Back to Journals » OncoTargets and Therapy » Volume 11
Reversine, a substituted purine, exerts an inhibitive effect on human renal carcinoma cells via induction of cell apoptosis and polyploidy
Authors Cheng L, Wang H , Guo KC, Wang ZC , Zhang ZY, Shen C, Chen L, Lin J
Received 27 November 2017
Accepted for publication 17 January 2018
Published 26 February 2018 Volume 2018:11 Pages 1025—1035
DOI https://doi.org/10.2147/OTT.S158198
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Li Cheng,1,2,* Hao Wang,3,* Kecun Guo,4 Zicheng Wang,1,2 Zhongyuan Zhang,1,2,5 Cheng Shen,1,2,5 Liang Chen,6 Jian Lin1,2,5
1Department of Urology, Peking University First Hospital, Beijing, China; 2Institute of Urology, Peking University, Beijing, China; 3Department of Obstetrics and Gynecology, Peking University First Hospital, Beijing, China; 4Department of Urology, The Second People’s Hospital of Liaocheng, Shandong, China; 5National Urological Cancer Center, Beijing, China; 6Medical Center of Reproductive and Genetics, Peking University First Hospital, Beijing, China
*These authors contributed equally to the paper
Background: Human renal cell carcinoma (RCC) is the most common type of kidney cancer that arises from the renal epithelium. Up to 33.3% of RCC patients treated with local tumor resections will subsequently develop recurrence or metastases. Thus, optimized therapeutic regimes are urgently needed to improve the prognosis of RCC. Reversine was recently reported to exert critical roles in cancer therapy.
Materials and methods: This study evaluated the anti-tumor effects of reversine on cell viability, colony formation, apoptosis, and cell cycle in 786-O and ACHN cell lines.
Results: It was demonstrated that reversine significantly inhibited the proliferation of both cell lines in time- and dose-dependent manners. Polyploidy formation was observed under high-concentration reversine treatment. In addition, reversine induced cell death via caspase-dependent apoptotic pathways, which could be partially inhibited by Z-VAD-FMK, a pan-caspase inhibitor.
Conclusion: Reversine could effectively suppress the proliferation of human RCC cells, and may serve as a novel therapeutic regimen for RCC in clinical practice.
Keywords: anti-cancer, apoptosis, Aurora kinase, human renal cell carcinoma, polyploidy, reversine
Introduction
Renal cell carcinoma (RCC), which is the most common form of adult kidney cancer, arises from the renal epithelium, and is comprised of three main histologic subtypes, namely, clear-cell RCC, papillary carcinoma, and chromophobe tumors.1,2 RCC is estimated to be the seventh most frequently diagnosed cancer in men, and the ninth in women, representing approximately 2%–3% of adult malignancies worldwide.3 In 2012, about 84,400 new cases of RCC were diagnosed in the European Union, and approximately 34,700 tumor-related deaths were recorded. Moreover, the incidence of RCC worldwide is steadily increasing, with an approximate annual rate of 2%.4,5 For patients with localized RCC, surgery is currently the golden standard of curative treatment with favorable prognosis. However, after surgery, up to 33.3% of the patients will subsequently develop metastases.6 Moreover, about 25%–30% of patients demonstrate locally advanced or metastatic stage by the first diagnosis with RCC.7 Thus, optimized diagnostic and therapeutic regimes are urgently needed to improve the prognosis of RCC.
Reversine, a substituted purine, was initially identified by Chen et al8 in 2004. This molecule induces the dedifferentiation of C2C12 cells, a murine myoblast cell line, into multipotent progenitor cells.8 Hence, various studies have focused on the roles of reversine in cell reprogramming and regenerative medicine.9–12 Reversine is supposed to reprogram somatic cells into a multipotent state, which could then be converted into different cell types. The impact of reversine on cell differentiation is also applicable to cancer therapy. Numerous studies have demonstrated the tumor-suppression activity of reversine against human cancer cells by induction of apoptosis, cell cycle arrest, polyploidy, and (or) autophagy.13–16 D’Alise et al17 reported that reversine could exert the same inhibitive influence in the colony formation of leukemic cells, such as VX-680, a potent Aurora kinase inhibitor which has entered phase II clinical trials. Reversine is superior to VX-680, because of its lower toxicity in this study. This derivative could cause a failure in cytokinesis and induce polyploidization by inhibiting the bioactivity of Aurora kinases A and B, which are involved in multi-aspects of mitosis. Moreover, using a tumor cell-specificity in vitro bioluminescence imaging assay, McMillin et al18 showed that reversine could inhibit multiple myeloma cells in vitro by suppressing the bioactivity of Aurora kinases, JAK2, and SRC.
