Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Reversible Affective Symptoms and Attention Executive Control Network Impairment Following Thyroid Function Normalization in Hyperthyroidism
Authors Yuan L , Zhang Y , Luan D , Xu X, Yang Q, Zhao S, Zhou Z
Received 15 August 2019
Accepted for publication 11 November 2019
Published 26 November 2019 Volume 2019:15 Pages 3305—3312
DOI https://doi.org/10.2147/NDT.S227386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Lili Yuan,1,* Yuanxiang Zhang,2,* Di Luan,1 Xiangjun Xu,1 Qian Yang,1 Shoucai Zhao,1 Zhiming Zhou1
1Department of Neurology, The First Affiliated Hospital of Wannan Medical College, Wuhu, Anhui Province, People’s Republic of China; 2Department of Clinical Pharmacy, The First Affiliated Hospital of Wannan Medical College, Wuhu, Anhui Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lili Yuan
Department of Neurology, The First Affiliated Hospital of Wannan Medical College, Wuhu, Anhui Province 241000, People’s Republic of China
Email [email protected]
Introduction: Affective symptoms and attention impairments are found in patients with hyperthyroidism. Our previous data have revealed that the patients with hyperthyroidism experience impairments of the attention networks, but it remains unclear whether these disorders persist after the treatment of hyperthyroidism.
Methods: Twenty healthy controls and 25 hyperthyroid patients were recruited and performed the attention network test (ANT) which can simultaneously examine the alertness, orientation and execution control components of the participants. The effect of treatment on affective symptom and attention networks impairments were examined in the patient group after 1-year anti-thyroid medication and reaching euthyroidism for at least 3 months.
Results: Anxiety and depression scores of patients with hyperthyroidism were significantly higher than those of the healthy control group. The patients with hyperthyroidism had impairments of the alerting and executive control networks. Meanwhile, the score of HAMA correlated significantly with thyroid hormone and TSH levels, and there was a negative significant correlation between the score of HAMD and TSH level in all subjects. There was a positive correlation between the value of the executive control network and thyroid hormones’ levels in all subjects and in the hyperthyroidism group. Anxiety and depression symptoms were improved with methimazole treatment after euthyroidism was reached. The value of the executive control network no longer differed from that of healthy controls, but deficits in the alerting network of hyperthyroidism still persisted after treatment.
Conclusion: The patients with hyperthyroidism existed affective symptoms and attention networks impairments. Affective symptoms and attention executive control network impairment were improved following thyroid function normalization in hyperthyroidism.
Keywords: hyperthyroidism, thyroid hormones, cognition, affective symptoms, attention network test
Introduction
Hyperthyroidism is one of the common endocrine diseases and can be treated with medication.1 Oral drug therapy is one of the main therapies in hyperthyroidism, and antithyroid drugs have been used to treat hyperthyroidism for more than 60 years. Methimazole is the first-line drug for the treatment of hyperthyroidism.2
Patients with hyperthyroidism often exhibit hypermetabolic symptoms due to excess thyroid hormone.3 These hypermetabolic symptoms are likely to disappear after anti-thyroid medication and reaching euthyroidism. Several clinical evidences have demonstrated that the patients with hyperthyroidism have affective and cognitive impairments such as irritability, anxiety, inability to concentrate, forgetfulness and so on.4–7 The patients with hyperthyroidism have higher scores of anxiety and depression than healthy control using various self-rating scales and other-rating scales.8–11 Our previous data have revealed that the patients with hyperthyroidism experience impairments of the attention networks.12 Following up on the newly diagnosed patients with hyperthyroidism, Vogel has found that their HAMA and HAMD scores at 3 months and 1 year after treatment were significantly lower than those without treatment.6 A patient with hyperthyroid dementia has attention, memory and behavior disorders, and his initial single-photon emission computed tomography (SPECT) has showed diffusion tracer uptake defect in the bilateral temporoparietal regions. After treatment with a beta-blocker (metoprolol tartrate) and an anti-thyroid drug (methimazole), his global cognitive dysfunction and abnormal SPECT findings have been normalized gradually when the hyperthyroid state has been corrected.13 However, there are some objective evidences that neuropsychological performances may be still damage to some degree in patients with hyperthyroidism even as an euthyroid state has been established after appropriate treatment. Previous investigation indicated that some of the patients with hyperthyroidism have long-term residual cognitive neuropsychological dysfunction using the Hyperthyroidism complained Questionnaire (HCQ), the Symptom checklist – 90 (SCL-90) and the Nottingham Health scale (NHP) in patients with abnormal thyroid function of Hyperthyroidism when their thyroid function has been normalized for less than 12 months or more than 12 months after treatment.8
In order to exclude the interference of other treatment methods and drugs, only hyperthyroidism patients treated with methimazole alone have been recruited in our study to compare whether their affective scores and attention networks appear changes before and after treatment with methimazole. Due to individual differences in the sensitivity of anti-thyroid drug and treatment compliance, thyroid hormones may fluctuate greatly. Therefore, only patients with hyperthyroidism who have been treated for more than 1 year and have reported euthyroidism for at least 3 months were included in the study during the follow-up.
Methods
Subjects
Twenty-five patients with hyperthyroidism (13 females and 12 males) and 20 healthy controls (10 females and 10 males) were recruited in the current study with informed consent and the approval of the ethics committee of Anhui Medical University. Their ages ranged from 18 to 50 and schooling ranged from 5 to 16 years. All patients with hyperthyroidism had never been treated with anti-thyroid medication. The exclusion criteria included left-handed, Mini-Mental State Examination (MMSE) score < 26, Hamilton Anxiety Scale (HAMA) score > 14, Hamilton Depression Scale (HAMD) score > 17, addiction to psychoactive substances, neurologic or psychiatric disorders, and other endocrine diseases.
Serum Measurements
Chemiluminescence immunoassay was used to measure serum hormone (T3, T4 and TSH) level. 0.92–2.79 nmol/L, 58.10–140.60 nmol/L and 0.550–4.780 µIU/mL were within the normal range of T3, T4 and TSH, respectively. Blood of the participants were drawn within 24 hrs before the neuropsychological tests.
Neuropsychological Background Tests
The MMSE, digit span test (DST), HAMA (version: 14-item) and HAMD (version: 17-item) were employed to measure globe cognitive ability, working memory, anxiety and depressive degree, respectively.
ANT
The ANT is an assessment instrument to assess alertness, orientation, and executive control functions.14 The participants were required to watch a line of five black horizontal arrowheads shown on a gray background of the computer screen and make the response via two different buttons according to the target arrows in the center. The experimental procedure is presented in Figure 1. At first, a cross was shown on the center of the screen and the time of duration (400–1600 ms) was varied randomly. After the duration of the cue condition (100 ms), another fixation period (400 ms) appeared. Then, the target item was presented until the participant pressing a response button (maximum time <2700 ms). The last fixation period depended on the first fixation period and time of making a response. Three target types, respectively, were neutral condition, congruent condition, incongruent condition throughout the task. There were four cue conditions: no-cue (only the cross was presented at the center of screen for 100 ms), central-cue (a cue was presented at the central cross), double-cue (two cues were simultaneously presented above and below the central cross, only warning the participant of the forthcoming target item), and spatial-cue (a cue was only presented above or below the central cross, providing the spatial information of target item) (see Figure 1).
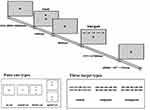 |
Figure 1 Experimental procedure of the ANT. |
The ANT task divided into two parts: a 24-trial practice set and 3 experimental sets. There are 96 trials (48 conditions: 4 cue types × 2 target locations × 2 target directions × 3 flanker conditions, with 2 repetitions) in each experimental set. The sequence of presentation of trials was completely randomized.
