventional treatment”.
Huang et al:7
- Conclusion: “IPL, MGP, and combined MGP-IPL are all effective methods for refractory o-MGD patients; however, the combination MGP-IPL method could maximize the therapeutic benefits which is especially helpful for patients who have severe meibomian gland obstruction and obvious intraductal or eyelid margin inflammation, who want to gain the greatest amelioration in all clinical signs and subjective symptoms or still remain frustrated to either MGP or IPL treatments”.
These conclusions by investigators, and the data shown in Table 1, demonstrate the powerful therapeutic effect of MGP on patients with refractory MGD. Each peer-reviewed study3–5,7–11,13,14 reported significant improvement in subjective symptoms and/or objective signs in patients receiving MGP without adverse sequelae. Therefore, MGP should be considered for patients with refractory MGD or as a first-line therapy.
MGP Effectiveness Compared to Prior Standard Treatment or Control Group
Compared to Prior Standard Treatment
First, as reported by investigators, our Table 1 column: Refractory to Prior Treatment shows that each study selected patients who were refractory to prior standard care treatment (some for at least 4 weeks4 and one patient up to 8 years13). These patients had already tried one or more of the following without showing improvement: lid hygiene, warm compresses, topical medications, systemic medication, lid massage, artificial tears, or other treatments. All investigators reported greatest improvement in patients only after MGP was added to the prior treatment protocol. In other words, MGP did outperform standard prior care in each study.
Compared to Control Groups Within a Study
Studies that compared MGP to standard care or sham probing all showed MGP outperformed controls or sham MGP in subjective and/or objective measures.3,7–9 (See our Table 1: Kheirkhah et al,3 Huang et al,7 Ma and Lu,8 Incekalan et al9).
Kheirkhah et al3 showed statistically significant improvement in subjective measures: MGP + Blephamide, SANDE (p=0.002), OSDI (p=0.02); MGP + GenTeal, SANDE (p=0.01). Sham MGP did not show statistically significant improvement in these measures (Table 1).
Huang et al7 showed improvement in subjective measures: 100% of MGP patients reported symptoms relief, compared to 85.7% of IPL patients. Plus, although no patient from any group displayed SPEED score ≤ 9 prior to treatment, 14.29% of IPL patients and 26.67% of MGP patients obtained post treatment score ≤ 9. Furthermore, no MGP patients had worsening of symptoms after treatment, whereas 14.8% of IPL patients had worsening of symptoms. Also, at 6 months, only 20% of MGP patients required retreatment, compared to 35.7% of IPL patients. In addition, for objective measures, 36.67% of MGP patients, compared to only 17.86% of IPL patients, had improvement in TBUT to >5 seconds (Table 1).
Ma and Lu8 compared the MGP + topical steroid group to baseline and to a control group using topical steroid only. MGP outperformed both as shown in our Table 1. The MGP group, improved in both subjective symptoms compared to baseline (p<0.01) and compared to control (p<0.001). In objective signs, the MGP group improved compared to baseline: lid margin abnormalities (p<0.05), meibum grade (p<0.05), TBUT (p<0.05), and FS (p<0.05); and compared to control: lid margin abnormalities (p<0.001), meibum grade (p<0.001) and TBUT (p=0.0293). In addition, 76% of patients in the MGP group experienced symptom relief in 1 day before the first drop of topical steroid was used (Table 1).
Incekalan et al9 compared MGP + conventional treatment (CT) to baseline and to a control group using conventional treatment alone. In subjective symptoms, the MGP + CT group compared to baseline improved in OSDI (p<0.05) and compared to control improved faster (p<0.0001). In objective signs, the MGP + CT group improved compared to baseline: Schirmer 1 (p<0.05), TBUT (p<0.05), Oxford score (p<0.05), meibum quality (p<0.05), and meibum expressibility (p=0.0001); and compared to control all parameters improved faster: Schirmer 1 Test (p=0.0001), TBUT (p=0.014), and Oxford score, p=0.0001; meibum quality, p=0.034; meibum expressibility, p=0.0001) (Table 1).