Aurora kinases have been reported to be frequently overexpressed in human tumors. These kinases have been deemed as valuable targets for cancer therapy because of their prominent role in mitotic regulation.19 Therefore, reversine, as a novel Aurora kinase inhibitor, might serve as an available agent in clinical cancer chemotherapy.17
To our knowledge, the anti-tumor mechanism of reversine against human RCC has not been elucidated yet. Here, we demonstrated the cytotoxic effect of reversine on two human RCC cells. This molecule could suppress cell viability and colony formation by inducing caspase-dependent apoptosis in 786-O (p53 mutant) and ACHN (p53 wide-type) cell lines. Moreover, polyploidy cells also formed after the cells were incubated with high-concentration reversine (0.8–1.6 μM). The data suggested that reversine might serve as a candidate chemotherapeutic agent against RCC.
Materials and methods
Cell lines and culture
Two human RCC cell lines, namely 786-O (p53 mutant) and ACHN (p53 wide-type), were used in this study. Both cell lines were purchased from the American Type Culture Collection and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco; Thermo Fisher Scientific, Waltham, CA, USA) with 10% fetal bovine serum (Corning Incorporated, Corning, NY, USA) and incubated in a 5% CO2 humidified atmosphere at 37°C.
Reagents
Reversine and Z-VAD-FMK were purchased from MCE (Middlesex County, NJ, USA) and dissolved in dimethyl sulfoxide (DMSO) according to the manufacturer’s recommendations. The 10 mmol/L stock solutions were stored at −80°C. Antibodies against cyclin A2, cyclin B1, cyclin D1, phosphor-Rb, p21, Bcl-2, Bcl-xL, and Bax were from Abcam (Cambridge, UK). Antibodies against cleaved-caspase-3 and cleaved-PARP were from Cell Signaling Technology (Danvers, MA, USA).
Cell proliferation assay
A 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was performed to evaluate cell proliferation. Briefly, 786-O and ACHN cells were plated in quadruplicate in a 96-well plate at 1×104/well density in a final volume of 100 μL of Dulbecco’s Modified Eagle’s Medium (DMEM) medium/well in a 5% CO2 environment at 37°C. After overnight attachment, the cells were incubated with DMEM (with 0.01% DMSO) or DMEM containing varied dosages of reversine at 0.1, 0.2, 0.4, 0.8, and 1.6 μM. After incubation for 24, 48, 72, and 96 h, MTS labeling reagent (Promega Corporation, Fitchburg, WI, USA) was mixed with DMEM and incubated with cells for 2 h. Absorbance at 490 nm and 690 nm was examined using a VARIOSCAN FLASH microplate reader (Thermo Fisher Scientific). Cell viability was represented by percentage values compared to a control.
Colony formation assay
Colony formation assay was performed to determine the long-range impact of reversine exposure on RCC cells. Briefly, the cells were seeded in a 6-well culture plate (1×103/well). After cell attachment for 6 h, DMEM with varied dosages of reversine or 0.01% DMSO was added into each well. Then, all experimental samples were incubated in a 5% CO2 environment at 37°C, until appropriately large colonies have formed. After approximately 10–14 days, the medium was removed. The samples were stained with crystal violet for 45 min and then washed three times with PBS. Three independent experiments were performed.
Analysis of apoptosis and cell death
Apoptosis and cell death were analyzed by flow cytometry using propidium iodide (PI) and Annexin-V-FITC staining kit (Nanjing Keygen Biotech. Co., Ltd., Nanjing China). The cells were washed with phosphate buffer saline (PBS) and then centrifuged at 2,000× g for 5 min, according to the manufacturer’s instructions. Next, the cell pellets were resuspended in 500 μL of binding buffer. Then, 5 μL of Annexin-V-FITC and 5 μL of PI were successively added. After incubating in the dark at room temperature for 15 min, Annexin-V or PI fluorescent intensities were analyzed by FACScan (Becton–Dickinson, Franklin Lakes, NJ, USA), 20,000 cells were evaluated in each sample.