Computation of Attention Network Efficiencies
The efficiencies of attention networks were calculated by the differences of reaction times (RT) under 12 conditions (4 cue types by 3 target types). The efficiency of alerting was computed by the average RT in no-cue condition minus the average RT in double-cue condition. The efficiency of orienting was computated by subtracting the average RT in spatial-cue condition from the average RT in center-cue condition. The efficiency of executive control was computated by the average RT in incongruent condition minus the average RT in congruent condition.
Follow-Up Assessments
The effect of treatment on affective symptom and attention networks impairments were examined in the patient group after 1-year anti-thyroid medication and reaching euthyroidism for at least 3 months. To eliminate the learning effect, the healthy controls were examined twice, and the interval between the two assessments was approximately 1 year.
Statistical Analysis
The data analysis was carried out with SPSS statistics software (version 13.0). The results were described as mean ± standard deviation. The independent sample t-test was employed to compare the difference of age, schooling, serum hormone level, scores of neuropsychological background tests and performances of the ANT of two groups. The differences of sex were compared by a Chi-square test. The changes in the attention networks and affective scores at baseline and at follow-up examinations were detected using paired sample t-test. Pearson and spearman correlation analyses were utilized to detect the correlation between thyroid hormone or TSH and the three networks. P < 0.05 was considered significant.
Results
Demographic, Clinical Characteristics and Neuropsychological Background Data
The data of the demographic, clinical and neuropsychological background of healthy controls and the patients with hyperthyroidism are shown in Table 1. No evidence found obvious differences in age, sex, schooling, scores of MMSE or DST between two groups. There wereremarkable differences in levels of T4, T3 and TSH and scores of HAMA and HAMD between two groups (see Table 1).
 |
Table 1 Background Characteristics of the Patients with Hyperthyroidism Before Treatment and Healthy Controls at Baseline |
Efficiencies of Three Networks
ANT performance of healthy controls and patients with hyperthyroidism before treatment are shown in Table 2. The alerting network efficiency of healthy controls was significantly higher than that of hyperthyroidism groups (t (43) = 2.391, p < 0.05). The value of the executive control network in patients with hyperthyroidism was statistically higher than that of healthy controls (t (43) = −2.244, p < 0.05). No remarkable differences in the orienting network efficiency, mean RT and accuracy between two groups (t (43) = 0.810, p > 0.05; t (43) = 0.851, p > 0.05; t (43) = −0.500, p > 0.05).
 |
Table 2 Attention Network RT and Accuracy (%) of Patients with Hyperthyroidism Before Treatment and Healthy Controls at Baseline |
Correlations Between Affective Symptoms, Efficiencies of Three Attention Networks and Thyroid Function
No significant correlations were observed between anxiety and depression symptoms and efficiencies of alerting (r = −0.169, p = 0.268; r = −0.238, p = 0.116), orienting (r = −0.205, p = 0.176; r = −0.029, p = 0.848) and executive control (r = 0.155, p = 0.311; r = −0.012, p = 0.937) networks in all subjects. Score of HAMA correlated significantly with T3 (r = 0.345, p = 0.020), T4 (r = 0.398, p = 0.007) and TSH (r = −0.561, p < 0.001) in all subjects. Score of HAMD correlated significantly with TSH (r = −0.482, p = 0.001), not correlate with T3 (r = 0.181, p = 0.234) and T4 (r = 0.249, p = 0.099) in all subjects. Value of alerting networks correlated significantly with TSH (r = 0.354, p = 0.017), not correlate with T3 (r = −0.124, p = 0.418) and T4 (r = −0.151, p = 0.321) in all subjects. There was no significant correlation between value of orienting networks and levels of T3 (r = −0.130, p = 0.394), T4 (r = −0.135, p = 0.377) and TSH (r = 0.097, p = 0.526). Value of executive control network correlated with levels of T3 (r = 0.474, p = 0.001) and T4 (r = 0.453, p = 0.002), not correlate with TSH (r = −0.254, p = 0.092) in all subjects.