Concepts and Insights Drawn from Experience
Definition of Successful MGP
SLM has written in recent papers2,18–21 that the purpose of MGP is restoring the integrity of the gland’s central duct by releasing fixed obstruction thought to be periductal fibrotic and fibrovascular scar tissue, thus establishing and/or confirming the patency of the duct, and concurrently equilibrating intraductal pressure as well as promoting gland functionality with improved meibum production.2 Periductal fibrosis is thought to arise from surface and lid inflammation and disrupts the external duct wall/basement membrane with secondary constriction of duct wall and lumen with compromise of outflow capacity.22
MGP is successful when the probe enters the gland through the natural orifice and either confirms or restores the gland’s patency. Not all successfully probed glands will immediately secrete meibum. Some acinar-ductular units may be dormant (or atrophic) resulting in reduced or no sequestered meibum behind the fixed obstruction (unpublished data).2,21 After gland probing and the equilibration of intraductal pressure, gland functionality and meibum production returns, characterized by increased gland tissue and a proliferation of duct wall epithelium suggestive of stem cell activation.18,23
Superficial (Distal) versus Deep (Proximal) Probing
Constriction from periductal fibroses can occur anywhere along a gland’s length at multiple foci. Shorter and stiffer 1mm probes are used initially to locate the angle of entry and restore integrity to the distal duct. Longer 2mm or 4mm probes are used to complete the procedure when there is indication of deeper obstruction, such as persistent lid tenderness after the 1mm probe is used. Longer probes are also used in lids with MGD if minimal obstruction is found with 1mm probes, based on our study showing more than 90% of glands in these lids have fixed obstruction deeper than 1 mm by using 2 or 4mm probes.2
Significance of Lid Tenderness
Lid tenderness elicited during the exam when the examiner applies pressure to the patient’s lid (after topical anesthetic is applied to the surface of the eye to ensure the sensation emanates from the lid and not the ocular surface), is a key indicator of obstructive MGD and elevated intraductal pressure in the setting of fixed periductal fibrotic obstruction.
SLM has found and published that immediately after MGP—literally instantaneously—lid tenderness disappeared with the release of periductal fibrotic obstruction and the equilibration of intraductal pressure. (Other symptoms like burning may take longer to resolve as gland functionality normalizes over 2–3 months after MGP.)1,19,20 If lid tenderness persists after MGP (the developer’s published and recommended MGP protocol is an evaluation of the lid for tenderness after completing a pass with a 1mm probe)19 a longer probe is then used to ensure that more proximal periductal obstruction is also released.
Supporting these findings some investigators reported that patients experienced immediate or near immediate relief of symptoms after MGP suggesting the release of periductal obstruction with MGP, leading to equilibration of intraductal pressure and resolution of lid tenderness. For example, Ma and Lu8 found 76% of patients had immediate relief of symptoms one day after MGP even before initiating topical steroid drops. Syed and Sutula4 reported 91.4% of patients had relief of symptoms at 1 week follow-up.
Huang et al7 reported improvement specifically in lid tenderness; MGP-alone showed a clear statistically significant superior result when directly compared to IPL-alone (Intense Pulsed Light) (p<0.001) for lid tenderness.
Kheirkhah et al3 reported statistically significant improvement in lid tenderness in the sham MGP group. However, in this study, lids receiving MGP were injected with anesthetic, which can cause microtrauma, hemorrhage and edema creating iatrogenic lid tenderness persisting for weeks. Sham MGP lids, in contrast, were not injected with anesthetic but rather received topical anesthetic only.3 Also, the study design did not describe how focal, regional and global lid tenderness findings were managed statistically: were they combined or was there an attempt to differentiate between focal and more diffuse tenderness? Furthermore, topical anesthetic should also have been used immediately prior to evaluating lid tenderness to rule out tenderness from the ocular surface.
Significance of Repeat MGP
Patients might be retreated after initial MGP for several reasons. For example, in the setting of inadequately treated or advanced comorbid ocular surface or systemic diseases, harsh environments, or a variety of work and lifestyle factors (such as heavy computer use), the duct will re-obstruct within 2–3 months.
Not following the published and recommended MGP protocol19 can also lead to early reprobing. For example, obstructive disease and symptoms will not be resolved when treating with only short probes when longer probes are needed. In addition, if all glands in both lids are not treated obstructive disease and symptoms may persist.