DNA content analysis
The Cellular DNA content of each well was determined by flow cytometry according to the protocol (KeyGEN Biotech). After harvesting and washing twice with ice-cold PBS, the cells were resuspended in 1 mL of 70% ethanol and fixed at 4°C overnight. Then, the fixed cells were washed twice with ice-cold PBS and treated with 100 μL RNase A for 30 min. Afterward, 400 μL of PI was added to the cells. Samples were acquired by flow cytometry using Millipore (Becton–Dickinson).
Western blot analysis
Briefly, RCC cell extracts were washed three times in cold PBS and lysed in lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100 (Beyotime, Jiangsu, China), supplemented with Protease Inhibitor Cocktail, and Phosphatase Inhibitor Cocktail (MCE) for 30 min on ice. The homogenates were centrifuged at 12,000× g for 10 min, and the extracted protein concentrations were determined by the Bio-Rad protein assay. The protein lysates (~20 μg) were electrophoresed in SDS-PAGE gels and transferred onto polyvinylidene fluoride membrane (Merck Millipore, Burlington, MA, USA). The membranes were blocked with TBST (tris-buffered saline with Tween® 20) containing 5% dried milk/BSA for 1 h at room temperature. After incubating in appropriate primary and fluorescent dye-labeled secondary antibodies, the blots were visualized using the ECL Western blot detection system (Syngene, Bangalore, India).
Statistical analysis
All data are presented as mean ± standard deviation for the indicated number of separate experiments. Statistical significance of the differences between the mean values was measured by the Student’s t-test with a paired, 2-tailed distribution. The data were considered significant when the P-value was less than 0.05 (*) or 0.01 (**).
Results
Reversine reduced cellular viability and inhibited cell colony formation of human RCC cells
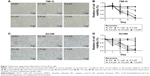
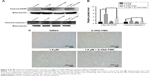
Human RCC cells 786-O and ACHN were used to evaluate the proliferation inhibitive effect of reversine. DMSO was used as a negative control. Microscopy results showed the inferior condition of both cell lines after reversine treatment for 48 h. Cell abundance of both cell lines decreased significantly compared with the negative control (Figure 1A and C). Moreover, we further verified the inhibitive effect of reversine on the cellular viability by MTS analysis. Figure 1B and D shows that 786-O and ACHN cells decreased in time- and dose-dependent manners after reversine treatment.
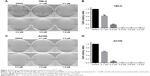
In addition, the growth modulatory effect of reversine in 786-O and ACHN cells was determined using a colony formation assay. Figure 2 shows that reversine could significantly inhibit the numbers of colony formations in both cell lines. Furthermore, cloning was virtually impossible under high-concentration reversine treatment (0.4–1.6 μM). Thus, these data indicated that reversine could reduce cell viability and inhibit cell colony formation of human RCC cells.
Reversine induced polyploidization in human RCC cells
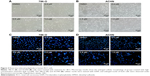
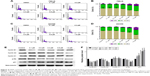
Reversine could therapeutically cause a failure in cytokinesis and induce polyploidization by suppressing Aurora kinases, which are prominent enzymes in mitotic regulation. This phenomenon has also been reported in multiple human cancer cells.14,20 Here, we explored whether reversine could cause an impaired mitosis in human RCC cell lines. Microscopy results showed that reversine treatment caused enlarged cell phenotypes (Figure 3A and B). We further confirmed this phenomenon by nucleo-staining with DAPI (4′,6-diamidino-2-phenylindole), and multinucleated cells were observed after reversine treatment in both cell lines (Figure 3C and D). In addition, flow cytometry was used to detect the DNA content of 786-O and ACHN cells after reversine treatment. The percentages of 2N, 4N, and ≥8N distribution of all cells were measured with CellQuest Pro software (BD Biosciences, San Jose, CA, USA), with DMSO as a negative control. Figure 4A–D indicates that polyploidization occurred and increased in a dosage-dependent manner in both cells with a higher drug concentration (0.8–1.6 μM). Furthermore, the failure of cell mitosis caused by reversine treatment was confirmed by determining the cell-cycle regulating proteins, namely, cyclin A2, cyclin B1, cyclin D1, p21, and p-Rb by Western blot in reversine-treated 786-O cells. The results indicated that reversine inhibited the expression levels of Cyclin A2, Cyclin B1, and p-Rb, but promoted p21 expression (Figure 4E and F). These data demonstrated that reversine-treated cells could exit from uncomplicated cytokinesis and re-enter into new rounds of cycle.