No significant correlations were observed between scores of HAMA and HAMD and efficiencies of alerting (r = 0.239, p = 0.250; r = 0.168, p = 0.423), orienting (r = −0.279, p = 0.176; r = −0.011, p = 0.958) and executive control (r = 0.094, p = 0.655; r = −0.204, p = 0.327) networks in hyperthyroidism group. No significant correlations were observed between scores of HAMA and HAMD and levels of T3 (r = 0.047, p = 0.822; r = 0.306, p = 0.137), T4 (r = −0.062, p = 0.770; r = −0.338, p = 0.098) and TSH (r = −0.046, p = 0.828; r = 0.022, p = 0.915) in hyperthyroidism group. There was no significant correlation between value of alerting and orienting networks and levels of T3 (r = 0.212, p = 0.308; r = 0.107, p = 0.609), T4 (r = 0.237, p = 0.255; r = −0.076, p = 0.719) and TSH (r = 0.257, p = 0.215; r = −0.090, p = 0.669) in hyperthyroidism group. Value of executive control network correlated with levels of T3 (r = 0.490, p = 0.013) and T4 (r = 0.488, p = 0.013), not correlate with TSH (r = −0.079, p = 0.707) in hyperthyroidism group.
For healthy controls, no significant correlations were observed between scores of HAMA and HAMD and efficiencies of alerting (r = −0.407, p = 0.075; r = −0.189, p = 0.425), orienting (r = 0.016, p = 0.948; r = 0.085, p = 0.723) and executive control (r = −0.227, p = 0.337; r = −0.194, p = 0.413) networks. No significant correlations were observed between scores of HAMA and HAMD and levels of T3 (r = −0.378, p = 0.101; r = −0.306, p = 0.190), T4 (r = −0.214, p = 0.366; r = −0.147, p = 0.537) and TSH (r = 0.320, p = 0.169; r = 0.340, p = 0.143) in healthy controls. There was no significant correlation between value of alerting, orienting and executive control networks and levels of T3 (r = 0.220, p = 0.352; r = 0.419, p = 0.066; r = 0.195, p = 0.410), T4 (r = 0.321, p = 0.168; r = −0.043, p = 0.856; r = −0.044, p = 0.855) and TSH (r = 0.151, p = 0.524; r = 0.335, p = 0.148; r = −0.376, p = 0.102) in healthy controls.
Thyroid Function, Affective Symptoms and Performance of ANT: Follow-Up Results
Thyroid function and the scores of HAMA and HAMD from the follow-up examinations are shown in Table 3. From Table 3, there was no significant difference in thyroid hormone, TSH and affective symptoms between hyperthyroidism after treatment and healthy controls after 1 year. There were remarkable differences in levels of T4, T3 and TSH and scores of HAMA and HAMD between the patients with hyperthyroidism at baseline and 1 year after treatment initiation (t (24) = 7.472, p < 0.01; t (24) = 10.866, p < 0.01; t (24) = −9.351, p < 0.01; t (24) = 5.564, p < 0.01; t (24) = 3.960, p < 0.01). There was no significant difference in thyroid function and affective symptoms of healthy controls from the baseline examination to the follow-up examination 1 year later (t (19) = 0.983, p = 0.338; t (19) = 0.663, p = 0.516; t (19) = −0.219, p = 0.829; t (19) = 0.596, p = 0.558; t (19) = 1.083, p = 0.292).