Furthermore, symptoms of MGD other than lid tenderness and soreness, such as burning, stinging and photophobia, usually improve in 2–3 months after MGP concurrent with ductal epithelial cell proliferation as seen on confocal microscopy suggestive of stem cell activation along with increased gland tissue noted on meibography as well as improved gland functionality.18,21,23 Thus, some study patients, like those retreated 1 week after initial treatment,10 may have been retreated prematurely.
Finally, 20% of MGP-alone patients in Huang et al7 required reprobing at 6 months follow up. But in the same study at 6 months follow up, 35.7% of IPL-alone patients required retreatment. That amounts to a 75% greater number of IPL patients requiring retreatment than MGP patients. Moreover, one IPL treatment consists of 3 sessions and yet the 3 individual sessions each administered several weeks apart, are considered one single treatment instead of 3 separate treatments. This means the reprobed patients required only 2 treatments whereas IPL patients required 6 total treatment sessions.
Seven of the independent studies reported on repeat probing as follows:
- Fermon et al14 reported that 12.5% (2 of 16 patients) required retreatment, once within the six-month follow-up.
- Nirupama et al11 reported that 26.6% (8 out of 30 patients) needed retreatment after 3 months.
- Incekalan et al9 reported that 0% (0 of 20 patients) were retreated at 3 months follow up.
- Wladis5 reported that 0% (0 of 10 patients) were retreated at 6 months follow up.
- Huang et al7 reported that 0% (0 of 14 MGP+IPL patients), 20% (3 of 15 MGP patients), and 35.7% (5 of 14 IPL patients), required retreatment.
- Sik Sarman et al10 reported that 60% (18 of 30 patients) were treated 2 times, 10% (3 of 30 patients) were treated 3 times, and 3.3% (1 of 30 patients) were treated 4 times. Together at 1 year follow up, 73.3% (22 of 30 patients) were retreated.
Taken together, across these studies that reported on MGP retreatment (which can occur for a variety of reasons) 25.9% (35 of 135 patients) required retreatment.
In addition:
- Syed and Sutula4 reported that 7.7% (1 of 13 lids with long term follow up) were retreated at 52 weeks after initial treatment.
The one outlier that reported more frequent reprobing was from Turkey. Sik Sarman et al10 (listed as #6 above) explained, “a single application was not sufficient, which was considered a result of the chronic, recurrent course of the disease and advanced MGD in the patients”. Sik Sarman et al10 also reported that all their patients who were previously refractory to medical therapy for 6 months and “who underwent more than one probing procedure, during probing, diffuse vascularization in meibomian ducts, fibrosis and minimal bleeding was observed”. This finding explains and confirms the investigators’ conclusions of advanced MGD and suggests deep multifocal periductal fibrosis and fibrovascular tissue. An aggressive co-morbid chronic inflammatory and fibrotic reaction requires combined environmental control and reprobing on subsequent visits. Still, despite managing these advanced MGD cases that were refractory to previous standard therapy for 6 months, Sik Sarman et al10 found with MGP, statistically significant improvement in TBUT (p< 0.001), decrease in conjunctival hyperemia (p< 0.0001), and eyelid margin vascularization (p=0.004), as well as a significant improvement in OSDI scores, leading the investigators to state, “In conclusion, for the treatment of MGD, meibomian duct probing performed with modified Maskin cannulas was effective and reliable in short term follow ups”.
Characteristic Probe Findings and Their Significance
Two characteristic probe findings and their significance have not always been understood. These are the “popping” sound2 and the post-MGP, self-limiting hemorrhage. Neither of these are side-effects, signs of adverse reactions to treatment, complication due to treatment, or indications of potential sequelae.
Popping Sound
The popping sound observed during probing is like the sound heard upon the release of canalicular fibrosis with a punctal dilator or the snap of an old rubber band and is thought to represent the release of the band of periductal fibrotic tissue constricting the gland duct. It is just a sound and not a side effect.
Multiple popping sounds in quick succession, that in the hand of the prober create a feeling of something gritty lodged within the gland, indicate the release of multiple bands of fibrotic tissue at different depths. When these fibrotic bands are present, the patient and prober will hear what sounds like nails on a washboard as the probe releases the bands of tissue in quick succession.