Reversine induced caspase-dependent apoptosis in human RCC cells
We also explored whether reversine could mediate apoptosis induction in human RCC cells. In this study, 786-O and ACHN were treated with reversine or DMSO, and flow cytometry analysis with AnnexinV and PI double staining were performed to determine apoptosis. The data showed that reversine could induce apoptosis in both human RCC cells in time- and dosage-dependent manners (Figure 5A–D). The p53-deficient 786-O cells were more sensitive to exposure to reversine than the p53-proficient ACHN cells. Furthermore, Western blot was performed to detect the activation of caspase-3 and PARP to explore the mechanism of reversine-mediated apoptosis in human RCC cells. As expected, the expression of cleaved-caspase-3 was significantly upregulated in the 786-O cells in a dosage-dependent manner after incubation with reversine for 48 h (Figure 5E and G). Moreover, the expression of cleaved PARP was also determined by Western blot to further confirm the increased apoptosis, and the result was consistent with cleaved-caspase-3 (Figure 5E and G). The expression levels of Bcl-2, Bcl-xL, and Bax after reversine incubation showed no significant difference compared with the control group (Figure 5F and G). Generally, our results demonstrated that reversine could induce apoptosis in human RCC cells through an extrinsic pathway, and this result is consistent with those of previous studies.13,21
In addition, Z-VAD-FMK, a pan-caspase inhibitor, was used to further elucidate the mechanism of apoptosis induced by reversine in RCC cells. After blocking the activation of caspase with Z-VAD-FMK, reversine-mediated activation of cleaved caspase-3 was partially suppressed (Figure 6A and B), and the cell number of 786-O cells was restored (Figure 6C). Moreover, the expression of cleaved-PARP was reduced (Figure 6A and B). These data indicated that reversine could reduce cell viability by inducing apoptosis in human RCC cells partially through a caspase-dependent pathway.
Discussion
Reversine, a 2,6-disubstituted purine, has been originally reported to induce dedifferentiation of C2C12 cells.8 Several studies have demonstrated that reversine could inhibit Aurora kinases and various kinds of cellular enzymatic (such as AKT, SRC, and JAK2) activities in vitro.9,18 The Aurora kinases comprised Aurora-A, Aurora-B, and Aurora-C, which play a vital role in regulating mitosis.22 The anti-tumor capability of reversine has been confirmed in numerous cancer cells.15–18,23 Thus, we investigated the growth modulatory effects of reversine in human RCC cells.
In the present study, we first demonstrated the anti-tumor capability of reversine in 786-O and ACHN RCC cells. Cell viability and colony formation were significantly inhibited in both cell lines. Moreover, reversine-mediated polyploidy formation and apoptosis were induced in a dose-dependent manner.
In our study, polyploidy formation was induced in both cell lines under reversine treatment. The formation was characterized by an enlargement of cell nuclei and an enrichment of 4N and 8N interphase populations. This finding is consistent with previous studies.13,14 Notably, polyploidy cancer cells show increased sensitivity to anti-tumor agents or gamma irradiation by inducing late-stage cell apoptosis and/or mitotic cell death.24,25
Endoreplication mechanisms may contribute to the induction of polyploidy formation in RCC cells in response to reversine treatment. We observed changes in the expression of cell cycle regulating proteins. The cell cycle is controlled by a complex bio-chemical-molecular system including cyclins, cdks, and other factors. Activation of CDK1-cyclin B1 is necessary for mitotic entry.26 We discovered that the downregulation of cyclin B1 was coordinated with an increased level of p21 in the 786-O cell line under reversine treatment. These changes may account for the exit from impaired cytokinesis followed by re-entering of the G1 phase and continuous DNA replication, and these processes subsequently increased polyploidy formation. Our results are in agreement with the results from other studies.16,20,27,28 Hence, the accumulation of polyploid cells may be one of the mechanisms of cytotoxicity in the treatment of RCC cells. Whether reversine-mediated polyploidy formation was through the inhibition of Aurora kinases and/or other factors in RCC cells deserves to be verified.