 |
Table 3 Thyroid Function and Affective Scores of Patients with Hyperthyroidism After Treatment and Healthy Controls After 1 Year |
ANT performance of healthy controls after 1 year and patients with hyperthyroidism after treatment are shown in Table 4. The alerting network efficiency of healthy controls was significantly higher than that of hyperthyroidism after treatment groups (t (43) = 2.466, p < 0.05). No remarkable differences in the efficiencies of orienting and executive control networks, mean RT and accuracy between two groups (t (43) = 1.407, p > 0.05; t (43) = −1.680, p > 0.05; t (43) = 0.932, p > 0.05; t (43) = −0.422, p > 0.05). From Figure 2A, there was the remarkably differences in executive control networks between the patients with hyperthyroidism before treatment and 1 year after treatment initiation (t (24) = 2.466, p = 0.014). No remarkable differences in the efficiencies of alerting and orienting networks between the patients with hyperthyroidism before treatment and 1 year after treatment initiation (t (24) = −0.813, p = 0.424; t (24) = 0.953, p = 0.350; t (24) = 0.632, p = 0.533; t (24) = −1.515, p = 0.143). No evidence found obvious differences in the performance (efficiencies of alerting, orienting and executive control networks) of healthy controls from the baseline ANT to the follow-up ANT 1 year later (t (19) = −0.262, p = 0.796; t (19) = 0.239, p = 0.814; t (19) = 1.162, p = 0.259; t (19) = 0.772, p = 0.540; t (19) = −1.473, p = 0.157) (see Figure 2B).
 |
Table 4 Attention Network RT and Accuracy (%) of Patients with Hyperthyroidism After Treatment and Healthy Controls After 1 Year |
Discussion
This current study clearly demonstrated that scores of HAMA and HAMD in patients with hyperthyroidism were higher than those in healthy controls, and there was a significant decline in these scores at the 1-year follow-up examination after antithyroid drug treatment. The score of HAMA correlated significantly with thyroid hormone and TSH levels, and there was a negative significant correlation between the score of HAMD and TSH level in all subjects. We observed impairments of alerting and executive control networks in patients with hyperthyroidism. There was a positive correlation between the value of the executive control network and thyroid hormone levels in all subjects and in the hyperthyroidism group. After 1 year of methimazole treatment, the value of the executive control network no longer differed from that of healthy controls, but deficits in alerting network of hyperthyroidism persisted.
Even though severe depression and anxiety were excluded, scores of HAMA and HAMD in hyperthyroidism group were higher than in healthy controls. The results were similar to Gulseren et al’s conclusion.9 They found that the levels of anxiety and depression in the overt hyperthyroidism group were higher than in the control group. The previous study demonstrated affective symptoms were very common in hyperthyroidism.11,15–17 Anxiety and depression symptoms were improved with methimazole treatment after euthyroidism was reached. Our results were in accordance with Vogel et al who described a significant decrease in affective symptoms when an euthyroid state was established.6
The patients with hyperthyroidism had deficits in alerting and executive control networks which were reported by our previous research.12 Neuroimage study showed lower activity in the limbic system, frontal lobes and temporal lobes of patients with hyperthyroidism before antithyroid therapy.18–20 It is speculated that abnormal metabolism in the limbic system and frontal cortex were caused by excessive thyroid hormone. Fukui et al13 thought that the integrity of the thyroid-locus ceruleus-frontal lobe system was damaged, and it was difficult to maintain attention and vigilance. The alerting and executive control networks related to norepinephrine and dopamine systems, respectively.21 Thyroid hormone played an important role in neurotransmitters including norepinephrine and dopamine.22–24 Preclinical research showed the levels of norepinephrine and dopamine were decreased in the cerebral cortex of hyperthyroidism rats.25 Treatment with methimazole lasted for 1-year improved attention executive control network function, although impairment of alerting network persisted. The result was a bit different from Fukui et al13 who reported overall cognitive functions and vigilance of a patient with hyperthyroid dementia were normalized in response to treatment. A study of Brain FDG-PET scans supported our conclusion and they found some cerebral hypometabolism in patients with hyperthyroidism could be improved after treatment of hyperthyroidism.26 Different brain regions had different responses to thyroid hormone levels. Specific brain regions or neurotransmitters associated with alertness might be less sensitive to changes in thyroid hormone than executive control networks. The investigation of Fahrenfort et al showed that regular treatment could alleviate many of the symptoms in patients with hyperthyroidism, but some of the patients with euthyroid function at least 1 year still had neurologic and psychological complaints.8 Meanwhile, by comparing patients with euthyroid function for less than 1 year and those with euthyroid function for more than 1 year, Fahrenfort et al found that patients with hyperthyroidism could benefit from the prolonged remission period.8 Therefore, it is unclear whether the alertness network function would gradually return to normal with the extension of the maintenance period of hyperthyroidism treatment.