All of these sounds, and the tactile sensation felt by the prober, are no more side effects than the sound of brushing and flossing one’s teeth. The sounds and tactile sensation are documented on a Probe Findings form (shown in Figure 2) used to monitor gland obstruction and improvement over time.
 |
Figure 2 Probe findings form of a patient. Notes: Reproduced from Maskin, SL, Warren NA. Your Dry Eye Mystery Solved: Reversing Meibomian Gland Dysfunction, Restoring Hope. Yale University Press, 2022 with permission from Yale University Press.22 |
Post-MGP Hemorrhage
Occasionally a microscopic self-limiting hemorrhage is seen at the gland orifice after MGP.
The droplet of blood is not an indication that the gland has been damaged.
Instead, the microscopic, self-limiting hemorrhage, along with the auditory pop or gritty sound described above, confirms that fixed obstruction consisting of abnormal fibrotic or fibrovascular tissue, that often wraps the glands and disrupts the external duct walls was released with probing (Figure 3).
One would expect a released fibrovascular stricture of a Meibomian gland, like a released fibrovascular stricture elsewhere in the body, to hemorrhage. This hemorrhage is a natural result of targeting and reversing the root cause of this disease and indicates the beginning of the rehabilitative process for the diseased glands. It is analogous to the small amount of hemorrhage sometimes seen when releasing a stricture of the urethra or esophagus.
Safety of MGP
MGP is inherently safe6,13 because:
- The probe enters the gland through the natural orifice opening at the lid margin, not through tissue of the lid margin.
- The probe advances into the hollow lumen of the gland, like an arm into a shirt sleeve, not into the gland’s acinar structures.
- The gland’s duct is not bony or rigid. The duct is flexible and the surrounding periglandular tissue is spongy and compressible, allowing the duct to flex when the probe is inserted while remaining intact.21
Furthermore, MGP has no contraindications (except for active infection), unlike other MGD treatments including IPL which poses numerous contraindications, such as uncontrolled ocular surface disease, history of ocular herpes simplex, and history of migraines with risks such as scarring, pain and discomfort, and change in pigmentation.24
Moreover, unlike patients treated with MGP alone, Huang et al7 noted that some patients treated with IPL alone reported worse symptoms, stating,
Surprisingly, instead of showing reduction in symptoms, 2 patients (14.8%) in the present study reported even more serious symptoms at the end of the IPL treatment course.
They suggested the post-IPL increase in pain was due to untreated or unreleased obstructions stating, “It can be speculated that this deterioration may relate to obstruction sites within the glands”.7 They further explained,
The heat released by IPL and the pressure caused by the forceps might paradoxically increase the intraductal pressure and exacerbate the inflammatory response; thus, treatment with IPL alone may not alleviate disease symptoms but instead irritate the condition.7
They went on to say,
This effect can also be indirectly observed in the present data in terms of the posttreatment lid tenderness of the IPL group, despite showing symptom alleviation compared with baseline, still being significantly higher than the MGP and MGP-IPL groups.7
Deviations from MGP Developer’s Published and Recommended Protocol
The 10 studies3–5,7–11,13,14 reported many deviations from MGP Developer’s Published and Recommended Protocol19 (see Table 1).
These deviations could affect outcomes and are discussed in the footnotes to Table 1:
- Inadequately anesthetizing lids.
- Injecting lids with excessively potent anesthetic that can cause toxic inflammatory side effects.
- Probing only 1 lid per eye.
- Probing a select few glands.
- Using large-diameter probes.
- Using probes not specifically designed for probing Meibomian glands.
- Not using a stiff short 1-mm probe to first locate the angle of entry.
- Probing with probes of only 1 length.
- Not checking for persistent lid tenderness after probing a lid with a 1mm probe.
- Prescribing adjunct treatment to patients regardless of comorbidities or sensitivities.
- Not identifying and successfully managing comorbid disease.
- Retreating prematurely.