Moreover, we first demonstrated that apoptosis was triggered by reversine in 786-O and ACHN RCC cells. Reversine has been reported to induce apoptosis in several human cancer cells. Consistent with previous studies,13,21 we exhibited that reversine induced apoptosis by activating caspase-dependent apoptotic pathways. Moreover, the mitochondrion-dependent apoptotic pathway was not involved in reversine-mediated RCC cell apoptosis. This phenomenon was confirmed by detection of Bcl-2, Bcl-xL, and Bax.
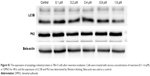
Previous studies have demonstrated that reversine could induce autophagosome formation in numerous cancer cells in vitro.15,16 However, no change of autophagy was observed in our study (Figure S1), which indicates that the induction of autophagosome formation by reversine was cell type-dependent.
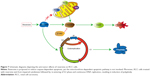
Several cancer cells have been reported to present TP53 mutations which lead to resistance against anti-cancer agents.29–33 Jemaà et al34 demonstrated that reversine killed p53-proficient cells more efficiently at low-concentration (~0.5 μM). Conversely, p53-deficient cells were more susceptible under high-concentrations (~5 μM) of reversine.34 In our study, we evaluated the cytotoxic profile of reversine (from 0.1–1.6 μM) in 786-O (p53 mutation) and ACHN (p53 wild-type) cell lines. Reversine exerted antiproliferative activity with the IC50 value of 1.61 μM in 786-O and 0.74 μM in ACHN (72 h), showing that ACHN cells were more susceptible than 786-O cells. Colony formations were completely inhibited in both cell lines under reversine treatment (≥0.4 μM). Moreover, at a concentration of 1.6 μM, reversine killed 786-O cells more efficiently than ACHN cells (28.70%±1.30%, compared with 13.84%±0.16%), while ACHN cells showed a higher distribution of polyploid (20.5%±2.7%, compared with 15%±3.1%). In general, the mechanism by which reversine exerts anti-tumor effects on RCC cells is shown in Figure 7.
Conclusion
We demonstrated that reversine is a potential therapeutic drug that can reduce cell viability by inducing apoptosis and polyploidy formation. Our study suggests that reversine may serve as a novel therapeutic drug for RCC patients.
Acknowledgments
This study was funded by the Natural Science Foundation of China (No. 81670617) and the Beijing Nature Science Foundation (No. 7142158).
Disclosure
The authors report no conflicts of interest in this work.
References
Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality.Eur Urol. 2015;67(3):519–530. | ||
Ning XH, Gong YQ, He SM, et al. Higher programmed cell death 1 ligand 1 (PD-L1) mRNA level in clear cell renal cell carcinomas is associated with a favorable outcome due to the active immune responses in tumor tissues. Oncotarget. 2017;8(2):3355–3363. | ||
Kurozumi A, Kato M, Goto Y, et al. Regulation of the collagen cross-linking enzymes LOXL2 and PLOD2 by tumor-suppressive microRNA-26a/b in renal cell carcinoma. Int J Oncol. 2016;48(5):1837–1846. | ||
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. | ||
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. | ||
Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353(23):2477–2490. | ||
Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–875. | ||
Chen S, Zhang Q, Wu X, Schultz PG, Ding S. Dedifferentiation of lineage-committed cells by a small molecule. J Am Chem Soc. 2004;126(2):410–411. | ||
Kim YK, Choi HY, Kim NH, et al. Reversine stimulates adipocyte differentiation and downregulates Akt and p70(s6k) signaling pathways in 3T3-L1 cells. Biochem Biophys Res Commun. 2007;358(2):553–558. | ||
Anastasia L, Sampaolesi M, Papini N, et al. Reversine-treated fibroblasts acquire myogenic competence in vitro and in regenerating skeletal muscle. Cell Death Differ. 2006;13(12):2042–2051. | ||