In our study, the significant correlations between affective score or attention network and hormone levels were found. The score of HAMA correlated significantly with thyroid hormone and TSH levels, and there was a negative significant correlation between the score of HAMD and TSH level in all subjects. There was a positive correlation between the value of the executive control network and thyroid hormones' levels in all subjects and in the hyperthyroidism group. There was a debate about which hormones, thyroid hormones or TSH had a greater effect on affective symptom and cognitive function. From our results, we thought that the TSH level was more closely related to depression than thyroid hormones, and thyroid hormones' levels were more closely related to executive control network function than TSH.
In conclusion, we observed affective symptoms and attention networks impairments in patients with hyperthyroidism. Depression was closely linked to the TSH level, and anxiety was closely linked to TSH and thyroid hormone in all subjects. Meanwhile, the executive control network was closely linked to thyroid hormones in all subjects and in the hyperthyroidism group. Follow-up data demonstrated a significant decrease in affective symptoms and value of the executive control network, but impairment of alerting network still persisted after 1 year of methimazole treatment. In the future research, the follow-up time was extended to observe whether the alert network returned to normal, and neuroimaging was employed to explore the mechanism of excessive thyroid hormone affecting emotion and attention network function.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
We obtained ethical approval to carry out this study from the Institutional Review Board (IRB) of Yijishan Hospital of Wannan Medical College, Wuhu, China. All patients or their relatives provided written informed consent for this research, which was carried out in compliance with the Helsinki Declaration. All individual information was strictly kept confidential and anonymous in the manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Kravets I. Hyperthyroidism: diagnosis and treatment. Am Fam Physician. 2016;93(5):363–370.
2. Azizi F, Yousefi V, Bahrainian A, Sheikholeslami F, Tohidi M, Mehrabi Y. Long-term continuous methimazole or radioiodine treatment for hyperthyroidism. Arch Iran Med. 2012;15(8):477–484. doi:10.12158/AIM.007
3. Lee JE, Lee DH, Oh TJ, et al. Validity and reliability of the Korean version of the hyperthyroidism symptom scale. Endocrinol Metab. 2018;33(1):70–78. doi:10.3803/EnM.2018.33.1.70
4. Yuan L, Tian Y, Zhang F, et al. Decision-making in patients with hyperthyroidism: a neuropsychological study. PLoS One. 2015;10(6):e0129773. doi:10.1371/journal.pone.0129773
5. Riguetto CM, Neto AM, Tambascia MA, Zantut-Wittmann DE. The relationship between quality of life, cognition, and thyroid status in Graves’ disease. Endocrine. 2019;63(1):87–93. doi:10.1007/s12020-018-1733-y
6. Vogel A, Elberling TV, Hording M, et al. Affective symptoms and cognitive functions in the acute phase of Graves’ thyrotoxicosis. Psychoneuroendocrinology. 2007;32(1):36–43. doi:10.1016/j.psyneuen.2006.09.012
7. Alvarez MA, Gomez A, Alavez E, Navarro D. Attention disturbance in Graves’ disease. Psychoneuroendocrinology. 1983;8(4):451–454. doi:10.1016/0306-4530(83)90026-4
8. Fahrenfort JJ, Wilterdink AM, van der Veen EA. Long-term residual complaints and psychosocial sequelae after remission of hyperthyroidism. Psychoneuroendocrinology. 2000;25(2):201–211. doi:10.1016/S0306-4530(99)00050-5