Despite the fact that each of these deviations from the published MGP protocol19 would have impacted patient outcomes and study results in some way, investigators reported improvement in patients after MGP. The question remains: how much more would the patients’ outcomes and/or tolerance for the procedure have improved if the investigators had followed the developer’s published and recommended protocol?19
To illustrate, Huang et al7 used 4% lidocaine for injection into the upper and lower eyelids which may cause toxic eyelid reactions compared to the recommended protocol19 of using topical 8% lidocaine in jojoba anesthetic ophthalmic ointment with, if indicated, the addition of nerve block at the supra and infraorbital foramen, which does not distort local tissues. Huang et al7 also used 120µm outer diameter probes and 160µm outer diameter tubes rather than Maskin devices, dimensioned specifically for Meibomian glands: the 76µm outer diameter Maskin Probe and the 110µm outer diameter Maskin MicroTube. Also, the protocol21 strongly recommends beginning with the 1mm-long probe to find the correct entry angle into the distal duct rather than starting off with a 4.5 mm probe, as Huang et al7 did in their study, and which could cause more patient discomfort.
Additionally, when indicated, irrigating or lavaging topical steroids or other therapeutics into the glands through the orifice after probing them is part of the MGP protocol.21 Once ductal integrity is established and constriction of the lumen has been released by probing, the duct is prepared for irrigation with corticosteroid or other therapeutics.21
Concluding Comments
This study provides a comprehensive summary and analysis of independent peer-reviewed studies on MGP.3–5,7–11,13,14 Despite variations in treatment protocols, as shown in Table 1, all studies showed that MGP consistently demonstrated positive results in patients refractory to standard treatments for MGD. We provide analysis of these variations and how they impact study results.
Many of the investigators suggested that independent larger studies with longer-term follow-up are needed. Nevertheless, positive study results with no adverse effects have led some investigators to propose MGP not only as a refractory treatment for MGD but also as a primary treatment for all patients diagnosed with MGD.9
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
SLM is a >5% owner of MGD Innovations, Inc. which holds patents on instrumentation and methods for intraductal diagnosis and treatment of meibomian gland disease (MGD). SLM also has patents on the use of jojoba-based treatment options for MGD. He also reports patent royalties from Katena (nos: 9510844, 10159599, and 11110003). NAW is chair of the Not A Dry Eye Foundation. The authors report no other conflicts of interest in this work.
References
1. Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29(10):8. doi:10.1097/ICO.0b013e3181d836f3
2. Maskin SL, Alluri S. Expressible meibomian glands have occult fixed obstructions: findings from meibomian gland probing to restore intraductal integrity. Cornea. 2019;38(7):880–887. doi:10.1097/ICO.0000000000001954
3. Kheirkhah A, Kobashi H, Girgis J, Jamali A, Ciolino JB, Hamrah P. A randomized, sham-controlled trial of intraductal meibomian gland probing with or without topical antibiotic/steroid for obstructive meibomian gland dysfunction. Ocul Surf. 2020;18(4):852–856. doi:10.1016/j.jtos.2020.08.008
4. Syed ZA, Sutula FC. Dynamic intraductal meibomian probing: a modified approach to the treatment of obstructive meibomian gland dysfunction. Ophthal Plast Reconstr Surg. 2017;33(4):307–309. doi:10.1097/IOP.0000000000000876
5. Wladis EJ. Intraductal meibomian gland probing in the management of ocular rosacea. Ophthal Plast Reconstr Surg. 2012;28(6):416–418. doi:10.1097/IOP.0b013e3182627ebc
6. Dongju Q, Hui L, Jianjiang X. Clinical research on intraductal meibomian gland probing in the treatment of patients with meibomian gland dysfunction. Chin J Optom Ophthalmol. 2014;16(10):615–621.
7. Huang X, Qin Q, Wang L, Zheng J, Lin L, Jin X. Clinical results of intraductal meibomian gland probing combined with intense pulsed light in treating patients with refractory obstructive meibomian gland dysfunction: a randomized controlled trial. BMC Ophthalmol. 2019;19(1):211. doi:10.1186/s12886-019-1219-6
8. Ma X, Lu Y. Efficacy of intraductal meibomian gland probing on tear function in patients with obstructive meibomian gland dysfunction. Cornea. 2016;35(6):725–730. doi:10.1097/ICO.0000000000000777
9. Incekalan TK, Harbiyeli II, Yagmur M, Erdem E. Effectiveness of intraductal meibomian gland probing in addition to the conventional treatment in patients with obstructive meibomian gland dysfunction. Ocul Immunol Inflamm. 2019;27(8):1345–1351. doi:10.1080/09273948.2018.1522357
10. Sik Sarman Z, Cucen B, Yuksel N, Cengiz A, Caglar Y. Effectiveness of intraductal meibomian gland probing for obstructive meibomian gland dysfunction. Cornea. 2016;35(6):721–724. doi:10.1097/ICO.0000000000000820
11. Nirupama D, Hymavathi B, Prathima L, Sanjay Reddy T, SR G. Meibomian gland probing in patients with meibomian gland dysfunction. Indian J Clin Exp Ophthalmol. 2019;5(1):78–81.