Saraiya M, Nasser R, Zeng Y, et al. Reversine enhances generation of progenitor-like cells by dedifferentiation of annulus fibrosus cells. Tissue Eng Part A. 2010;16(4):1443–1455. | ||
Jung DW, Williams DR. Novel chemically defined approach to produce multipotent cells from terminally differentiated tissue syncytia. ACS Chem Biol. 2011;6(6):553–562. | ||
Kuo CH, Lu YC, Tseng YS, et al. Reversine induces cell cycle arrest, polyploidy, and apoptosis in human breast cancer cells. Breast Cancer. 2014;21(3):358–369. | ||
Lu YC, Lee YR, Liao JD, et al. Reversine induced multinucleated cells, cell apoptosis and autophagy in human non-small cell lung cancer cells. PloS One. 2016;11(7):e0158587. | ||
Lu CH, Liu YW, Hua SC, Yu HI, Chang YP, Lee YR. Autophagy induction of reversine on human follicular thyroid cancer cells. Biomed Pharmacother. 2012;66(8):642–647. | ||
Lee YR, Wu WC, Ji WT, et al. Reversine suppresses oral squamous cell carcinoma via cell cycle arrest and concomitantly apoptosis and autophagy. J Biomed Sci. 2012;19:9. | ||
D’Alise AM, Amabile G, Iovino M, et al. Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells. Mol Cancer Ther. 2008;7(5):1140–1149. | ||
McMillin DW, Delmore J, Weisberg E, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16(4):483–489. | ||
Keen N, Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat Rev Cancer. 2004;4(12):927–936. | ||
Hsieh TC, Traganos F, Darzynkiewicz Z, Wu JM. The 2,6-disubstituted purine reversine induces growth arrest and polyploidy in human cancer cells. Int J Oncol. 2007;31(6):1293–1300. | ||
Hua SC, Chang TC, Chen HR, et al. Reversine, a 2,6-disubstituted purine, as an anti-cancer agent in differentiated and undifferentiated thyroid cancer cells. Pharm Res. 2012;29(7):1990–2005. | ||
Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2(1):21–32. | ||
Qin HX, Yang J, Cui HK, et al. Synergistic antitumor activity of reversine combined with aspirin in cervical carcinoma in vitro and in vivo. Cytotechnology. 2013;65(4):643–653. | ||
Lanzi C, Cassinelli G, Cuccuru G, et al. Cell cycle checkpoint efficiency and cellular response to paclitaxel in prostate cancer cells. Prostate. 2001;48(4):254–264. | ||
Kim JY, Kim CH, Stratford IJ, Patterson AV, Hendry JH. The bioreductive agent RH1 and gamma-irradiation both cause G2/M cell cycle phase arrest and polyploidy in a p53-mutated human breast cancer cell line. Int J Radiat Oncol Biol Phys. 2004;58(2):376–385. | ||
Lindqvist A, van Zon W, Karlsson Rosenthal C, Wolthuis RM. Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol. 2007;5(5):e123. | ||
Harrington EA, Bebbington D, Moore J, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–267. | ||
Perreira M, Jiang JK, Klutz AM, et al. “Reversine” and its 2-substituted adenine derivatives as potent and selective A3 adenosine receptor antagonists. J Med Chem. 2005;48(15):4910–4918. | ||
Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Commun. 2005;331(3):834–842. | ||
Park WS, Lee JH, Shin MS, et al. Inactivating mutations of the caspase-10 gene in gastric cancer. Oncogene. 2002;21(18):2919–2925. | ||
Soung YH, Lee JW, Kim SY, et al. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 2005;65(3):815–821. | ||
Soung YH, Lee JW, Kim SY, et al. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene. 2005;24(1):141–147. | ||
You J, He Z, Chen L, et al. CH05-10, a novel indinavir analog, is a broad-spectrum antitumor agent that induces cell cycle arrest, apoptosis, endoplasmic reticulum stress and autophagy. Cancer Sci. 2010;101(12):2644–2651. | ||
Jemaà M, Galluzzi L, Kepp O, et al. Preferential killing of p53-deficient cancer cells by reversine. Cell Cycle. 2012;11(11):2149–2158. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.