9. Gulseren S, Gulseren L, Hekimsoy Z, Cetinay P, Ozen C, Tokatlioglu B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch Med Res. 2006;37(1):133–139. doi:10.1016/j.arcmed.2005.05.008
10. Larisch R, Kley K, Nikolaus S, et al. Depression and anxiety in different thyroid function states. Horm Metab Res. 2004;36(9):650–653. doi:10.1055/s-2004-825925
11. Calissendorff J, Mikulski E, Larsen EH, Moller M. A prospective investigation of Graves’ disease and selenium: thyroid hormones, auto-antibodies and self-rated symptoms. Eur Thyroid J. 2015;4(2):93–98. doi:10.1159/000381768
12. Yuan L, Tian Y, Zhang F, et al. Impairment of attention networks in patients with untreated hyperthyroidism. Neurosci Lett. 2014;574:26–30. doi:10.1016/j.neulet.2014.05.016
13. Fukui T, Hasegawa Y, Takenaka H. Hyperthyroid dementia: clinicoradiological findings and response to treatment. J Neurol Sci. 2001;184(1):81–88. doi:10.1016/S0022-510X(00)00487-1
14. Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–347. doi:10.1162/089892902317361886
15. Ittermann T, Volzke H, Baumeister SE, Appel K, Grabe HJ. Diagnosed thyroid disorders are associated with depression and anxiety. Soc Psychiatry Psychiatr Epidemiol. 2015;50(9):1417–1425. doi:10.1007/s00127-015-1043-0
16. Li L, Zhi M, Hou Z, Zhang Y, Yue Y, Yuan Y. Abnormal brain functional connectivity leads to impaired mood and cognition in hyperthyroidism: a resting-state functional MRI study. Oncotarget. 2017;8(4):6283–6294. doi:10.18632/oncotarget.14060
17. Fischer S, Ehlert U. Hypothalamic-pituitary-thyroid (HPT) axis functioning in anxiety disorders. A systematic review. Depress Anxiety. 2018;35(1):98–110. doi:10.1002/da.2018.35.issue-1
18. Schreckenberger MF, Egle UT, Drecker S, et al. Positron emission tomography reveals correlations between brain metabolism and mood changes in hyperthyroidism. J Clin Endocrinol Metab. 2006;91(12):4786–4791. doi:10.1210/jc.2006-0573
19. Elberling TV, Danielsen ER, Rasmussen AK, Feldt-Rasmussen U, Waldemar G, Thomsen C. Reduced myo-inositol and total choline measured with cerebral MRS in acute thyrotoxic Graves’ disease. Neurology. 2003;60(1):142–145. doi:10.1212/01.WNL.0000038911.07643.BF
20. Bhatara VS, Tripathi RP, Sankar R, Gupta A, Khushu S. Frontal lobe proton magnetic-resonance spectroscopy in Graves’ disease: a pilot study. Psychoneuroendocrinology. 1998;23(6):605–612. doi:10.1016/S0306-4530(98)00028-6
21. Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi:10.1146/annurev-neuro-062111-150525
22. Claustre J, Balende C, Pujol JF. Influence of the thyroid hormone status on tyrosine hydroxylase in central and peripheral catecholaminergic structures. Neurochem Int. 1996;28(3):277–281. doi:10.1016/0197-0186(95)00088-7
23. Bernal J, Guadano-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13(11):1005–1012. doi:10.1089/105072503770867174
24. Accorroni A, Chiellini G, Origlia N. Effects of thyroid hormones and their metabolites on learning and memory in normal and pathological conditions. Curr Drug Metab. 2017;18(3):225–236. doi:10.2174/1389200218666170116112407
25. Mano T, Sakamoto H, Fujita K, et al. Effects of thyroid hormone on catecholamine and its metabolite concentrations in rat cardiac muscle and cerebral cortex. Thyroid. 1998;8(4):353–358. doi:10.1089/thy.1998.8.353
26. Miao Q, Zhang S, Guan YH, et al. Reversible changes in brain glucose metabolism following thyroid function normalization in hyperthyroidism. AJNR Am J Neuroradiol. 2011;32(6):1034–1042. doi:10.3174/ajnr.A2449
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