12. Cárdenas Díaz T, Guerra Almaguer M, Hernández López I, Cruz Izquierdo D, Cuan Aguilar Y. Efficacy of intraductal probing in the dysfunction of the meibomian glands. Rev Cubana Oftalmología. 2016;30(2):725–730.
13. Nakayama N, Kawashima M, Kaido M, Arita R, Tsubota K. Analysis of meibum before and after intraductal meibomian gland probing in eyes with obstructive meibomian gland dysfunction. Cornea. 2015;34(10):1206–1208. doi:10.1097/ICO.0000000000000558
14. Fermon S, Zaga IH, Alvarez Melloni D. Intraductal meibomian gland probing for the treatment of blepharitis. Arch Soc Esp Oftalmol. 2015;90(2):76–80. doi:10.1016/j.oftal.2014.04.014
15. Prozornaia LP, Brzhevskii VV. Эффективность физиотерапевтических и гигиенических процедур в лечении детей и взрослых с хроническим блефаритом и синдромом [Efficacy of physiotherapy and hygienic procedures in treatment of adults and children with chronic blepharitis and dry eye syndrome]. Vestn Oftalmol. 2013;129(3):68–63. Russian.
16. Magno M, Moschowits E, Arita R, Vehof J, Utheim TP. Intraductal meibomian gland probing and its efficacy in the treatment of meibomian gland dysfunction. Surv Ophthalmol. 2021;66(4):612–622. doi:10.1016/j.survophthal.2020.11.005
17. Maskin SL. Comments on: intraductal meibomian gland probing and its efficacy in the treatment of meibomian gland dysfunction. Surv Ophthalmol. 2021;66(4):680–685. doi:10.1016/j.survophthal.2021.02.007
18. Maskin SL, Testa WR. Growth of meibomian gland tissue after intraductal meibomian gland probing in patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2018;102(1):59–68. doi:10.1136/bjophthalmol-2016-310097
19. Maskin SL, Alluri S. Intraductal meibomian gland probing: background, patient selection, procedure, and perspectives. Clin Ophthalmol. 2019;13:1203–1223. doi:10.2147/OPTH.S183174
20. Maskin SL, Alluri S. Intraductal meibomian gland probing: background, patient selection, procedure, and perspectives [Erratum]. Clin Ophthalmol. 2019;13:1475.
21. Maskin SL, Alluri S. Meibography guided intraductal meibomian gland probing using real-time infrared video feed. Br J Ophthalmol. 2020;104(12):1676. doi:10.1136/bjophthalmol-2019-315384
22. Maskin SL, Warren NA. Your Dry Eye Mystery Solved: Reversing Meibomian Gland Dysfunction, Restoring Hope. Yale University Press; 2022.
23. Alluri S, Maskin SL. Intraductal meibomian gland probing (MGP) leads to ductal epithelial proliferation with increased duct wall thickness. Invest Ophthalmol Vis Sci. 2020;61(7):96.
24. Lumenis. Website of Lumenis. Available from: https://lumenis.com/medical/eye-care-products/optilight.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Eyelid Warming Devices: Safety, Efficacy, and Place in Therapy
Bzovey B, Ngo W
Clinical Optometry 2022, 14:133-147
Published Date: 5 August 2022
Analysis of Treatment Efficacy of Intense Pulsed Light (M22) for Meibomian Gland Dysfunction with Demodex Mites
Zhang W, Cao X, Yang L, Duan Y, Zhang W
Clinical, Cosmetic and Investigational Dermatology 2023, 16:3743-3751
Published Date: 27 December 2023
Meibomian Gland Probing Stimulates a Proliferative Epithelial Response Resulting in Duct Regeneration
Maskin SL, Toland C
Clinical Ophthalmology 2024, 18:631-645
Published Date: 1 March 2024